Abstract
Background
While disease-modifying antirheumatic drugs (DMARDs) are a mainstay of therapy for rheumatoid arthritis (RA), some patients with early RA refuse DMARDs. In anthroposophic medicine (AM), a treatment strategy for early RA without DMARDs has been developed. Preliminary data suggest that RA symptoms and inflammatory markers can be reduced under AM, without DMARDs.
Patients and methods
Two hundred and fifty-one self-selected patients aged 16–70 years, starting treatment for RA of <3 years duration, without prior DMARD therapy, participated in a prospective, non-randomized, comparative Phase IV study. C-patients were treated in clinics offering conventional therapy including DMARDs, while A-patients had chosen treatment in anthroposophic clinics, without DMARDs. Both groups received corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs). Primary outcomes were intensity of RA symptoms measured by self-rating on visual analog scales, C-reactive protein, radiological progression, study withdrawals, serious adverse events (SAE), and adverse drug reactions in months 0–48.
Results
The groups were similar in most baseline characteristics, while A-patients had longer disease duration (mean 15.1 vs 10.8 months, p<0.0001), slightly more bone destruction, and a much higher proportion of women (94.6% vs 69.7%, p<0.0001). In months 0–12, corticosteroids were used by 45.7% and 81.6% (p<0.0001) and NSAIDs by 52.8% and 68.5% (p=0.0191) of A- and C-patients, respectively. During follow-up, both groups not only had marked reduction of RA symptoms and C-reactive protein, but also some radiological disease progression. Also, 6.2% of A-patients needed DMARDs. Apart from adverse drug reactions (50.4% and 69.7% of A- and C-patients, respectively, p=0.0020), none of the primary outcomes showed any significant between-group difference.
Conclusion
Study results suggest that for most patients preferring anthroposophic treatment, satisfactory results can be achieved without use of DMARDs and with less use of corticosteroids and NSAIDs than in conventional care.
Limitation
Because of the non-randomized study design, with A-patients choosing anthroposophic treatment, one cannot infer how this treatment would have worked for C-patients.
Supplementary materials
Figure S1 Patient ratings of therapy tolerability (“poor”–“average”–“good”).
Note: Percentage of patients with the rating “good tolerability” are as follows: in months 0 (Anthroposophic group: n=99, Conventional group: n=86), 3 (122+107), 6 (115+105), 9 (114+95), 12 (109+95), 15 (107+92), 18 (105+87), 21 (98+88), 24 (95+86), 27 (87+61), 30 (84+61), 33 (84+60), 36 (82+56), 39 (70+53), 42 (73+56), 45 (69+53), and 48 (68+57).

Figure S2 Physician ratings of therapy tolerability.
Note: Physician ratings of therapy tolerability on a VAS (0= no adverse reactions, 100= most severe adverse reactions) in months 0 (Anthroposophic group: n=99, Conventional group: n=86), 3 (126+111), 6 (122+110), 9 (118+107), 12 (117+102), 15 (115+99), 18 (112+94), 21 (103+94), 24 (100+92), 27 (92+69), 30 (89+68), 33 (85+68), 36 (83+65), 39 (75+60), 42 (72+61), 45 (71+59), and 48 (71+64).
Abbreviation: VAS, visual analog scale.
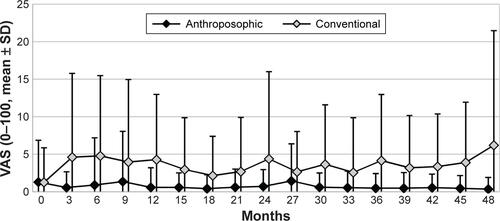
Figure S3 Use of corticosteroids in months 0–48.
Note: Use is calculated as average dose in prednisolone equivalents among steroid users in each 3-month period (“3” indicating “months 1–3” and so on).
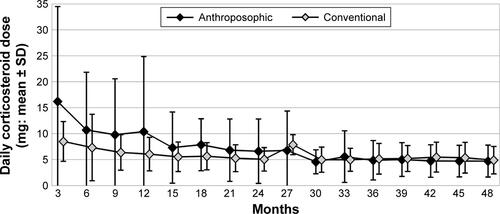
Figure S4 Use of NSAIDs in months 0–48.
Note: Use is calculated as average dose in diclofenac equivalents among NSAID users in each 3-month period (“3” indicating “months 1–3” and so on).
Abbreviation: NSAID, nonsteroidal anti-inflammatory drug.
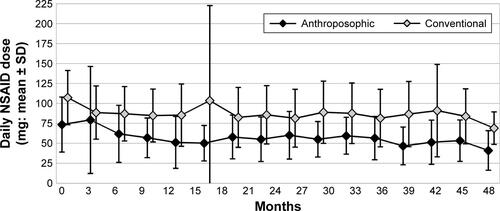
Figure S5 Disease Activity Score (0–10).
Note: Disease Activity Score (0–10) in months 0 (Anthroposophic group: n=129, Conventional group: n=108), 3 (176+104), 6 (123+103), 9 (118+99), 12 (118+99), 15 (115+94), 18 (111+90), 21 (105+90), 24 (101+89), 27 (93+66), 30 (89+64), 33 (85+64), 36 (86+63), 39 (74+56), 42 (73+59), 45 (72+55), and 48 (71+63).
Table S1 Patient numbers at each follow-up
Table S2 Dropout analyses
Table S3 RA-VAS and C-reactive protein, alternative analyses
Acknowledgments
Current affiliations: CK (family practice, Hamburg, Germany), JK (Klinikum Stephansplatz, Hamburg, Germany), US (rheumatology practice, Bad Münder, Germany), LG (Anthromed Öschelbronn, Niefern-Öschelbronn, Germany), AD (Clinical Trials Support, Münster, Germany).
The late Ulrich Schmidt was responsible for study monitoring and data transfer, and the late Hartwig Mathies functioned as an independent rheumatologist, confirming rheumatoid arthritis diagnosis after 0 and 48 months.
The study was funded mainly by the Federal Ministry of Education and Research (Grant FKZ 01 KT 9414/9501/9502/9503/9504/9505) with subsequent additional support from the Software-AG Stiftung (Grant P-4291), the Mahle-Stiftung, and the German Society of Anthroposophic Physicians (Grant GAÄD-Sonderfonds-CPAK, 2007). This publication was funded by the Förderstiftung Anthroposophische Medizin and the Christophorus-Stiftung (Grant 176-CST). The German Ministry of Health approved the study protocol, while the sponsors had no influence on the planning or conduct of the study, analysis or interpretation of data, or writing of this paper.
Author contributions
LS, JK, and US wrote the study protocol. LS and CK were the study physicians. RR calculated the Ratingen Score. VNP and AD analyzed data, and HJH performed a final audit of the analyses. HJH and LS wrote the first publication draft. LS was the principal investigator, had access to all data, was guarantor until May 2016 (followed by HJH), and approved the penultimate manuscript version before his death in June 2016. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
In the past 3 years, the following authors received funding from manufacturers of medications used for RA therapy: HJH (research grants: Wala, Weleda), JK (lecturing fee: Weleda), and US (lecturing fee: AbbVie, Berlin-Chemie, Pfizer). The authors report no other conflicts of interest in this work.
