Abstract
Purpose
Despite American College of Rheumatology recommendations, appropriate and timely initiation of biologic therapies does not always occur. This study examined openness to and preference for attributes of biologic therapies among patients with rheumatoid arthritis (RA), differences in patients’ and rheumatologists’ perceptions, and discussions around biologic therapy initiation.
Patients and methods
A self-administered online survey was completed by 243 adult patients with RA in the US who were taking disease-modifying antirheumatic drugs (DMARDs) and had never taken, but had discussed biologic therapy with a rheumatologist. Patients were recruited from a consumer panel (n=142) and patient advocacy organization (n=101). A separate survey was completed by 103 rheumatologists who treated at least 25 patients with RA per month with biologic therapy. Descriptive and bivariate analyses were conducted separately for patients and rheumatologists. Attributes of biologic therapy included route of administration (intravenous infusion or subcutaneous injection), frequency of injections/infusions, and duration of infusion.
Results
Over half of patients (53.1%) were open to both intravenous infusion and subcutaneous injection, whereas rheumatologists reported 40.7% of patients would be open to both. Only 26.3% of patients strongly preferred subcutaneous injection, whereas rheumatologists reported 35.2%. Discrepancies were even more pronounced among specific patient types (eg, older vs younger patients and Medicare recipients). Among patients, 23% reported initiating discussion about biologics and 54% reported their rheumatologist initiated the discussion. A majority of rheumatologists reported discussing in detail several key aspects of biologics, whereas a minority of patients reported the same.
Conclusion
Preferences differed among patients with RA from rheumatologists’ perceptions of these preferences for biologic therapy, including greater openness to intravenous infusion among patients than assumed by rheumatologists and relative lack of discussion about key aspects of biologic therapy perceived by patients. There is a need for more open communication about treatment options, which may encourage more appropriate, timely transition to biologic therapy.
Introduction
Rheumatoid arthritis (RA), a chronic progressive autoimmune condition, is estimated to affect between 0.5% and 1.0% of the adult population globally.Citation1 The prevalence of arthritis overall has been rising, with RA accounting for 17.9% of those diagnosed with arthritis, according to 1999–2008 data from the US National Health and Nutrition Examination survey.Citation2 RA is most prevalent among older individuals and females.Citation1 RA has been found to have an extensive impact on mortality and morbidity.Citation3 For instance, RA leads to pain, morbidity, joint damage, a decline in functional abilities, a high rate of comorbidities, and higher rates of mortality.Citation1,Citation4,Citation5 In addition to the direct costs of health care associated with managing these negative outcomes, significant indirect costs are incurred by RA, such as a loss in work productivity.Citation6,Citation7
Traditional disease-modifying antirheumatic drugs (DMARDs), most commonly methotrexate, have demonstrated efficacy in the management of RA and are used by a majority of patients. However, the American College of Rheumatology recommends initiation of biologic therapy in patients who are not adequately responding to DMARDs (ie, moderate to high disease activity with methotrexate mono-therapy lasting 3 months or DMARD combination therapy).Citation3,Citation8 Biologic therapies have been shown to be highly effective in treating RA, including managing symptomsCitation9,Citation10 and halting progression of structural damage and physical disability due to RA.Citation11,Citation12 This reduction in symptom burden and structural damage, in turn, leads to decreases in the level of disabilityCitation9 and cost of careCitation10 faced by patients with RA. Biologic therapies have also been associated with more quality-adjusted life years, in comparison with traditional DMARDs.Citation10 Moreover, biologic therapies have been associated with reduced disease activity and improved functional abilities of RA sufferers, as well as increased quality of life when used alone and in combination with methotrexate or other DMARDs.Citation13–Citation15 It is anticipated that demand for biologic therapies will increase from both patients and prescribers as more evidence is presented about the associated outcomes.Citation10
Nonetheless, research indicates that appropriate and timely escalation of treatment from DMARDs to biologic therapies does not always occur, in spite of their demonstrated effectiveness.Citation16 Also, rheumatologists and patients have been found to differ with respect to instrumental factors in care escalation decisions.Citation16 Biologic agents are currently administered via two routes: subcutaneous injection (SQ) and intravenous infusion (IV). SQ and IV administration of biologic agents differs not only in routes of administration but also in dosing schedulesCitation17 and costs,Citation18 which are associated with patients’ preferences and corresponding persistence in treatment utilization. Additionally, injection issues (depending on route of administration and agent type) have been shown to influence patients’ utilization of biologic therapies.Citation19 Prior research suggests variations in dosing frequency, delivery type, pH levels, and needle size may all influence whether patients discontinue using a particular biologic agent.Citation19 Factors such as safety, efficacy, and patients’ preferences for various aspects of the biologic therapies have also been identified as integral to patients’ decision making regarding RA treatment.Citation20
Frequency of dosing and route of administration have also been demonstrated to play a significant role not only in persistence with therapies but also in the initial decision-making process regarding biologic therapy.Citation3 For example, one study found that route of administration was the most important feature for patients in choosing a biologic therapy.Citation21 This study also found that SQ was the most preferred route of administration by both those currently receiving and those not yet receiving biologic therapies. In addition to preferring SQ route of administration, patients preferred the longest possible interval between administrations of treatment. Another two studies identified frequency of dosing as the second or third most preferred attribute of biologic therapy reported by patients with RA.Citation22,Citation23
Physician guidance is another factor influencing patients’ decisions regarding RA treatment.Citation24 In fact, a rheumatologist’s experience with a particular therapy has been identified as one of the most important factors taken into consideration by both patients and rheumatologists when deciding on a biologic therapy.Citation22 Many studies have examined how patients and rheumatologists approach the decision to escalate treatment to biologic therapies.Citation25–Citation27 However, a majority of these studies represented either rheumatologists’ or patients’ perspectives alone, and many of these studies examined only a limited number of factors influencing the decision-making process. Other studies have noted the importance of information for patients with RA regarding their treatment-related decision making, with a high level of need for information identified.Citation28,Citation29 Thus, there is a need for open communication and for rheumatologists to have a clear understanding of patients’ preferences.
The primary aim of the current study was to examine openness to and preference for attributes of biologic therapies among patients with RA prior to biologic therapy initiation. A secondary aim was to examine rheumatologist perceptions of patient openness to and preference among biologic therapies. A related aim was to identify gaps between rheumatologist and patient perceptions about biologic therapies and corresponding discussions about biologic therapies, as well as implications for patient–rheumatologist dialogue and the decision-making process surrounding biologic therapy treatment for RA.
Materials and methods
Sample
The sample for this study consisted of 243 patients with RA in the US recruited from a consumer panel, Lightspeed Research (n=142), and a patient advocacy organization, Creaky Joints (n=101). Patient inclusion criteria were as follows: self-reported diagnosis of RA by a rheumatologist, visited a rheumatologist for RA in the past 6 months, currently taking DMARDs, never taken a biologic therapy, aware of and discussed biologic therapy with rheumatologist, aged 18 years or older, residing in the US, able to read/write English, and provided online informed consent. Rheumatologist inclusion criteria were as follows: specialty in rheumatology, board certified or eligible, 2–25 years postresidency practice as a rheumatologist, at least 50% time spent providing direct patient care, at least 50 patients with RA treated per month, at least 25 treated with biologic therapy, not employed by or providing consulting services to any pharmaceutical company, and not working for government. The study protocol was reviewed and approved by the Essex Institutional Review Board (Lebanon, NJ, USA).
Measures
Patient data were collected via a self-administered online survey ~30 minutes in length. Rheumatologist data were also collected via a self-administered online survey ~15 minutes in length (see Table S1 for an outline of survey items).
Openness to route of administration (IV and SQ) was assessed for patients via a 5-point Likert scale (1= not at all open; 2= not very open; 3= somewhat open; 4= very open; and 5= extremely open), with 3–5 categorized as “open”. Patient openness to route of administration as reported by rheumatologists was assessed by requiring rheumatologists to report the proportion of their patients estimated to be open to IV only, SQ only, both, or neither. Patient openness to specific biologic therapy attributes (ie, duration of infusions and frequency of injections/infusions based upon the characteristics of approved biologic treatments in the US at the time of the study) was also assessed for both patients and rheumatologists via the 5-point Likert scale described above. Mean scores were calculated to determine patient and rheumatologist perceptions of biologic treatment characteristics.
Patient preference was assessed from both the patients’ and rheumatologists’ perspective via allocation of 100 points across various biologic therapy options. Mean scores were calculated to determine patient and rheumatologist perceptions of preferences. Preference for route of administration (IV and SQ) was assessed for patients via a 5-point Likert scale ranging from 1= strongly prefer self-injection to 5= strongly prefer IV infusion, with 1–2 or 4–5 categorized as preferring one route or the other. Patient preference for route of administration was assessed for rheumatologists via proportion of their patients estimated to fall within each of the five aforementioned preference categories.
Level of discussion around treatment was rated by both patients and rheumatologists as “not discussed at all”, “discussed somewhat”, or “discussed in detail”.
Statistical analyses
Descriptive analyses (means and standard deviations for continuous variables and percentages and frequencies for categorical variables) were conducted for patient demographics and openness to and preference among biologic therapies and routes of administration, as well as other patient and rheumatologist attitudes related to treatment perceptions and decision-making preferences. The statistical significance (two-tailed P<0.05) of differences across groups on measures assessed in a compatible manner (ie, consumer panel vs advocacy group comparisons) was assessed via pairwise t-tests for mean (continuous variables) and chi-square or binomial proportion tests for frequencies (categorical variables).
Patients recruited via Creaky Joints and the consumer panel were initially compared in terms of various characteristics in order to understand sample differences and inform the breadth of representativeness captured in the current study. However, as the objective of the study was to analyze patients on the whole vis-à-vis rheumatologists, patients were subsequently included as a single category for the majority of the analysis.
Results
The mean age of patients in the total sample was 52.5 years (). Mean years since onset of RA symptoms reported by patients was 12.90 years. Patients in this sample had been discussing biologic therapies with their rheumatologists on average for 1.32 years over an average of 2.67 visits. Patients recruited through the consumer panel were on average older (mean=57.3 years) than those recruited through Creaky Joints, the patient advocacy group (mean=45.8 years), P<0.05. The majority of the total patient sample was female (85.2%). However, Creaky Joints patients were more frequently female (93.1%) in comparison with the consumer panel (79.6%), P<0.05. The majority of both Creaky Joints and consumer panel patients were white (88.1% and 83.8%, respectively). About a third of patients (32.9%) were insured with Medicare, a lower proportion in the Creaky Joints (20.8%) than in the consumer (41.5%) panel, P<0.05. Nearly half of patients (49.8%) had at least a college degree (59.4% of Creaky Joints vs 43.0% of consumer panel, P<0.05). About a third (34.2%) of patients were employed full-or part-time, a greater proportion in the Creaky Joints (47.5%) than in the consumer (24.6%) panel, P<0.05. At the same time, a lower proportion of patients were retired in the Creaky Joints (6.9%) than in the consumer (33.1%) panel, with 22.2% retired in total, P<0.05.
Table 1 Patient demographics and disease characteristics
The mean age of rheumatologists was 49.2 years (). Female rheumatologists composed 27.2% of the sample. The majority were white (72.8%). Mean years in practice as a rheumatologist was 14.4 years. Mean patients with RA treated per month was 125.6. The majority of rheumatologists reported having an infusion suite in their office (80.6%).
Table 2 Rheumatologist characteristics
Openness to route of administration and attributes of biologic therapies
Over half of patients were open to both IV and SQ administration (53.1%) (). In contrast, rheumatologists reported that only a mean 40.7% of their patients would be open to both IV and SQ. Whereas rheumatologists thought 34.0% of patients would be open to SQ only, only 16.5% of patients reported openness to SQ only.
Table 3 Patients’ and rheumatologists’ perceptions of patient openness to route of administration and attributes of biologic therapies
Openness to attributes of biologic therapy administration frequency was highest for SQ administration every 4 weeks or monthly for both patients (mean=3.37) and rheumatologists (mean=4.29) (). Mean openness to an IV administration time of 30 minutes every 8 weeks was the third highest among both patients (mean=2.86) and rheumatologists (mean=3.75), below SQ administered every 2 weeks or 4 weeks and above preference for SQ once a week.
Preferences for route of administration and attributes of biologic therapy
A similar pattern emerged in the preference point allocations. The most preferred therapy option was SQ administered every 4 weeks or monthly by both patients (mean=35.1) and rheumatologists (mean=23.3) (). The second most preferred therapy option by patients was IV treatment administered for 30 minutes every 8 weeks (mean=13.9). However, rheumatologists reported that SQ treatment administered every 2 weeks would be the second most preferred therapy option by their patients (mean=14.6), followed by IV treatment administered for 30 minutes every 8 weeks (mean=13.1).
Table 4 Patient and rheumatologist perceptions of patient preferences for route of administration and attributes of biologic therapies
Whereas rheumatologists reported that 35.2% of their patients would strongly prefer SQ, only 26.3% of patients actually reported a strong preference for SQ (). In contrast, whereas rheumatologists reported that 31.3% of their patients would have no preference between SQ and IV administration, only 22.2% of patients reported no preference between the two routes of administration.
Discrepancies between rheumatologist perceptions and patient preferences
Several discrepancies emerged between rheumatologist perceptions and patient reports when stratified by key patient characteristics (). Rheumatologists believed that nearly half (48.6%) of their older patients (aged 65 and older) would prefer IV, compared with none (0.0%) of their younger patients. However, among older patients, only 21.0% reported a preference for IV, compared with 30.0% of younger patients. Whereas rheumatologists believed that only 9.7% of their older patients would prefer SQ, 51.1% of older patients actually reported a preference for SQ. Rheumatologists reported that 70.9% of patients insured with Medicare would prefer IV, whereas only 20.6% of Medicare patients actually reported a preference for IV. In contrast, rheumatologists reported low preference for SQ among Medicare patients (3.9%), whereas 58.6% of Medicare patients reported preferring SQ. Rheumatologists reported low preference for IV among those who were employed (1.9%), whereas 24.2% of employed patients reported preferring IV.
Table 5 Rheumatologist perceptions of patient preferences versus patient preferences, stratified by patient characteristics
Treatment initiation and treatment decision making
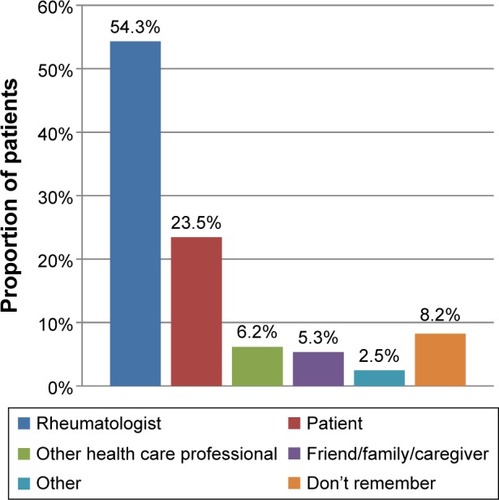
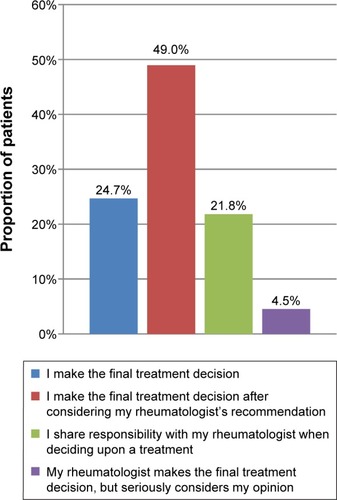
With respect to treatment initiation, over half of patients surveyed (54.3%) reported that the discussion regarding biologic therapy was initiated by their rheumatologist (). Nearly a quarter of patients (23.5%) reported being the ones to initiate discussion. Nearly half of patients surveyed reported that they would make the final decision regarding biologic therapies after considering their rheumatologist’s recommendation (49.0%) (). However, only 21.8% reported sharing the decision-making process equally with their rheumatologist, and 24.7% reported making the decision themselves.
Aspects of biologic therapies discussed by patients and rheumatologists
Several discrepancies emerged with respect to aspects of biologic therapies discussed by patients and rheumatologists. The majority of rheumatologists reported discussing in detail the ability of biologics to slow (78.6%) or stop (76.7%) joint damage (). In contrast, a minority of patients reported discussing in detail biologics’ ability to slow (28.8%) or stop (23.5%) joint damage. Whereas 77.7% of rheumatologists reported discussing in detail the ability of biologics to improve well-being and daily function, only 30.9% of patients reported the same level. Whereas 68.0% of rheumatologists reported discussing in detail the effectiveness of biologics compared with other treatments, only 26.3% of patients reported the same. About a third of patients reported discussing at least somewhat their IV (30.9%) and SQ (34.2%) experience. In contrast, the vast majority of rheumatologists reported discussing the IV (95.1%) or SQ (99.0%) experience with their patients. Frequency of biologic therapy administration was reported being discussed by the majority of patients (74.1%) and rheumatologists (100.0%).
Table 6 Aspects of biologic therapy discussed by patients and rheumatologists
Factors considered when making treatment decisions
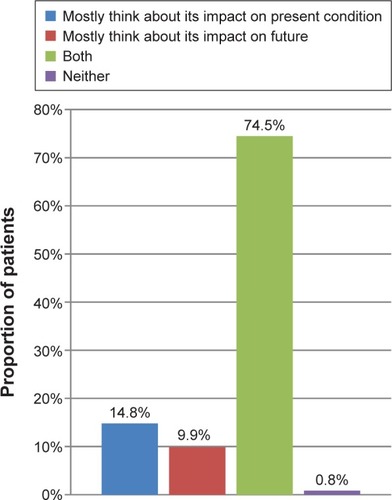
The majority of patients considered the impact of treatments on both their present and future condition when making treatment decisions (74.5%), with only a small percentage considering the impact on present (14.8%) or future (9.9%) condition alone ().
Discussion
The current study found that more patients may be open to both SQ and IV routes of biologic therapy administration than rheumatologists assume. Similarly, more patients may prefer the IV route of administration than rheumatologists perceive. The discrepancies between rheumatologists’ perceptions of patients’ preferences and actual patients’ preferences regarding biologic therapies are consistent with prior studies indicating similar discrepancies.Citation30 For instance, Willeke et alCitation30 found that 94% of rheumatologists reported believing their patients would prefer SQ injections whereas the majority of patients (63%) reported that they would prefer an infusion every 6–9 months.
Findings from the present study also demonstrated substantial variations in preferences among routes of administration across various types of patients based on demographic characteristics (eg, age) and insurance status, as well as in terms of rheumatologists’ expectations. Consistent with the present findings, prior research has also shown that individual preferences among routes of administration were driven by patient characteristics, including: expectations of safety and convenience, past experience, and patients’ perceptions of their disease state.Citation31 The current findings build on this literature with a better understanding of the interplay between patient characteristics and preferences.
The present study examined separately patients’ openness to and preferences among routes of administration (SQ and IV) and various attributes of biologic therapy, as these refer to distinct dimensions worthy of evaluation. On the one hand, our preference measures allow us to determine what patients would choose in a comparative, binary sense. Whereas, our openness measures allow us to determine in a noncomparative way what patients would evaluate as acceptable versus unacceptable, allowing the possibility of openness to multiple treatment options versus none. In tandem, these sets of results provide for a more comprehensive, transparent understanding of patients’ attitudes than either dimension alone. For example, our results indicate that over half of patients were open to both IV and SQ, while at the same time, patients tended to prefer SQ over IV.
Whereas most studies of nondisease specific populations have found that a majority of patients prefer to leave decision making to their physicians, the current study found that nearly a quarter of patients reported initiating the discussion with their rheumatologist.Citation28,Citation29 However, over half of patients reported that their rheumatologist initiated the discussion to start biologic therapy. Most patients reported making treatment decisions after considering their rheumatologist’s recommendation (approximately half) or sharing responsibility (about a fifth). This finding is in line with prior research, which demonstrates that the majority of patients with RA (95%) report using a paternalistic decision-making model immediately after their diagnosis, but then many (74%) of these patients evolve to utilizing shared decision making.Citation32 These findings suggest that rheumatologists play a significant role in treatment decision making for patients with RA.
Despite rheumatologists’ role in treatment decision making, a study by van Hulst et alCitation16 indicated that patients and rheumatologists prioritize very different factors when making decisions regarding treatments. As such, it is critical to understand better the dialogue that occurs between patients and rheumatologists. When examining this dialogue, the present study found several discrepancies between patients’ and rheumatologists’ perceptions of the level of discussion provided around aspects of biologic therapy. Specifically, a larger proportion of rheumatologists than patients reported detailed discussion of potential side effects as well as the ability of biologics to slow or stop joint damage and improve well-being and daily functioning with their patients. Additionally, the majority of patients reported no discussion at all with their rheumatologist regarding SQ or IV infusion experience or the patient’s ability to attend infusion appointments, whereas the majority of rheumatologists reported discussing these topics at least somewhat with their patients. Moreover, a higher proportion of rheumatologists than patients reported that all aspects of biologic therapies were discussed. This inconsistency may highlight a gap in communication in which rheumatologists either do discuss or perceive discussing aspects of biologic therapies more than patients either perceive hearing or do hear these aspects discussed. Future research should examine actual clinical encounters to pinpoint the source of the discrepancy and whether misperceptions reside primarily among rheumatologists, patients, or both, as well as whether issues, such as lack of comprehension on the part of patients and lack of clarity on the part of physicians may contribute to any biases. Improved understanding of communications within clinical encounters can help inform which aspects of communication should be targeted for the most effective intervention.
These discussions between patients and their rheumatologists are critical, as they influence patients’ and rheumatologists’ decisions regarding biologic therapies. Inconsistencies in patient and rheumatologist preferences and beliefs indicate a break in communication that needs to be addressed in order to improve patient care. Not only have patients indicated a high need for information about RA and treatment optionsCitation28,Citation29 but also prior research indicates that a high level of information received was strongly correlated with patients’ involvement in the treatment decision-making process.Citation33 The discrepancies in communication outlined in the present study could exert a substantial influence on the decision to initiate biologic therapy. Thus, providing patients with the appropriate information regarding these critical aspects of biologic therapies could empower more patients to make the decision to escalate care to biologic therapy in a timely fashion. In fact, a systematic review of the literature (n=15 studies) found that most patients required more information related to their treatment and disease.Citation34 Communicating this information might alleviate some patient concerns, such as the discontinuation of therapy, which has been known to affect patients’ decision making regarding RA treatment.Citation35
The differences in reports of what was discussed as well as patients’ actual preferences for which aspects of biologic therapy to discuss with their rheumatologist might influence patients’ final decision regarding which biologic therapy to utilize. Because of discrepancies in reports of which aspects of biologic therapy were discussed in these clinical contexts, shared decision making is all the more important in this context. Prior research on shared decision making indicates that critical steps include both patients and physicians sharing information, building consensus about preferred treatments, and coming to a shared agreement on which treatment to implement.Citation36 Furthermore, shared decision-making models acknowledge that patients and physicians may have different values and preferences – hence the need to build toward reaching consensus on the appropriate treatment.Citation37 Shared decision-making approaches could thus overcome differences between information that patients would like regarding biologic therapies and information the rheumatologists share, ensuring that patients are able to engage in treatment decisions aligned with their own values and preferences. The current study was not designed to assess the association between discussion topic preferences and patients’ final decisions regarding biologic treatment; therefore, future research should examine this link to further inform opportunities for alignment in patient–provider communication.
In regard to treatment decision making, the majority (nearly three-quarters) of patients in the present study reported considering primarily the impact of treatment on their current condition as a factor taken into consideration. This finding is consistent with a previous study showing that 63.8% of respondents would not change their therapy as long as their condition did not worsen.Citation26 Furthermore, results of the present study are consistent with prior research indicating that patients are more motivated to maintain their current condition than to think about future prognosis.Citation26 Namely, only 9.9% of patients in the present study reported thinking about their future health alone when making treatment decisions.
Despite the novel addition of this study to our understanding of the openness to, preferences for, and discrepancies in communication regarding biologic therapy for RA, there are limitations that should be considered. One of the main limitations of this study is the use of an online data collection method. This approach did not yield a random sample; however, this was minimized by the recruitment of the sample from two sources, an advocacy group and a consumer panel. This sampling approach yielded substantial variation across the groups and prevented a bias toward a single patient type. Another limitation of the study is that the surveys were cross-sectional, depicting patients’ and rheumatologists’ perspectives at a single point in time. As such, these perspectives could potentially change based on symptom changes or functional declines. Additionally, this sample was highly educated, which could potentially affect patients’ preferences among treatment options. A majority of the patients in both samples were white and, therefore, noted racial disparities between white and black patients in terms of treatment aggressiveness (eg, lower among black patients with similar disease severityCitation38) may not have been captured in these data. Rheumatologists’ prior experiences with specific biologic agents may have influenced their perceptions and preferences among these therapies; this in turn could have introduced bias into our results, to the extent that 1) our physician sample was not representative of the overall population of physicians and 2) physician and practice characteristics that differed from those of the population were correlated with different preferences among therapeutic options. This potential bias would not be expected among patients, given that they had no prior experience using biologic agents. Finally, patient and rheumatologist data were not linked in this study, thus limiting insight into a precise estimation of the source of disconnect between patients and their corresponding rheumatologist in discussions regarding their treatment options. A patient–rheumatologist link would also have enabled an understanding of the potential influence of the relationship itself on the decision-making process.
Conclusion
Studies have found that patients with RA trust and would benefit from the guidance of their rheumatologists in the decision-making process.Citation20 Thus, there is a strong need for thorough discussions of all available treatment options and patient preferences between patients and their rheumatologists. Additionally, profiles of patients based on demographic variables should not influence rheumatologists’ perceptions of patients’ preferences or priorities, as expectations may frequently be misaligned. Given discrepancies in patient–rheumatologist perceptions around discussions, and considering the substantial role of rheumatologists in the decision-making processCitation32 (both initiating treatment and guiding patients), it is important to address dialogue between patients and rheumatologists to ensure transparency and improve understanding of patients’ needs and preferences.
Author contributions
All authors contributed to the study design, interpretation of results, and review, revision, and approval of the final manuscript. Authors have access to the study data supporting this publication. Duncan Brown, Amir Goren, and Susan C Bolge contributed to data analysis.
Acknowledgments
The authors acknowledge Hema Kannan Gandhi, MPH, for her contribution to literature review and developing the detailed outline of the manuscript with funding from Janssen Scientific Affairs, LLC. The authors acknowledge Megan Shen, PhD, assistant professor at Weill Cornell Medicine, for her contribution to literature review and writing on behalf of Kantar Health with funding from Janssen Scientific Affairs, LLC.
Janssen Scientific Affairs, LLC provided funding for this manuscript and the study upon which it is based. Kantar Health conducted the study with funding from Janssen Scientific Affairs, LLC.
Disclosure
Susan C Bolge is an employee of Janssen Scientific Affairs, LLC, which funded this study and a stockholder of parent company Johnson & Johnson. Seth Ginsberg is an employee of Global Healthy Living Foundation (GHLF), which is the parent nonprofit of Creaky Joints. GHLF received funding from Janssen Scientific Affairs, LLC for data collection and consulting on this study. Isabel Allen is an employee of UCSF. Amir Goren and Duncan Brown were employees of Kantar Health at the time the study was conducted. Kantar Health received funding from Janssen Scientific Affairs, LLC for conducting, analyzing, and reporting on this study. The authors report no other conflicts of interest in this work.
References
- KvienTKEpidemiology and burden of illness of rheumatoid arthritisPharmacoeconomics200422111215157000
- OngKLWuBJCheungBMBarterPJRyeK-AArthritis: its prevalence, risk factors, and association with cardiovascular diseases in the United States, 1999 to 2008Ann Epidemiol2013232808623218811
- BartonJLPatient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapyPatient Prefer Adherence2009333534420016797
- FiresteinGSEvolving concepts of rheumatoid arthritisNature2003423693735636112748655
- DougadosMSoubrierMAntunezAPrevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: Results of an international, cross-sectional study (COMORA)Ann Rheum Dis2014731626824095940
- Newhall-PerryKLawNRamosBDirect and indirect costs associated with the onset of seropositive rheumatoid arthritisJ Rheumatol20002751156116310813281
- FurneriGMantovaniLBelisariASystematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritisClin Exp Rheumatol2012304S7223072761
- SinghJAFurstDEBharatA2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritisArthritis Care Res2012645625639
- SinghJAChristensenRWellsGABiologics for rheumatoid arthritis: an overview of Cochrane reviewsCochrane Database Syst Rev200944CD00784819821440
- DoanQVChiouC-FDuboisRWReview of eight pharmacoeconomic studies of the value of biologic DMARDs (adalimumab, etanercept, and infliximab) in the management of rheumatoid arthritisJ Manag Care Pharm200612755556916981801
- IannoneFLopalcoGCantariniLGaleazziMLapadulaGEfficacy and safety of combination therapy for preventing bone damage in rheumatoid arthritisClin Rheumatol2016351192326581205
- SmolenJSLandewéRBreedveldFCEULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 updateAnn Rheum Dis201365820052014
- KeystoneECKavanaughAFSharpJTRadiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: A randomized, placebo-controlled, 52-week trialArthritis Rheum20045051400141115146409
- SaagKGTengGGPatkarNMAmerican College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritisArthritis Rheum200859676278418512708
- ScottDBiologics-based therapy for the treatment of rheumatoid arthritisClin Pharmacol Ther2012911304322166850
- Van HulstLKievitWVan BommelRvan RielPFraenkelLRheumatoid arthritis patients and rheumatologists approach the decision to escalate care differently: results of a maximum difference scaling experimentArthritis Care Res2011631014071414
- HuynhTKØstergaardAEgsmoseCMadsenORPreferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritisPatient Prefer Adherence20148939924470758
- CurkendallSPatelVGleesonMCampbellRZagariMDuboisRCompliance with biologic therapies for rheumatoid arthritis: Do patient out-of-pocket payments matter?Arthritis Rheum200859101519152618821651
- BolgeSCGorenATandonNReasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: A patient perspectivePatient Prefer Adherence2015912113125653505
- LisickiRChuLWhat matters to patients and physicians when considering biologic therapy for rheumatoid arthritisPostgrad Med2008120315416018824833
- WilliamsEEdwardsCPatient preferences in choosing anti-TNF therapies-R1Rheumatology (Oxford)200645121575157617085468
- AugustovskiFBeratarrecheaAIrazolaVPatient preferences for biologic agents in rheumatoid arthritis: A discrete-choice experimentValue Health201316238539323538191
- PoulosCHauberABGonzálezJMOgaleSTurpcuAPatient tradeoffs between frequency and duration of biologic treatments for rheumatoid arthritis. Poster presented at: The ISPOR 17th Annual International Meeting. Washington, DC, on June 2–6, 2012. [abstract]Value in Health2012154A42
- ThompsonAPractical aspects of therapeutic intervention in rheumatoid arthritisJ Rheumatol Suppl200982394119509329
- KievitWVan HulstLVan RielPFraenkelLFactors that influence rheumatologists’ decisions to escalate care in rheumatoid arthritis: Results from a choice-based conjoint analysisArthritis Care Res2010626842847
- WolfeFMichaudKResistance of rheumatoid arthritis patients to changing therapy: Discordance between disease activity and patients’ treatment choicesArthritis Rheum20075672135214217599730
- BenhamouMRinchevalNRoyCThe gap between practice and guidelines in the choice of first-line disease modifying antirheumatic drug in early rheumatoid arthritis: results from the ESPOIR cohortJ Rheumatol200936593494219286850
- LevinsonWKaoAKubyAThistedRANot all patients want to participate in decision making: A national study of public preferencesJ Gen Intern Med200520653153515987329
- StevensonFABarryCABrittenNBarberNBradleyCPDoctor–patient communication about drugs: The evidence for shared decision makingSoc Sci Med200050682984010695980
- WillekePBeckerHWassenbergSPavenstädtHJacobiAPatient/rheumatologist evaluation of infusion treatment for rheumatoid arthritisZ Rheumatol201170323223421359555
- ScarpatoSAntivalleMFavalliEGPatient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study)Rheumatology (Oxford)201049228929419920093
- MathewsALColeskaABurnsPBChungKCThe evolution of patient decision-making regarding medical treatment of rheumatoid arthritisArthritis Care Res (Hoboken)201668331832426315611
- NeameRHammondADeightonCNeed for information and for involvement in decision making among patients with rheumatoid arthritis: a questionnaire surveyArthritis Rheum200553224925515818715
- DillaTRenteroMLComellasMLizanLSacristánJAPatients’ preferences for rheumatoid arthritis treatments and their participation in the treatment decision-making process. A systematic review of the literatureValue Health2015187A65226533657
- SandersonTCalnanMMorrisMRichardsPHewlettSThe impact of patient-perceived restricted access to anti-TNF therapy for rheumatoid arthritis: a qualitative studyMusculoskeletal Care20097319420919127529
- CharlesCGafniAWhelanTShared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango)Soc Sci Med19974456816929032835
- MontgomeryAFaheyTHow do patients’ treatment preferences compare with those of clinicians?Qual Health Care200110suppl 1i39i4311533437
- ConstantinescuFGoucherSWeinsteinAFraenkelLRacial disparities in treatment preferences for rheumatoid arthritisMed Care200947335035519165120