Abstract
Background
Pulmonary arterial hypertension (PAH) is a devastating disease and ultimately leads to right heart failure and premature death. A total of four classical targeted drugs, prostanoids, endothelin receptor antagonists (ERAs), phosphodiesterase 5 inhibitors (PDE-5Is), and soluble guanylate cyclase stimulator (sGCS), have been proved to improve exercise capacity and hemodynamics compared to placebo; however, direct head-to-head comparisons of these drugs are lacking. This network meta-analysis was conducted to comprehensively compare the efficacy of these targeted drugs for PAH.
Methods
Medline, the Cochrane Library, and other Internet sources were searched for randomized clinical trials exploring the efficacy of targeted drugs for patients with PAH. The primary effective end point of this network meta-analysis was a 6-minute walk distance (6MWD).
Results
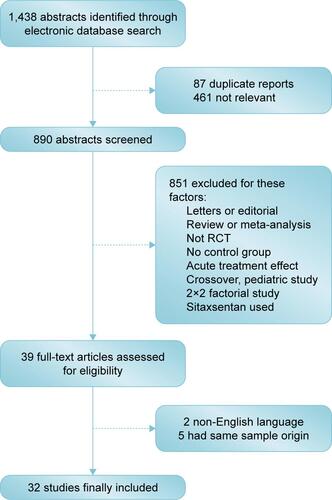
Thirty-two eligible trials including 6,758 patients were identified. There was a statistically significant improvement in 6MWD, mean pulmonary arterial pressure, pulmonary vascular resistance, and clinical worsening events associated with each of the four targeted drugs compared with placebo. Combination therapy improved 6MWD by 20.94 m (95% confidence interval [CI]: 6.94, 34.94; P=0.003) vs prostanoids, and 16.94 m (95% CI: 4.41, 29.47; P=0.008) vs ERAs. PDE-5Is improved 6MWD by 17.28 m (95% CI: 1.91, 32.65; P=0.028) vs prostanoids, with a similar result with combination therapy. In addition, combination therapy reduced mean pulmonary artery pressure by 3.97 mmHg (95% CI: −6.06, −1.88; P<0.001) vs prostanoids, 8.24 mmHg (95% CI: −10.71, −5.76; P<0.001) vs ERAs, 3.38 mmHg (95% CI: −6.30, −0.47; P=0.023) vs PDE-5Is, and 3.94 mmHg (95% CI: −6.99, −0.88; P=0.012) vs sGCS. There were no significant differences in all-cause mortality and severe adverse events between prostanoids, ERAs, PDE-5Is, sGCS, combination therapy, and placebo.
Conclusion
All targeted drugs for PAH are associated with improved clinical outcomes, especially combination therapy. However, all these drugs seem to show less favorable effects on survival in the short-term follow-up, suggesting further clinical trials are required.
Supplementary materials
Table S1 General characteristics of the included studies
References
- RubinLJMendozaJHoodMTreatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trialAnn Intern Med19901124854912107780
- OlschewskiHSimonneauGGalieNInhaled iloprost for severe pulmonary hypertensionN Engl J Med200234732232912151469
- GalieNHumbertMVachieryJLEffects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trialJ Am Coll Cardiol2002391496150211985913
- GalieNOlschewskiHOudizRJAmbrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2Circulation20081173010301918506008
- BadeschDBTapsonVFMcGoonMDContinuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trialAnn Intern Med200013242543410733441
- BarstRJRubinLJLongWAA comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertensionN Engl J Med19963342963018532025
- BarstRJMcGoonMMcLaughlinVBeraprost therapy for pulmonary arterial hypertensionJ Am Coll Cardiol2003412119212512821234
- RubinLJBadeschDBBarstRJBosentan therapy for pulmonary arterial hypertensionN Engl J Med200234689690311907289
- HumbertMBarstRJRobbinsIMCombination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2Eur Respir J20042435335915358690
- GalieNBeghettiMGatzoulisMABosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled studyCirculation2006114485416801459
- ChannickRNSimonneauGSitbonOEffects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled studyLancet20013581119112311597664
- GalieNRubinLHoeperMTreatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trialLancet20083712093210018572079
- JingZCYuZXShenJYVardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled studyAm J Respir Crit Care Med20111831723172921471085
- TapsonVFTorresFKermeenFOral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trialChest20121421383139022628490
- TapsonVFJingZCXuKFOral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trialChest201314495295823669822
- JingZCParikhKPulidoTEfficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trialCirculation201312762463323307827
- HoeperMMLeuchteHHalankMCombining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertensionEur Respir J20062869169417012628
- McLaughlinVVGaineSPBarstRJEfficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertensionJ Cardiovasc Pharmacol20034129329912548091
- McLaughlinVVOudizRJFrostARandomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertensionAm J Respir Crit Care Med20061741257126316946127
- GhofraniHAGalieNGrimmingerFRiociguat for the treatment of pulmonary arterial hypertensionN Engl J Med201336933034023883378
- BarstRJOudizRJBeardsworthATadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertensionJ Heart Lung Transplant20113063264321256048
- WilkinsMRPaulGAStrangeJWSildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) studyAm J Respir Crit Care Med20051711292129715750042
- PulidoTAdzerikhoIChannickRNMacitentan and morbidity and mortality in pulmonary arterial hypertensionN Engl J Med201336980981823984728
- SimonneauGBarstRJGalieNContinuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trialAm J Respir Crit Care Med200216580080411897647
- SimonneauGRubinLJGalieNAddition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trialAnn Intern Med200814952153018936500
- GalieNGhofraniHATorbickiASildenafil citrate therapy for pulmonary arterial hypertensionN Engl J Med20053532148215716291984
- McLaughlinVVBenzaRLRubinLJAddition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trialJ Am Coll Cardiol2010551915192220430262
- HiremathJThanikachalamSParikhKExercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trialJ Heart Lung Transplant20102913714920022264
- GalieNBarberaJAFrostAEInitial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial HypertensionN Engl J Med201537383484426308684
- ZhuangYJiangBGaoHZhaoWRandomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertensionHypertens Res20143750751224646647
- GalieNMullerKScaliseAVGrunigEPATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertensionEur Respir J2015451314132225657022
- McLaughlinVChannickRNGhofraniHABosentan added to sildenafil therapy in patients with pulmonary arterial hypertensionEur Respir J20154640541326113687
Author contributions
CSL had the original idea and designed the study. GXF and ZJJ performed the systematic literature search, study identification, data extraction, and quality assessment. JXM and GZ undertook the statistical analysis. WZM, LB, and MWX drafted the article. All authors revised and approved the final report. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The study is supported by the Jiangsu Provincial Special Program of Medical Science (BL2013001).