Abstract
Purpose
Medication nonadherence is common in the treatment of patients with severe mental illness and is a frequent cause of relapse. Different formulations have been developed in an effort to improve medication adherence. The aim of this study was to explore whether there are differential clinical outcomes between two different formulations of olanzapine (orodispersible tablets [ODTs] vs standard oral tablets [SOT]) for the treatment of nonadherent patients with schizophrenia or bipolar disorder.
Methods
Data for this analysis were from an observational study conducted in Europe (N=903). Adult schizophrenia and bipolar disorder patients in outpatient settings who initiated or changed to either olanzapine ODT or SOT according to physician decision within the last 45 days were eligible for enrollment. The follow-up period was 1 year. Of the 903 participants, 266 nonadherent patients (Medication Adherence Rating Scale score 0–4 at baseline) were included in the analysis. Clinical outcomes of interest were: 1) hospitalization and 2) relapse identified by the participating psychiatrist or hospitalization. An adjusted logistic regression model was fitted.
Results
Patients taking ODT had more severe illness at baseline (P<0.001) as assessed with the Clinical Global Impression with mean (standard deviation [SD]) scores of ODT 4.63 (1.03) and SOT 4 (1.16). In the regression models adjusted for potential confounders, patients taking ODT had significantly lower odds for hospitalization (odds ratio =0.355; 95% confidence interval =0.13–0.974) and relapse or hospitalization (odds ratio =0.368; 95% confidence interval =0.183–0.739), respectively.
Conclusion
Nonadherent patients with schizophrenia or bipolar disorder treated with the orodispersible formulation were less likely to be hospitalized or suffer relapse compared to those patients taking the standard oral coated tablets.
Introduction
A substantial proportion of patients with severe mental illnesses such as schizophrenia and bipolar disorder do not fully benefit from the wide availability of effective drugs to treat and prevent the symptoms of these conditions due to nonadherence. Although the adherence rate varies widely between studies, it is generally known to be about 40%–60% in patients with schizophrenia,Citation1 whilê40% and 75% of patients have been reported to stop taking medication within 1 and 2 years, respectively.Citation2 Furthermore, a high rate of suboptimal adherence has also been reported in patients with bipolar disorder (69%).Citation3 In a recent systematic review, positive attitudes to medication and illness insight were the only factors consistently associated with better adherence in patients with schizophrenia, while mixed results were found for other factors such as symptom severity and side effects.Citation1
Nonadherence in severe mental illness may exacerbate symptoms and lead to relapse and hospitalization.Citation3,Citation4 For instance, in patients with a first episode of psychosis, non-adherence was identified as the strongest single predictor of relapse,Citation5 while a recent study from China showed that nonadherent patients were 2.5 times more likely to relapse in the year following discharge than adherent patients.Citation6 Furthermore, compared to patients with no gaps in medication therapy, those patients with gaps of >30 days over a 1-year period had a nearly fourfold risk of being hospitalized, with even a gap of 1–10 days doubling the risk.Citation7
Given that nonadherence is a potentially preventable cause of adverse clinical outcomes including relapse and hospitalization, different types of formulations have been developed in an attempt to improve adherence. These delivery systems include long-acting injections, liquid formulations, oral granules, transdermal patches, and orodispersible tablets (ODTs).Citation8 For example, olanzapine is a well-established drug used to treat patients with schizophrenia and bipolar disorder. Oral olanzapine is available in the form of ODTs and standard oral tablets (SOT). The ODT formulation of olanzapine is designed to dissolve upon contact with saliva and cannot be easily spat out,Citation8 and may be preferable for patients who are reluctant or unable to swallow tablets.Citation9 Previous studies have shown that the ODT formulation is associated with higher adherence rates,Citation8,Citation9 increased patient preference,Citation10,Citation11 and improved ease of administration, which may reduce the burden of treatment not only on patients but also on caregivers.Citation12
Despite the potential benefits associated with ODT,Citation13 particularly in terms of adherence, there are no studies to date which have compared the effects of different oral forms of olanzapine on the hospitalization and relapse rates in patients with severe mental disorders, while studies on the use and effectiveness of different oral formulations of olanzapine in natural settings (eg, routine care in outpatient settings) are scarce. Furthermore, data on nonadherent patients are limited as they are rarely included in clinical trials.Citation14 Thus, the aim of this study was to assess whether nonadherent patients with schizophrenia or bipolar disorder have different hospitalization and relapse rates when treated with ODT compared to SOT within a follow-up period of 12 months in the outpatient settings of three European countries (France, Germany, and Greece).
Methods
Study design
Details regarding the study design have been published previously.Citation15–Citation17 Briefly, data for this post hoc analysis were obtained from a prospective, observational (noninterventional), naturalistic, multicenter, multicountry study (France, Germany, and Greece) designed to compare medication adherence between two oral forms of olanzapine (ODT vs SOT) in patients with schizophrenia or bipolar disorder. Patients were followed up for 1 year, with up to five study visits at ~3-month (±1 month) intervals. Data collection occurred when the patients attended their regular clinic visits. The participating psychiatrists or their designees conducted the assessments. All investigators attended a start-up meeting which consisted of training in the study procedures and questionnaire administration. Each participating psychiatrist was asked to enroll, consecutively, up to eight eligible patients, in order to limit selection bias.
Patients
Patients were eligible for enrollment if they met both of the following inclusion criteria: 1) adult patients diagnosed with schizophrenia or any type of bipolar disorder based on the Diagnosis and Statistical Manual of Mental Disorders, Fourth Edition, and 2) patients for whom their physician decided to begin antipsychotic treatment with olanzapine (either ODT or SOT), according to the approved marketing authorization, within the last 45 days (either as treatment initiation or as switch from another antipsychotic). The exclusion criteria were the following: 1) currently receiving treatment with investigational drugs or procedures at enrollment (or during the study period) and 2) any contraindication for olanzapine (eg, hypersensitivity, risk for narrow angle glaucoma).
A total of 903 patients with schizophrenia or any type of bipolar disorder from outpatient or hospital settings were enrolled in this study between April 2007 and April 2008. The last study visit occurred in May 2009. Patients with schizoaffective disorder were not included in the analysis, as olanzapine is not approved specifically for its treatment in the countries included in the study. The protocol did not restrict use to antipsychotic monotherapy or combination therapy, in order to obtain an unbiased sample. This was also done to reflect medication decisions in real-world settings where combination therapy is not uncommon. All treatment decisions were made at the discretion of the treating physician and patient, including the choice of olanzapine formulation. Of the 903 patients, 266 nonadherent patients, defined as having a baseline rating of 0–4 in the Medication Adherence Rating Scale (MARS), constituted the final analytical sample (details of this scale can be found below). Although the MARS does not have an established cut-off, in line with a previous study using the same dataset,Citation16 we used 4 as the cutoff for MARS. This corresponded to the lowest tertile of the MARS score distribution, and we judged that scores in this range are highly likely to have clinical implications.
Ethical approval
The study was approved by ethical review boards as required by local law and was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki. The specific ethics committees that approved the study are: 1) Comité consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé (France), 2) Landesärztekammer Hessen Ethik-Kommission (Germany), and 3) Papageorgiou Regional General Hospital of Thessaloniki (Greece). All patients provided written informed consent.
Outcome
Two clinical outcomes during the follow-up period (12 months) were assessed in this analysis: 1) inpatient hospitalization for any reason and 2) relapse. Relapse was defined as either an inpatient hospitalization or a relapse identified by the participating psychiatrist. Information on these events was recorded during follow-up at each visit.
Other variables
Clinical severity was measured with the Clinical Global Impression (CGI) scale for Bipolar Disorder (CGI-BP)Citation18 or Schizophrenia (CGI-SCH).Citation19 However, for this study, we only used the general severity score, which is included in both scales and is equivalent to the original CGI score, for the sake of consistency. Medication adherence was measured with the MARS, which is a ten-item self-reported measure ranging from 0 to 10, with higher scores representing better adherence. The MARS has demonstrated a high level of validity compared to existing self-report measures, and assesses a range of behaviors and attitudes linked to adherence.Citation20 Information on physical comorbidities such as diabetes, hypertension, hyperlipidemia, and obesity was also obtained. Those who had at least one physical comorbidity were considered to have any physical condition.
Statistical analysis
Baseline patient characteristics and by treatment and by relapse were described and compared using the chi-squared test (or Fischer’s exact test) and Mann–Whitney U tests for categorical and continuous variables, respectively. To study the effect of the baseline treatment form on the post-baseline hospitalization as well as the relapse rates, multivariable logistic regression models were employed. The models were adjusted for relevant baseline covariates (CGI, diagnosis, sex, country, age, any physical condition, and time since first episode). CGI, age, and time since first episode were included in the models as continuous variables, whereas other variables were included as categorical variables. Odds ratios (ORs) and 95% confidence intervals (CIs) are presented. A P-value <0.05 was considered to be statistically significant. Data analysis was carried out with SAS® software (SAS Institute, Cary, NC, USA), version 9.3.
Results
A total of 266 nonadherent patients (116 females and 150 males) at baseline were included in the analysis, of whom 195 and 71 had schizophrenia and bipolar disorder, respectively. The mean age (standard deviation [SD]) was 38.7 (12.6) years. SOT and ODT were administered to 89 and 177 patients, respectively. Of these 266 individuals, at the final visit, only 33.7% continued to be nonadherent, and there were 8 individuals who had to discontinue the study for incomplete compliance (n=3; SOT 2 vs ODT 1), patient request (n=4; SOT 3 vs ODT 1), and intolerability (n=1; ODT 1). The mean (SD) MARS scores at the final visit were not significantly different between the two treatment groups (SOT 5.5 [2.6] and ODT 5.9 [2.5]; Student’s t-test P=0.2994).
There was a significant difference in terms of the proportion of those taking different forms of tablets by country; ODTs were used more than twice as frequently in Greece as in France (). Furthermore, the mean CGI was significantly higher (ie, higher disease severity) among those taking ODT compared to those taking SOT (4.63 vs 4; P<0.001). There were no significant differences in terms of sex, diagnosis, any physical condition, age, years since first treatment, and MARS score between the two treatment groups. The relapse rates by the baseline characteristics are provided in . Patients who experienced relapse during the follow-up period were significantly more likely to have at least one physical health condition, longer time since first treatment, and higher CGI scores at baseline.
Table 1 Patient baseline demographic and clinical characteristics by treatment form
Table 2 Baseline demographics and clinical characteristics of patients by relapse
The overall hospitalization and relapse rates were 9.8% and 28.6%, respectively. In the unadjusted analysis, the hospitalization rate was significantly higher in the SOT group compared to the ODT group (16.9% vs 6.2%; P=0.0058). Although the relapse rate was also higher among patients on SOT compared to ODT (36% vs 24.9%), the difference was of borderline statistical significance (P=0.059; ). The effect of treatment form on hospitalization () and relapse () estimated by multivariate logistic regression revealed that patients taking ODT had significantly (64.5% and 63.2%, respectively) lower odds for hospitalization (OR =0.355; 95% CI =0.13–0.974) and relapse (OR =0.368; 95% CI =0.183–0.739), respectively.
Figure 1 Hospitalization and relapse rate by medication formulation at baseline.
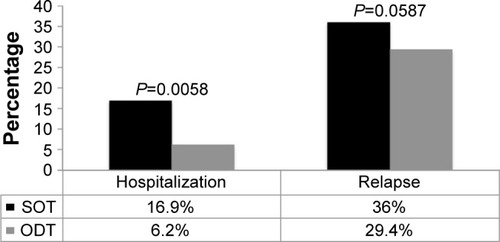
Table 3 Effect of treatment form on hospitalization estimated by multivariable logistic regression
Table 4 Effect of treatment form on relapse estimated by multivariable logistic regression
Our study protocol included individuals who were on polypharmacy (ie, taking another antipsychotic in conjunction with olanzapine). In order to assess whether this affected our results, we conducted a sensitivity analysis by excluding those who were on polypharmacy from the analysis. The sensitivity analysis showed that there were no appreciable changes in the results, meaning that polypharmacy was unlikely to have affected our results.
Discussion
This post hoc analysis examined the clinical outcomes of non-adherent schizophrenia and bipolar disorder patients treated with either SOT or ODT at baseline over a 1-year period. The study found that patients on ODT had 64.5%–63.2% lower odds for hospitalization and/or relapse compared to those on SOT, despite the fact that disease severity was more pronounced in the ODT group at baseline. Our study results point to a possible clinical benefit of ODT in nonadherent patients – a group for which only limited data exist due to their tendency to not participate in clinical trials.
Our finding that those on ODT had more severe disease at baseline is in line with a previous studyCitation9 and may reflect attitudes of psychiatrists who tend to prescribe ODT to patients who are more likely to be noncompliant, severely ill, or aggressive. The lower hospitalization/relapse rate among patients on ODT, despite more severe illness at baseline, may be attributable to improvements in adherence, given that nonadherence has been reported to be the single strongest predictor of relapse.Citation5
In an observational study with a follow-up period of 6 weeks, ODT was not only significantly associated with improvements in clinical symptoms, but also with improvements in adherence, attitude, and nursing care burden in acutely ill nonadherent patients with schizophrenia.Citation8 In particular, improvements in adherence were observed at as early as 1 week. Also in this study, although the sample consisted of noncompliant patients at baseline, the majority (75.3%) of patients completed the 6-week study and expressed positive opinions about taking ODT throughout the study period. It has been suggested that ODT tablets may increase acceptance of medication due to ease of intake and the difficulty in discarding the dose, and the evident improvements in psychotic symptoms accompanied by minimal side effects.
Furthermore, in another observational study of acutely ill patients with schizophrenia requiring emergency treatment, increase in the rate of medication acceptance was more pronounced in the ODT group compared to the SOT group as follows: (baseline vs after 2 weeks) positive attitude toward medication (ODT 31.6% vs 68.4%; SOT 58.0% vs 78.3%), ingestion (ODT 48.9% vs 83.4%; SOT 75.4% vs 89.4%), and nursing effort (ie, no extensive nursing effort was needed to administer medication; ODT 53.9% vs 86.2%; SOT 81.1% vs 92.8%).Citation9
In addition, it is of particular importance to consider the patient’s preference in terms of treatment in disorders such as schizophrenia and bipolar disorder, which frequently require long-term treatment, as this may influence longer-term adherence with treatment and subsequent clinical outcomes. In a 12-week randomized, crossover, open-label study, patients with stable schizophrenia who were on olanzapine SOT monotherapy were randomly assigned to ODT or SOT to assess patient preference for these different formulations. At the end of the study, a significantly higher proportion of patients claimed to prefer ODT over SOT (61% vs 27%; P<0.001).Citation11
The study results should be interpreted in light of several limitations. First, we were unable to conduct stratified analyses by disorder type (ie, schizophrenia or bipolar disorder) due to the small sample size. Second, the data were drawn from an observational study in which allocation of treatment form was not at random. While we adjusted for potential confounders, it is possible that there are other factors related to clinical outcome and physician’s choice of drug formulation that were not measured in the study. Thus, our results should not be considered as a direct comparison of the effects of the two different formulations of olanzapine. Third, medication adherence is notoriously difficult to measure, as the simple act of measuring adherence via self-administered questionnaires, physician assessment, or pill counts may influence patient behavior.Citation21 Despite this, the fact that our results were based on routine care provides us with the unique opportunity to observe the effectiveness of treatments in natural settings, which may be different from studies conducted under experimental conditions, as in a clinical trial. Fourth, the selection of the patients included in the study was based on the MARS, which measures general attitudes toward medication. Although 82% of the patients included in the analysis were taking antipsychotics immediately before study entry, the rest may have based their answers for the MARS on experiences with other types of medication. Finally, assessment was not blind. However, this was a post hoc analysis in which the investigators were not aware of the study question.
Conclusion
This analysis from a real-world study found that in nonadherent patients with schizophrenia or bipolar disorder, those patients treated with the orodispersible formulation were less likely to be hospitalized and/or suffer relapse compared to those treated with the standard oral coated tablets, in spite of having more severe disease at baseline. More research is needed to uncover the factors which may underlie our findings. Specifically, features of ODT such as ease of use, patient preference, and better adherence may be responsible for our findings, and these warrant further investigation.
Acknowledgments
This study was funded by Eli Lilly and Co.
Disclosure
Diego Novick, William Montgomery, Tamas Treuer, and Susanne Kraemer are Lilly employees. Jaume Aguado conducted the statistical analyses under a contract of CIBER-SAM with Lilly. Josep Maria Haro has received personal fees from Eli Lilly and Co., Roche, Lundbeck, and Otsuka. The authors report no other conflicts of interest in this work.
References
- SendtKVTracyDKBhattacharyyaSA systematic review of factors influencing adherence to antipsychotic medication in schizophrenia-spectrum disordersPsychiatry Res20152251–2143025466227
- PerkinsDOAdherence to antipsychotic medicationsJ Clin Psychiatry199960Suppl 212530
- MontesJMMaurinoJde DiosCMedinaESuboptimal treatment adherence in bipolar disorder: impact on clinical outcomes and functioningPatient Prefer Adherence20137899423378745
- American Psychiatric AssociationPractive Guidelines for the Treatment of Patients with Schizophrenia2nd edWashington, DCAmerican Psychiatric Association2004
- CaseiroOPerez-IglesiasRMataIPredicting relapse after a first episode of non-affective psychosis: a three-year follow-up studyJ Psychiatr Res20124681099110522721546
- XiaoJMiWLiLShiYZhangHHigh relapse rate and poor medication adherence in the Chinese population with schizophrenia: results from an observational survey in the People’s Republic of ChinaNeuropsychiatr Dis Treat2015111161116726056450
- WeidenPJKozmaCGroggALocklearJPartial compliance and risk of rehospitalization among California Medicaid patients with schizophreniaPsychiatr Serv200455888689115292538
- KinonBJHillALLiuHKollack-WalkerSOlanzapine orally disintegrating tablets in the treatment of acutely ill non-compliant patients with schizophreniaInt J Neuropsychopharmacol2003629710212890301
- CzekallaJWagnerTSchachtAKlugeMKinonBEffectiveness and medication acceptance of olanzapine disintegrating tablets compared to standard olanzapine tablets in acutely treated psychiatric patientsPatient Prefer Adherence20071192719956444
- ChuePJonesBTaylorCCDicksonRDissolution profile, tolerability, and acceptability of the orally disintegrating olanzapine tablet in patients with schizophreniaCan J Psychiatry200247877177412420656
- BitterITreuerTDilbazNPatients’ preference for olanzapine orodispersible tablet compared with conventional oral tablet in a multinational, randomized, crossover studyWorld J Biol Psychiatry201011789490320653494
- SanLCasillasMCiudadAGilaberteIOlanzapine orally disintegrating tablet: a review of efficacy and complianceCNS Neurosci Ther200814320321418801113
- MontgomeryWTreuerTKaragianisJAscher-SvanumHHarrisonGOrally disintegrating olanzapine review: effectiveness, patient preference, adherence, and other propertiesPatient Prefer Adherence2012610912522346347
- BarnettPGScottJYRosenheckRAGroupCSPSHow do clinical trial participants compare to other patients with schizophrenia?Schizophr Res20111301–3343921514794
- KraemerSChartierFAugendre-FerranteBEffectiveness of two formulations of oral olanzapine in patients with schizophrenia or bipolar disorder in a natural setting: results from a 1-year European observational studyHum Psychopharmacol201227328429422473831
- NovickDMontgomeryWTreuerTAguadoJKraemerSHaroJMComparative effectiveness in terms of treatment discontinuation of orodispersable versus standard oral olanzapine tablets in non-adherent patients: results from a 1-year European Outpatient Observational StudyValue Health2014177A766
- NovickDMontgomeryWTreuerTAguadoJKraemerSHaroJMRelationship of insight with medication adherence and the impact on outcomes in patients with schizophrenia and bipolar disorder: results from a 1-year European outpatient observational studyBMC Psychiatry20151518926239486
- SpearingMKPostRMLeverichGSBrandtDNolenWModification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BPPsychiatry Res19977331591719481807
- HaroJMKamathSAOchoaSThe Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophreniaActa Psychiatr Scand Suppl2003416162312755850
- ThompsonKKulkarniJSergejewAAReliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychosesSchizophr Res200042324124710785582
- LamWYFrescoPMedication adherence measures: an overviewBiomed Res Int2015201521704726539470
