Abstract
Purpose
Simponi® (golimumab, MSD) is a fully human monoclonal antibody against tumor necrosis factor alpha administered subcutaneously using an autoinjector or a prefilled syringe. This study examined preference for administration of golimumab by autoinjector or prefilled syringe in patients with moderate-to-severe ulcerative colitis (UC).
Patients and methods
This was a multicenter, open-label, randomized crossover trial (EudraCT no 2014-000656-29). Patients with moderate-to-severe UC were randomized 1:1 to receive 2 subcutaneous injections of 50 mg golimumab with the autoinjector followed by 2 injections of 50 mg with the prefilled syringe or the same 4 injections administered in the opposite order. Patients assessed preference, ease of use, and discomfort immediately after the injections and 2 weeks later.
Results
Ninety-one patients were included (median age=42.7 years [range, 19.7–93.7]; 58% male). The autoinjector was preferred by 76.9% of patients immediately after injections and by 71.4% 2 weeks later. The autoinjector was more often considered extremely easy or easy to use (94.5%) than the prefilled syringe (73.6%). Moderate discomfort or worse was reported by more patients when using the prefilled syringe (20.9%) than when using the autoinjector (5.5%), and severe discomfort or discomfort preventing injection of future doses was reported by 8.8% for the pre-filled syringe but not at all when using the autoinjector. A favorable or extremely favorable overall impression was reported by 89.0% for the autoinjector and 72.5% for the prefilled syringe.
Conclusion
Most patients with moderate-to-severe UC preferred to self-administer golimumab with the autoinjector over a prefilled syringe.
Introduction
Ulcerative colitis (UC) is a chronic and incurable autoimmune inflammatory bowel disorder characterized by continuous inflammation and ulceration of the mucosa of the rectum and, to a variable extent, the colon.Citation1,Citation2 The disease affects 2.5 million people worldwide and is usually diagnosed when individuals are in their 20s and peaks again in their 60s or 70s, but it can occur at any age. UC is generally characterized by flares that alternate with periods of remission, although some patients have continuous activity. The severity of flares and their response to treatment are difficult to predict, although the frequency of flares decreases with time. Between 25% and 40% of people living with UC will require surgery at some point in their life.Citation1
Simponi® (golimumab, MSD, Janssen Biotech, Inc., Horsham, PA, USA) is a fully human monoclonal antibody against tumor necrosis factor α (TNF-α) available in the European Union since 2013 for the treatment of moderate-to-severe active UC in adult patients who have had an inadequate response to conventional therapy or who are intolerant to or have medical contraindications for such therapies.Citation3 Golimumab is also indicated for the treatment of moderate-to-severe active rheumatoid arthritis (RA) (in combination with methotrexate), active psoriatic arthritis (alone or in combination with methotrexate), and active ankylosing spondylitis.
Golimumab is administered with the SmartJect® autoinjector (MSD, Janssen Biotech, Inc.) or with a prefilled syringe (). The SmartJect autoinjector was developed to simplify self-injection of golimumab for patients suffering from RA, psoriatic arthritis, and ankylosing spondylitis, with the objective of optimizing treatment adherence.Citation4 Autoinjectors offer several advantages, including portability, convenience, and flexible scheduling, and they have been shown to improve treatment adherence.Citation5–Citation7 In the GO-MORE study, which examined the efficacy and safety of golimumab in biologic-naive RA patients, two-thirds of those who self-injected chose to use the autoinjector over a prefilled syringe.Citation4 In addition, most of the patients using the autoinjector had a favorable impression of it, considered it easy to use, and reported that it caused little pain or discomfort. Device preferences for UC patients, however, have not been reported and may be different from RA patients who often suffer from hand pain, swelling, and deformities. Here, we report the results of a study examining whether patients with moderate-to-severe UC prefer administration of golimumab using the SmartJect autoinjector or a prefilled syringe.
Patients and methods
Study design
This was a multicenter, open-label, randomized, crossover trial comparing preference for administration of golimumab using the SmartJect autoinjector or a prefilled syringe in patients with moderate-to-severe UC (EudraCT no 2014-000656-29). The trial was conducted at 20 sites in Belgium (19 sites recruited patients) between July 2014 and October 2015. The primary objective was to determine whether UC patients prefer to administer golimumab using the autoinjector, using a prefilled syringe, or are undecided. Secondary objectives were to determine which of the 2 administration devices patients consider the easiest to use and result in the least discomfort, and how patient characteristics influence device preference.
The study included adults with an established diagnosis of UC and moderate-to-severe disease (Mayo score ≥6, including an endoscopic subscore ≥2) and a previous conventional therapy of at least 3 months with aminosalicylates and at least 3 months with corticosteroids, 6-mercaptopurine, or azathioprine unless intolerant to or contraindicated for such therapies. Individuals who previously self-injected any agent or who were using other biological agents were excluded.
Patients completed a questionnaire to collect demographic data, and investigators collected information about the patient’s medical history. Patients were then randomized 1:1 to receive 2 injections of golimumab 50 mg (Simponi®, MSD) with the autoinjector (SmartJect®, MSD) followed by 2 injections of 50 mg with the prefilled syringe or 2 injections of 50 mg golimumab with the prefilled syringe followed by 2 injections with the autoinjector (total 200 mg golimumab administered). All injections were subcutaneous, were in the same part of the body, and were performed on the same day (day 0). The first of the 2 injections with each device was performed by the physician, and the second by the patient under the physician’s supervision.
Immediately after using each device, patients answered a questionnaire assessing the hand used to self-inject (left, right, both), ease of use for self-injection (extremely easy, easy, neither easy nor difficult, difficult, or extremely difficult), overall discomfort (none, mild, moderate, severe, or such discomfort that I cannot inject future doses), and overall impression of the self-injection experience (extremely favorable, favorable, neither favorable nor unfavorable, unfavorable, or extremely unfavorable). After completing all 4 injections, patients completed a questionnaire on their preference for the devices (prefilled syringe, autoinjector, or undecided). After 2 weeks, the patient was contacted to complete the device preference questionnaire again to capture the effect of any delayed adverse events on the patient’s responses.
Investigators recorded adverse events according to the International Conference on Harmonization Guidelines for Good Clinical Practice including the following: adverse event/diagnosis; dates of onset and resolution; severity (mild, moderate, severe), whether the event was serious (yes/no) and, if so, why; potential relationship to the study drug (yes/no); and action taken. Adverse events were considered serious if they resulted in death, were life threatening, required hospitalization or prolongation of an existing inpatient hospitalization, resulted in a persistent or significant disability or incapacity, were a congenital abnormality or birth defect, were cancer, were associated with an overdose, or were any other important medical event.
Ethics
The study was approved by each site’s independent ethics committee () and was conducted in accordance with International Conference on Harmonization Guideline for Good Clinical Practice. All included patients provided written informed consent.
Study size estimate
A power calculation was not performed. Instead, a study size estimate of 100 subjects was planned based on sample sizes that yielded relevant results in similar studies.Citation8–Citation11 Assuming 10% dropout, approximately 110 subjects were to be screened.
Statistical analysis
The primary outcome measure (device preference) was analyzed in the per-protocol set, defined as all subjects who met the inclusion and exclusion criteria, received all 4 injections, and completed the device preference questionnaire. Statistical analyses of preference included frequency distribution overall immediately and 2 weeks after injection; by order of device administration; 2 weeks after injection by preference immediately after injection; and by age, sex, ethnicity, marital status, education level, employment status, and total Mayo score category. Statistical analysis of secondary outcome measures (ease of use, discomfort, and overall impression) included frequency distribution overall and by order of injection. As stipulated in the study protocol, only descriptive statistics were calculated, although in a post hoc analysis, preferences were analyzed according to subject baseline characteristics by Cochrane–Armitage trend test or Fisher’s exact test, with p-values below 0.05 considered to indicate statistical significance. Missing data were not replaced. Calculations were made using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patients
Between July 11, 2014, and September 17, 2015, 100 patients were included in the study, and the study was completed on October 5, 2015. Of the 100 recruited patients, 99 were treated. Another 8 patients did not fulfill the selection criteria. Thus, 91 patients were included in the analysis. Most of these patients were between 30 and 60 years of age, slightly more than half were male, and all but 2 were White/Caucasian (). On average, patients had been diagnosed with UC for 8.6 years. Disease severity was moderate in about two-thirds and severe in about one-third, although precise proportions depended slightly on the assessment (Mayo score, physician’s global assessment, and sigmoidoscopic and endoscopic findings). Most were being treated with 5-aminosalicylic acid and corticosteroids, and most had not previously received anti-TNF therapy.
Device preference
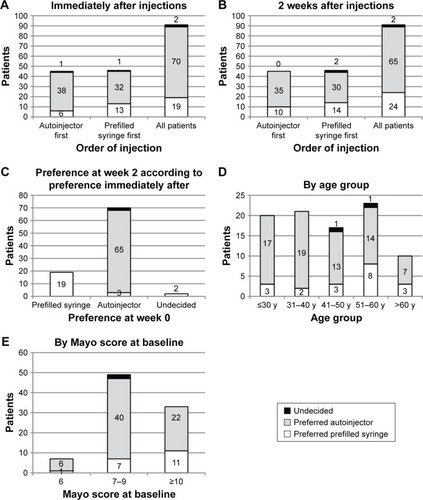
Immediately after injections, approximately three-quarters of patients (76.9%) indicated that they preferred administering golimumab with the autoinjector (). This was similar to the preference reported 2 weeks later, with 71.4% reporting that they preferred the autoinjector. This was also the case irrespective of the order of injection, although more patients who started with the autoinjector preferred it (84.4%) than patients who started with the prefilled syringe (69.6%). Also, most patients (92.3%) did not change their preference 2 weeks later: of those who preferred a prefilled syringe immediately after the injections, all still preferred it 2 weeks later, and of those that preferred the autoinjector immediately after the injections, 92.9% still preferred it 2 weeks later. Results were similar when analyzed for all 99 patients completing the questionnaire (data not shown). A post hoc analysis showed that preference for the autoinjector was not significantly affected by patient age, disease severity as measured by the total Mayo score, sex, marital/cohabitation status, level of education, employment status, or time since UC diagnosis (data not shown).
Figure 2 Device preference.
Ease of use, discomfort, and overall impression of the devices
More patients considered the autoinjector extremely easy or easy to use (94.5%) than the prefilled syringe (73.6%) (). Moderate discomfort or worse was reported by only 5 patients (5.5%) when using the autoinjector but by 19 patients (20.9%) when using the prefilled syringe. Severe discomfort or discomfort such that the patient could not inject future doses was reported by 8 patients (8.8%) when using the prefilled syringe but was not reported by any of the patients using the autoinjector.
Table 1 Ease of use, discomfort, and overall impression of the devices
In agreement with these findings, 89.0% of patients had an overall extremely favorable or favorable impression of the autoinjector, while 72.5% had an extremely favorable or favorable impression of the prefilled syringe. An unfavorable impression of the device or worse was reported by only 2 patients (2.2%) when using the autoinjector but by 8 patients (8.8%) when using the prefilled syringe.
The order of use of the 2 different devices appeared to slightly bias the subjects toward the device they used first. For example, the proportion of patients with an extremely favorable or favorable impression of the autoinjector was 93.3% when it was used first vs 84.8% when it was used second. Likewise, the proportion of patients with an extremely favorable or favorable impression of the prefilled syringe was 84.8% when it was used first vs 60.0% when it was used second.
Safety
None of the patients experienced a serious adverse event. Treatment-emergent adverse events, all mild or moderate in severity, were reported by 10 patients (10.1%) (). These events included injection-site hematoma (n=2), injection-site pain (n=1), palpitations (n=1), UC flare (n=1), dyspepsia (n=1), flatulence (n=1), hemorrhoidal hemorrhage (n=1), tooth sensitivity (n=1), parotid gland enlargement (n=1), viral upper respiratory tract infection (n=1), headache (n=1), and hot flush (n=1).
Discussion
This study showed that most patients with moderate-to-severe UC preferred to self-administer golimumab with the autoinjector over a prefilled syringe. More patients found the autoinjector easier to use and to less often cause discomfort than the prefilled syringe. This preference for the autoinjector did not change when measured again 2 weeks later, during which time most delayed reactions would have appeared. Demographic characteristics did not significantly affect preference. Order of presentation did not affect the overall preference for the autoinjector, although it biased the preference somewhat toward the device first presented. The results of this study are strengthened by the fact that data were collected in a real-life clinical setting at multiple sites. In addition, although the absolute sample size was small, this study included a relatively large population for UC and was enough to observe meaningful differences in preference.
Overall opinions were also favorable in the GO-MORE trial, in which patients with active RA self-injected or had someone else administer subcutaneous golimumab with the same device.Citation4 Although individuals with RA often suffer from hand pain, swelling, and deformities, in GO-MORE, two-thirds of patients who chose to self-inject selected the autoinjector over a prefilled syringe. Most of the patients in the GO-MORE study who used the autoinjector had a favorable impression of it, considered it easy to use, and reported that it caused little pain or discomfort. However, the study did not directly assess preference or compare patient experiences between the autoinjector and injection with the prefilled syringe. A preference for the golimumab autoinjector over previous injection devices was also reported by 70.6% of patients in the GO-SAVE trial, which included patients with active RA who were switched from adalimumab or etanercept to golimumab.Citation12
Prefilled pens or other autoinjectors are also preferred over and considered easier to use and less painful than syringes for self-administration of darbepoetin by chronic kidney disease patients,Citation11 methotrexateCitation13 and adalimumabCitation14 for RA patients, and insulin for diabetes patients.Citation15–Citation18 Similarly, a study in healthy volunteers found that subcutaneous injection by autoinjector was preferred over syringe injection by a nurse.Citation19
A systematic review in 2013 found that treatment adherence to anti-TNF biologics in UC patients was only 52.7%.Citation20 It also found that administration with a syringe vs a pen was a predictor of nonadherence. Although the SmartJect autoinjector might therefore be expected to improve adherence to golimumab, we did not assess adherence in this study.
Conclusion
This study showed that patients with UC generally prefer to administer golimumab with an autoinjector. Although this should help inform prescribers about what their patients may expect, they should be aware that some patients might still prefer using a prefilled syringe.
Author contributions
S Vermeire participated in developing a first draft of the manuscript together with the medical writer. All authors contributed to patient recruitment, analysis and interpretation of the results, critical revision of the manuscript, and approval of the final version.
Acknowledgments
Medical writing was provided by Dr Phillip Leventhal (4Clinics, Paris, France) and funded by MSD Belgium. The work was funded by MSD. The study funder participated in designing the study; and collecting, analyzing, and interpreting the data; writing the report; and in the decision to submit it for publication.
Supplementary materials
Table S1 List of participating ethics committees
Table S2 Patient demographic characteristics
Table S3 Treatment-emergent adverse events
Disclosure
S Vermeire has received consulting fees from AbbVie, MSD, Takeda, Ferring, Genentech/Roche, Shire, Pfizer Inc, Galapagos, Mundipharma, Hospira, Celgene, Second Genome, and Janssen; research grants from AbbVie, MSD, and Takeda; and speaker fees from AbbVie, MSD, Takeda, Ferring, Dr Falk Pharma, Hospira, and Tillots. F Baert received research grants from AbbVie, Chiesi, MSD, and Ipsen, Roche; and speaker and consultancy fees from Abbvie, Hospira, Janssen (Johnson & Johnson), MSD, Mundipharma, Falk, Ferring, and Takeda. O Dewit received research grants from Abbvie and MSD; consulting fees from Ferring, Mundipharma, Hospira, Takeda, and Janssen; and speaker fees from MSD, Ferring, Mundipharma, Hospira, and Takeda. P Van Hootegem received consultancy fees from Janssen (Johnson & Johnson) and Takeda; and speaker fees from Falk and Ferring. D Franchimont received honorarium fee for lectures; consulting fees and/or educational grants from Amgen, Pfizer, Mundipharma, AbbVie, MSD, Takeda, Ferring, Falk, Merck Serono, Roche and Janssen. E Louis has received grants from MSD and AbbVie; speaker fees from AbbVie, Ferring, MSD, Chiesi, Mitsubishi Pharma, Hospira, Janssen, and Takeda; and is on the advisory board of AbbVie, Ferring, MSD, Takeda, Mitsubishi Pharma, Celltrion, and Prometheus. The authors report no other conflicts of interest in this work.
References
- CosnesJGower-RousseauCSeksikPCortotAEpidemiology and natural history of inflammatory bowel diseasesGastroenterology201114061785179421530745
- StangeEFTravisSPVermeireSEuropean evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosisJ Crohns Colitis20082112321172194
- GilardiDFiorinoGAlloccaMBravataIDaneseSGolimumab: clinical update on its use for ulcerative colitisDrugs Today (Barc)201551317118425876561
- Schulze-KoopsHGiacomelliRSamborskiWFactors influencing the patient evaluation of injection experience with the SmartJect autoinjector in rheumatoid arthritisClin Exp Rheumatol201533220120825664404
- KivitzASeguradoOGHUMIRA pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumabExpert Rev Med Devices20074210911617359217
- LasalviaPBarahona-CorreaJERomero-AlverniaDMPen devices for insulin self-administration compared with needle and vial: systematic review of the literature and meta-analysisJ Diabetes Sci Technol201610495996626920639
- RidyardCHDawoudDMTuersleyLVHughesDAA systematic review of patients’ perspectives on the subcutaneous route of medication administrationPatient20169428129226792584
- WongMAbdulnabiRCareyMAFuHA randomized, cross-over comparison of preference between two reusable insulin pen devices in pen-naive adults with diabetesCurr Med Res Opin201329546547323438454
- PfutznerASchipperCNiemeyerMComparison of patient preference for two insulin injection pen devices in relation to patient dexterity skillsJ Diabetes Sci Technol20126491091622920818
- PhillipsJTFoxEGraingerWTuccilloDLiuSDeykinAAn open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex® prefilled syringe in multiple sclerosis subjectsBMC Neurol20111112621999176
- LimWHChanDBoudvilleNPatients’ perceptions of subcutaneous delivery of darbepoetin alfa by autoinjector prefilled pen versus prefilled syringe: a randomized, crossover studyClin Ther20123491948195322902097
- TandonNBolceRNaimAPMS66 satisfaction with and preference for golimumab and its auto-injector among rheumatoid arthritis patients switched from adalimumab or etanerceptValue Health2012154A45A46
- DemaryWSchwenkeHRockwitzKSubcutaneously administered methotrexate for rheumatoid arthritis, by prefilled syringes versus prefilled pens: patient preference and comparison of the self-injection experiencePatient Prefer Adherence201481061107125125973
- KivitzACohenSDowdJEClinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trialClin Ther200628101619162917157117
- PfutznerABaileyTCamposCAccuracy and preference assessment of prefilled insulin pen versus vial and syringe with diabetes patients, caregivers, and healthcare professionalsCurr Med Res Opin201329547548123402225
- CamposCLajaraRDeluzioTUsability and preference assessment of a new prefilled insulin pen versus vial and syringe in people with diabetes, physicians and nursesExpert Opin Pharmacother201213131837184622873943
- PfutznerAAsakuraTSommavillaBLeeWInsulin delivery with FlexPen: dose accuracy, patient preference and adherenceExpert Opin Drug Deliv20085891592518713000
- KorytkowskiMBellDJacobsenCSuwannasariRA multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitusClin Ther200325112836284814693308
- BerteauCSchwarzenbachFDonazzoloYEvaluation of performance, safety, subject acceptance, and compliance of a disposable autoinjector for subcutaneous injections in healthy volunteersPatient Prefer Adherence2010437938821049090
- LopezABillioudVPeyrin-BirouletCPeyrin-BirouletLAdherence to anti-TNF therapy in inflammatory bowel diseases: a systematic reviewInflamm Bowel Dis20131971528153323518810

