Abstract
Objectives
We examined rheumatologists’ motivation for prescribing biosimilars, assessed their treatment preferences in relation to prescribing behavior and explored patient attitudes to biosimilars.
Methods
Data were taken from the Adelphi Real World Biosimilars Programme, a real-world, cross-sectional study undertaken with German rheumatologists and patients with rheumatoid arthritis, ankylosing spondyloarthritis or psoriatic arthritis in 2015–2016. Rheumatologists provided data on their prescribing behavior and attitudes toward biosimilars and invited the next eight eligible consecutive consulting patients to complete a questionnaire. Rheumatologists were split into “investigative”, “conservative” and “other” groups.
Results
Overall, 50 rheumatologists and 261 patients participated. Biosimilars accounted for <10% of all biologic therapy prescriptions, and >95% of rheumatologists would prescribe a biooriginator rather than biosimilar as the first- or second-line therapy if unrestricted. Patients showed some reluctance to accept biosimilars, and a small proportion of patients were unhappy when switched from a biooriginator to a biosimilar. Satisfaction with treatment was highest in patients who started treatment with a biooriginator prior to biosimilar availability. Patient concerns when starting treatment with a biooriginator or a biosimilar included not knowing enough about the drug (25%–41%), potential side effects (26%–32%) and potential long-term problems (19%–30%).
Conclusion
Study results demonstrate that there is some reluctance from patients to accept biosimilars and the need to educate patients who are unsure to allow them to be involved in decision making, highlighting the importance of patient and physician communication. There remains a need for further research into nonclinical switching and the long-term impact of prescribing biosimilars.
Supplementary materials
Questions asked of physicians were as follows:
– What percentage of your patients with each condition are currently receiving each product?
– What percentage of your patient do you expect will be receiving each product in 12 months’ time?
– Assuming there were no restrictions on your prescribing or guidelines you need to follow, what advanced therapy would you prefer to use first, second and third for each condition?
– Why did you prescribe the biosimilar(s) rather than the originator biologic(s)?
○ I wanted to get experience with the new product(s)
○ I am convinced of equivalent efficacy compared with biologic originators
○ Due to the lower cost
○ I believe this to be economic prescribing
○ Using biosimilars allows me to make savings which can be used elsewhere
○ Due to formulary/hospital guidelines
○ Insurance reasons
○ Other, specify
– Once biosimilars are more widely available, how do expect you might use them?
○ When a treatment change* is required I will select the biosimilar version of a molecule I would otherwise have prescribed.
○ When a treatment change* is required I will select a biosimilar of a different molecule rather than the branded biologic molecule I would otherwise have prescribed.
○ In controlled patients currently receiving a branded biologic, I will switch the branded biologic to the biosimilar version.
○ In controlled patients currently receiving a branded biologic, I will switch the branded biologic to a bio-similar version of a different molecule.
– Thinking of all the patients who had never before received a biologic originator or biosimilar (ie, biologic/biosimilar naive) for whom you tried to prescribe a biosimilar, what proportion?
○ Accepted the switch onto a biosimilar without any reluctance.
○ Were reluctant to take a biosimilar but finally accepted as they had no other choice.
○ Refused the biosimilar and remained on their existing biologic originator.
○ Refused the biosimilar and either switched to an alternative biologic originator or discontinued biologic therapy completely.
– Thinking of all the patients who were receiving an originator biologic who, when they needed a switch of therapy you tried to prescribe a biosimilar, what proportion?
○ Accepted the switch onto a biosimilar without any reluctance.
○ Were reluctant to take a biosimilar but finally accepted as they had no other choice.
○ Refused the biosimilar and remained on their existing biologic originator.
○ Refused the biosimilar and either switched to an alternative biologic originator or discontinued biologic therapy completely.
– Thinking of all the patients who were receiving an originator biologic who did not clinically need a switch of therapy but you tried to prescribe a biosimilar, what proportion?
○ Accepted the switch onto a biosimilar without any reluctance.
○ Were reluctant to take a biosimilar but finally accepted as they had no other choice.
○ Refused the biosimilar and remained on their existing biologic originator.
○ Refused the biosimilar and either switched to an alternative biologic originator or discontinued biologic therapy completely.
Questions asked of patients were as follows:
– How happy were you to switch from a biooriginator to your current treatment?
○ Extremely unhappy
○ Very unhappy
○ Somewhat unhappy
○ Indifferent
○ Somewhat happy
○ Very happy
○ Extremely happy
– Which option best describes your satisfaction with how well your current treatment is controlling your condition/symptoms?
○ Very dissatisfied
○ Dissatisfied
○ Neither satisfied or dissatisfied
○ Satisfied
○ Very satisfied
– What concerns, if any, did/do you have about taking this medication at the times indicated at the top of the columns?
○ Don’t know enough about the drug
○ I think there are better medications
○ The medicine is too expensive
○ Potential side effects
○ Potential long term problems
○ Doesn’t help my symptoms overall
○ I don’t feel confident that this drug is tried and tested
○ I think this version is a cheaper and less-effective version
○ Other
Note: *We are defining a treatment change as either initiation of 1st biologic or biologic switch.
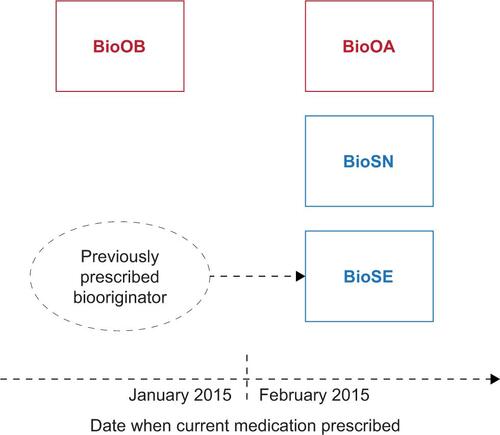
Figure S1 Patient analysis groups based on current medication.
Notes: BioOA, patient receiving biooriginator who was initiated after February 2015; BioOB, patient receiving biooriginator who was initiated before January 2015; BioSN, patient receiving biosimilar who was previously biologic naive; BioSE, patient receiving biosimilar who has an experience of a biooriginator.
Acknowledgments
This study was funded by Merck & Co.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
CMB and SK are employees of Merck & Co. and may hold stock and/or options. JW, ES and JP are employees of Adelphi Real World. The authors report no other conflicts of interest in this work.
