Abstract
Purpose
The risk of venous thromboembolism (VTE) recurrence is high following an initial VTE event, and it persists over time. This recurrence risk decreases rapidly after starting with anticoagulation treatment and reduces by ~80%–90% with prolonged anticoagulation. Nonpersistence with anticoagulants could lead to increased risk of VTE recurrence. This systematic review aimed to estimate persistence at 3, 6, and 12 months with anticoagulants in patients with VTE, and to evaluate the risk of VTE recurrence in nonpersistent patients.
Methods
PubMed and Embase® were searched up to May 3, 2014 and the search results updated to May 31, 2015. Studies involving patients with VTE aged ≥18 years, treatment with anticoagulants intended for at least 3 months or more, and reporting data for persistence were included. Proportions were transformed using Freeman–Tukey double arcsine transformation and pooled using the DerSimonian–Laird random-effects approach.
Results
In total, 12 observational studies (7/12 conference abstracts) were included in the review. All 12 studies either reported or provided data for persistence. The total number of patients meta-analyzed to estimate persistence at 3, 6, and 12 months was 71,969 patients, 58,940 patients, and 68,235 patients, respectively. The estimated persistence for 3, 6, and 12 months of therapy was 83% (95% confidence interval [CI], 78–87; I2=99.3%), 62% (95% CI, 58–66; I2=98.1%), and 31% (95% CI, 22–40; I2=99.8%), respectively. Only two studies reported the risk of VTE recurrence based on nonpersistence – one at 3 months and the other at 12 months.
Conclusion
Limited evidence showed that persistence was suboptimal with an estimated 17% patients being nonpersistent with anticoagulants in the crucial first 3 months. Persistence declined over 6 and 12 months. Observational data on persistence with anticoagulation treatment, especially direct oral anticoagulants, in patients with VTE and its effect on risk of VTE recurrence were scarce and further research is required.
Introduction
Venous thromboembolism (VTE) is a disease that includes both deep vein thrombosis (DVT) and pulmonary embolism (PE). VTE associated with known acquired risk factors is referred to as “provoked;” if there is no known clinical risk factor present, it is referred to as “unprovoked”.Citation1 It is estimated that there are 300,000–600,000 incident VTE cases annually in the USA.Citation2 Across six major European countries, the estimated incidence per 100,000 person-years was 148 for DVT and 95 for PE.Citation3 Subsequent VTE events after an index VTE are referred to as “recurrent VTE”. The risk of recurrent VTE decreases rapidly after starting with anticoagulation treatment.Citation4 Risk of recurrent VTE is 50% higher if anticoagulation is interrupted within 4–6 weeks compared with 3 months or later.Citation5 Prolonged anticoagulation treatment beyond 3 months reduces the risk of recurrent VTE by ~80%–90% when treated with vitamin K antagonist (VKA)Citation6,Citation7 or direct oral anticoagulants (DOACs).Citation8 The risk of VTE recurrence is high in the first 6–12 months and does not return to baseline;Citation9 therefore, persistence with the prescribed regimen of anticoagulation treatment is crucial. Persistence reflects “the duration of time from initiation to discontinuation of therapy” in an individual patient,Citation10 being defined as the proportion of patients who continue the treatment for a specified period of time without exceeding a prespecified gap. Premature discontinuation of treatment indicates nonpersistence. Evaluating the persistence in routine clinical practice is important because deviation from a prescribed regimen may lead to subtherapeutic responses and increased risk of VTE recurrence. This report summarizes the evidence on persistence with anticoagulation treatment and its effect on the risk of VTE recurrence.
Objectives
The objective of this systematic review was to estimate persistence at 3, 6, and 12 months after initiation of anticoagulant treatment in patients with VTE, and to evaluate the risk of VTE recurrence in nonpersistent patients.
Methods
Search strategy and study screening
A comprehensive search strategy was formulated using keywords such as “deep vein thrombosis”, “pulmonary embolism”, “venous thromboembolism”, “thrombosis”, “patient compliance”, “medication adherence”, “persistence”, “discontinuation”, “non-compliance”, “non-persistence”, “non-adherence”, “health behaviour”, “concordance”, “drug regimen”, “withdrawal”, “patient drop-outs”, “warfarin”, “coumarin”, “heparin”, “low molecular weight heparin”, “acenocoumarol”, “phenprocoumon”, “fondaparinux”, “dalteparin”, “enoxaparin”, “nadroparin”, “dabigatran”, “rivaroxaban”, “anticoagulants”, “vitamin K antagonists”, “VKA”, “observational study”, “retrospective study”, “prospective study”, “cohort study”, longitudinal studies”, “case control study”, “meta-analysis”, “systematic review” (full strategy available with the authors). A broad search strategy was used to ensure that outcomes of interest were captured even if reported as secondary outcomes. PubMed and Embase® were searched up to May 3, 2014, along with Google Scholar. Additional search was carried out up to May 31, 2015 and the results were used to update this review. The identified references were screened by the first author (PV) using EndNote® X7 (Thomson Reuters, USA).
Inclusion and exclusion criteria
Observational studies (full-text/conference abstracts) were included if they fulfilled the following criteria:
Patients ≥18 years of age
Patients diagnosed with VTE which includes DVT and PE with or without DVT
Treatment with anticoagulants intended for at least 3 months or more
Data reported for persistence.
Studies of pregnant patients, prophylaxis, and other therapy/indications were excluded. Guidelines, case reports, case series, reviews, clinical trials, randomized controlled trials, and editorials were also excluded. Review articles and systematic reviews were screened for references before exclusion.
Data analysis
The methodological quality of the studies was assessed using a tool developed by the Cochrane Bias Method Group.Citation11 Time points of 3, 6, and 12 months were used for persistence, as they reflect different recurrence risk periods. The 3-month time point reflects the period of active treatment to suppress acute events of thrombosis with the highest risk of recurrence, whereas the 6- and 12-month time points reflect recurrence risk periods with prolonged anticoagulation, which is based on the type of index VTE and individual risk factors.Citation1,Citation12 The proportion of patients persistent with treatment, if not reported, was calculated using data published in the studies. Freeman–Tukey double arcsine transformation was used to stabilize the variances of the raw proportions before pooling them.Citation13 The DerSimonian–Laird random-effects approach was used to calculate the between-study variance and pooled persistence.Citation14 This approach gave an overall weighted estimate of persistence. Analyses were conducted in R version 3.2.0 using the R packages “meta” and “metafor”.Citation15,Citation16 The I2 index was used as a measure of the variability in effect estimates due to between-study heterogeneity. Sensitivity analysis was performed to determine the effect of sample size on the final pooled estimate. Possible sources of heterogeneity were explored by univariate meta-regression using treatment type (VKA vs DOAC), where P-values of <0.05 were considered significant. Meta-regression was performed using transformed proportions and transformed within-study standard errors.
Results
Study characteristics
The screening process and the final number of studies included are shown in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart (). After the search update (May 31, 2015), two conference abstractsCitation17,Citation18 were published as full-text articles and no new references were found relevant to be included in the review. In total, 12 observational studies were included, of which five were full-text articlesCitation19–Citation23 and seven were conference abstracts.Citation24–Citation30 Five studies were from the USA,Citation19–Citation23 five from Germany,Citation25,Citation27–Citation30 and one each from CanadaCitation24 and the UK.Citation26 All 12 studies either reported or provided data for persistence. Of the 12 studies, five reported on DOAC (rivaroxaban),Citation25,Citation27–Citation30 six reported on VKAs,Citation19–Citation21,Citation23,Citation24,Citation26 and one reported on multiple anticoagulants (93% were warfarin users).Citation22 The total number of patients meta-analyzed to estimate persistence was 71,969 at 3 months, 58,940 at 6 months, and 68,235 at 12 months. Eleven studies reported patients with VTE with average age ranging 52–67 years; one study involving nursing home residents reported median age of 80 years.Citation20 Six studies included patients with recurrent VTE (24%–57% of the study populations)Citation21,Citation25,Citation27–Citation30 and the remaining six included index VTE patients. Five studies included patients with cancer (11%–21% of the study population),Citation19–Citation23 two included noncancer patients,Citation24,Citation26 and the cancer status could not be determined for the remaining five studies. Out of the 12 studies, four studies categorized VTE into provoked and unprovoked; two studies had 63% and 62% unprovoked VTE patients, respectively,Citation22,Citation26 one study had 37.3% unprovoked VTE patients,Citation24 one study was totally based on unprovoked VTE patients (n=37,664),Citation23 and the remaining eight studies did not categorize VTE. The characteristics of the studies are illustrated in . The summary of risk of bias for each of the 12 studies (available on request from the authors).
Figure 1 PRISMA flow diagram.
Table 1 Characteristics of studies included in the review
Threshold and time points
In six studies, persistence was determined using a pre-specified gap which varied from 37 to 90 days based on prescription refills, and exceeding this gap meant discontinuation (nonpersistence).Citation19–Citation23,Citation26 Five studies reported premature discontinuation of the treatment (nonpersistence)Citation25,Citation27–Citation30 and one study did not report the gap (conference abstract).Citation24 Most studies reported persistence at multiple time points within the same study: six studies reported all three time points (3, 6, and 12 months),Citation20,Citation22–Citation26 two studies reported two time points (3 and 6 months),Citation29,Citation30 one study reported two time points (3 and 12 months),Citation21 two studies reported one time point (6 months),Citation27,Citation28 and one study reported one time point (12 months).Citation19 Therefore, nine studies had 3-month persistence data,Citation20–Citation26,Citation29,Citation30 ten had 6-month persistence data,Citation20,Citation22–Citation30 and eight had 12-month persistence data.Citation19–Citation26
Persistence
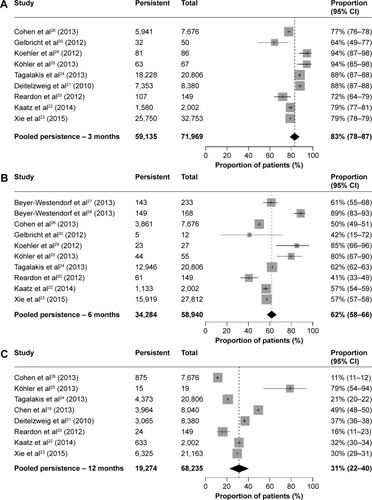
The estimated persistence with anticoagulation treatment at 3 months was 83% (95% confidence interval [CI], 78–87; I2=99.3%) (), at 6 months was 62% (95% CI, 58–66; I2=98.1%) (), and at 12 months was 31% (95% CI, 22–40; I2=99.8%) (). Univariate meta-regression for 3 months persistence revealed that treatment type (VKA vs DOAC) did not significantly contribute to heterogeneity (P=0.4074); however, it was significant for 6 months (P=0.0068) and 12 months (P=0.0065).
Figure 2 Pooled proportion of patients persistent with anticoagulation treatment.
Abbreviation: CI, confidence interval.
Two studies reported persistence to anticoagulation treatment for 3, 6, and 12 months stratified by provoked noncancer VTE and unprovoked VTE. For provoked noncancer VTE, persistence at 3 months was 76.8% and 86.9%, at 6 months was 49% and 59.5%, and at 12 months was 10.3% and 19.9% for each study, respectively. For unprovoked VTE, persistence at 3 months was 77.8% and 88.8%, at 6 months was 51.1% and 66.8%, and at 12 months was 11.9% and 22.9% for each study, respectively.Citation24,Citation26 One study that was based only on unprovoked VTE patients reported that 78.6%, 57.2%, and 29.9% were persistent at 3, 6, and 12 months, respectively.Citation23
Sensitivity analysis
Four studies (3-month persistence),Citation20,Citation25,Citation29,Citation30 six studies (6-month persistence),Citation20,Citation25,Citation27–Citation30 and two studies (12-month persistence)Citation20,Citation25 with small sample size were excluded to evaluate the effect of sample size on pooled estimates. After exclusion, the estimates of 3-, 6-, and 12-month persistence remained almost the same (3 months, 82% [95% CI, 77–87; I2=99.6%]; 6 months, 57% [95% CI, 52–61; I2=99.1%]; 12 months, 29% [95% CI, 20–39; I2=99.9%]).
Risk of recurrence
Only two studies reported the risk of recurrence based on discontinuation (nonpersistence) – one at 3 months and the other at 12 months;Citation19,Citation21 therefore, these data could not be pooled for analysis. Deitelzweig et alCitation21 reported that patients who discontinued VKA (warfarin) treatment within 3 months had a 45% increased risk of VTE recurrence compared with patients who discontinued at or after 3 months (hazard ratio, 1.45; 95% CI, 1.18–1.77). Chen et alCitation19 reported the risk of VTE recurrence after 12 months of treatment with VKA (warfarin) and found that patients who were nonpersistent with or discontinued VKA treatment within 12 months had a 48% increased risk of VTE recurrence compared with patients who were persistent with VKA for 12 months (hazard ratio, 1.48; 95% CI, 1.09–2.01).
Discussion
The findings of this systematic review suggest that, in routine clinical practice, the estimated persistence with anticoagulation treatment for the first 3 months after a VTE event was 83% and it declined to 31% at 12 months. Clinical guidelines suggest that patients with VTE should be treated with anticoagulants for at least 3 months as the risk of recurrence is high during this early period.Citation1,Citation12 In this review, an estimated 17% of patients were nonpersistent with anticoagulation treatment at 3 months, placing them at high risk of recurrence. After 3 months, the treatment duration is extended based on the type of VTE (provoked and unprovoked), individual patient factors, and weighing benefits–risks (eg, bleeding risk).Citation1,Citation12 Guidelines also suggest that the risk of recurrence reduces after 3 months of anticoagulant therapy but persists over time, especially for unprovoked and cancer-related VTE.Citation1 The present review estimated that 38% and 69% were nonpersistent at 6 and 12 months, respectively. The results of this study suggest that persistence with anticoagulant therapy in routine clinical practice was suboptimal, indicating patients might be at risk of recurrent VTE. Of the studies included in this review, only two explored the risk of VTE recurrence based on persistence. Although the number of studies included was limited, data suggest that the risk of VTE recurrence is high in nonpersistent patients. Another finding of this review was that studies investigating persistence or discontinuation rates in VTE patients were available only for VKA and rivaroxaban (preliminary data as conference abstracts) and were lacking for heparins and other DOACs such as dabigatran and apixaban. To the best of our knowledge, there are no previous reviews that have summarized and pooled data from observational studies on persistence in VTE patients at different time points and the risk of VTE recurrence in nonpersistent patients.
There are some limitations to this review and its findings. First, subgroup analysis based on type of VTE (provoked vs unprovoked), type of treatment (DOAC vs VKA), and other variables was not feasible because of limited data. Unprovoked VTE has a higher risk of recurrence than provoked VTE and future studies should investigate these subgroups separately.Citation31 Second, the threshold for persistence was reported as a prespecified gap in six studies.Citation19–Citation23,Citation26 The prespecified gap used varied across the studies, which may be due to differences in pattern of prescriptions and pack size across countries. Third, complexity of the treatment regimen should be taken into consideration when estimating real-world persistence. In most of the studies included in this review, patients were taking VKA, which is a well-established therapy for VTE; however, it requires routine anticoagulation monitoring and is associated with food and drug interactions which might affect long-term persistence.Citation32 Seven out of the 12 studies were based on administrative or claims database that rely on pharmacy refill records which might not reflect actual consumption.Citation19–Citation24,Citation26 Five studies included in this review were preliminary data of ongoing studies on rivaroxaban with small sample sizes and presented as conference abstracts.Citation25,Citation27–Citation30 Also, 6/12 included studies did not examine all the three time points (3, 6, and 12 months) for persistence within the same study.Citation19,Citation21,Citation27–Citation30 Heterogeneity was high for the pooled estimates and remained high in the sensitivity analysis, suggesting that a subgroup analysis is needed. The limited number of studies and missing information precluded a complete meta-regression and subgroup meta-analysis. Covariates such as mean age, proportion of women, and threshold for persistence could not be included in the meta-regression because of limited or missing information. Consequently, heterogeneity could not be explored, and therefore, all results must be interpreted with caution.
Conclusion
This systematic review revealed that data on real-world persistence were limited, but the available data showed that persistence was suboptimal with an estimated 17% patients being nonpersistent with anticoagulants in the crucial first 3 months and at high risk of recurrence. Being persistent beyond 3 months of anticoagulation treatment is important; however, persistence declined with increased duration of treatment. Data on the risk of VTE recurrence in nonpersistent patients were limited, but two studies showed an increased risk of VTE recurrence. Recurrent VTE is associated with substantial health care resource utilization, increased morbidity and mortality, and impacts patient’s quality of life. As a result, it is important to educate patients about the importance of remaining on the anticoagulation treatment to prevent recurrent VTE. Therefore, further research is required to evaluate the risk of VTE recurrence in nonpersistent patients, reasons for poor persistence, and steps to improve it, thus creating a robust body of evidence to inform decision making and management of individual patients.
Author contributions
All authors made a substantial contribution to the concept and design of the review and interpretation of results. P Vora was responsible for the search strategy, extraction of data, statistical analysis, interpretation of results, and drafting of the manuscript. G Persson Brobert was the main supervisor for this project. K Suzart and M Soriano-Gabarró co-supervised the project. All authors were involved in critical revision of the manuscript. All authors gave final approval of the submitted version of the manuscript.
Acknowledgments
The authors are grateful to Jesse Alderson (Oxford PharmaGenesis Ltd) for editorial support (funded by Bayer) and Ronald Herrera-Clavijo for technical support.
Disclosure
All authors are employees of Bayer Pharma AG. The authors report no other conflicts of interest in this work.
References
- KearonCAklEAComerotaAJAntithrombotic therapy for vte disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest20121412 supple419Se494S22315268
- BeckmanMGHooperWCCritchleySEOrtelTLVenous thromboembolism: a public health concernAm J Prev Med2010384 SupplS495S50120331949
- CohenATAgnelliGAndersonFAVenous thromboembolism (VTE) in Europe – The number of VTE events and associated morbidity and mortalityThromb Haemost2007981075676417938798
- HeitJASilversteinMDMohrDNThe epidemiology of venous thromboembolism in the communityThromb Haemost200186745246311487036
- BoutitieFPinedeLSchulmanSInfluence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: Analysis of individual participants’ data from seven trialsBMJ20113427810
- MiddeldorpSPrinsMHHuttenBADuration of treatment with vitamin K antagonists in symptomatic venous thromboembolismCochrane Database Syst Rev20148CD00136
- KearonCGentMHirshJA comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolismN Engl J Med19993401290190710089183
- HirschlMKundiMNew oral anticoagulants in the treatment of acute venous thromboembolism – a systematic review with indirect comparisonsVASA201443535336425147012
- HeitJAMohrDNSilversteinMDPettersonTMO’FallonWMeltonLPredictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort studyArch Intern Med2000160676176810737275
- CramerJARoyABurrellAMedication compliance and persistence: terminology and definitionsValue Health2008111444718237359
- Cochrane-Bias-Methods-Group. Tool to Assess Risk of Bias in Cohort Studies2013 Available from: http://bmg.cochrane.org/sites/bmg.cochrane.org/files/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdfAccessed July 2, 2014
- HowardLSHughesRJNICE guideline: management of venous thromboembolic diseases and role of thrombophilia testingThorax201368439139323234857
- FreemanMFTukeyJWTransformations related to the angular and the square rootProject Euclid1950214607611
- DerSimonianRLairdNMeta-analysis in clinical trialsControlClin Trials198673177188
- SchwarzerGMeta: meta-analysis with R. R package version2010215 Available from http://CRAN.R-project.org/package=meta
- ViechtbauerWConducting meta-analyses in R with the metafor packageJ Stat Softw2010363148
- KaatzSFuACAbuDaggaADoes anticoagulant treatment duration vary by the risk of venous thromboembolism recurrence in clinical practice?J Thromb Haemost201311553554
- LiuXXieLPhatakHPersistence on warfarin therapy in patients with venous thromboembolism: A large US insurance database analysisCirculation Epub 2013724
- ChenSYWuNGulsethMOne-year adherence to warfarin treatment for venous thromboembolism in high-risk patients and its association with long-term risk of recurrent eventsJ Manag Care Pharm201319429130123627575
- ReardonGPandyaNNutescuEAUse of warfarin therapy among residents who developed venous thromboembolism in the nursing homeAm J Geriatr Pharmacother201210636137223217529
- DeitelzweigSBLinJKreilickCHusseinMBattlemanDWarfarin therapy in patients with venous thromboembolism: Patterns of use and predictors of clinical outcomesAdv Ther201027962363320680533
- KaatzSFuACAbuDaggaAAssociation between anticoagulant treatment duration and risk of venous thromboembolism recurrence and bleeding in clinical practiceThromb Res2014134480781325127013
- XieLLiuXPhatakHWarfarin discontinuation in patients with unprovoked venous thromboembolism: a large US insurance database analysisAm J Ther Epub2015724
- TagalakisVPatenaudeVKahnSRSuissaSOral anticoagulation treatment and persistence after venous thromboembolism in a real world population: The Q-VTE study cohortBlood2013122212386
- KöhlerCWerthSTittlLBeyer-WestendorfJAcute treatment of pulmonary embolism with rivaroxaban – Real life data from the prospective Dresden NOAC registry (NCT01588119)Blood201312221238023929856
- CohenAMartinezCWallenhorstCBamberLVitamin K antagonist treatment patterns and persistence after venous thromboembolism in noncancer patients: VTE Epidemiology Group (VEG) StudyJ Thromb Haemost201311262723140188
- Beyer-WestendorfJEbertzFGelbrichtVForsterKKohlerCWerthSTreatment of acute VTE with rivaroxaban. Updated results of the prospective Dresden NOAC registry (NCT01588119)J Thromb Haemost201311314315
- Beyer-WestendorfJEbertzFForsterKChronic VTE treatment VTE with rivaroxaban. Updated results of the prospective Dresden NOAC registry (NCT01588119)J Thromb Haemost201311439
- KoehlerCWerthSGelbrichtVReal life efficacy and safety of rivaroxaban for extended vte treatment-first results of the prospective NOAC registry (NCT01588119)Blood2012120212267
- GelbrichtVKoehlerCWerthSReal life efficacy and safety of rivaroxaban for acute vte treatment-first results of the prospective NOAC registry (NCT01588119)Blood2012120211159
- IorioAKearonCFilippucciERisk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: A systematic reviewArch Intern Med2010170191710171620975016
- MekajYHMekajAYDuciSBMiftariEINew oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic eventsTher Clin Risk Manag20151196797726150723
