Abstract
Introduction
Pulmonary arterial hypertension (PAH) is a rare, incurable disease associated with decreased life expectancy and a marked impact on quality of life (QoL). There are three classes of drugs available for treatment: endothelin receptor antagonists (ERA), drugs acting on nitric oxide pathway (riociguat and phosphodiesterase type 5 inhibitors [PDE5i]), and drugs acting on prostacyclin pathway. The latter have widely different modes of administration – continuous intravenous infusion, continuous subcutaneous infusion, inhaled, and oral – each associated with variable treatment burden, and implications for health economic assessment. This study aimed to establish utility values associated with different modes of administration of drugs acting on the prostacyclin pathway for use in economic evaluations of PAH treatments.
Methods
A UK general public sample completed the EQ-5D-5L and valued four health states in time trade-off interviews. The health states drafted from literature and interviews with PAH experts (n=3) contained identical descriptions of PAH and ERA/PDE5i treatment, but differed in description of administration including oral (tablets), inhaled (nebulizer), continuous subcutaneous infusion, and continuous intravenous infusion.
Results
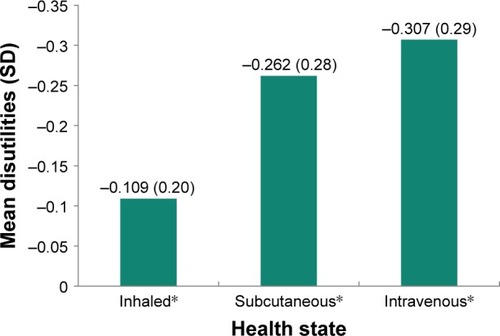
A total of 150 participants (63% female; mean age 37 years) completed interviews. Utilities are presented as values between 0 and 1, with 0 representing the state of being dead and 1 representing being in full health. The mean (SD) utility for oral health state was 0.85 (0.16), while all other health states were significantly lower at 0.74 (0.27) for inhaled (p=0.001), 0.59 (0.31) for subcutaneous (p<0.001) and 0.54 (0.32) for intravenous (p<0.001), indicating that there are disutilities (negative differences) associated with non-oral health states. Disutilities were −0.11 for inhaled, −0.26 for subcutaneous, and −0.31 for intravenous administration.
Conclusion
The results demonstrate quantifiable QoL differences between modes of administration of drugs acting on the prostacyclin pathway. QoL burden should be considered for economic evaluation of drugs for PAH treatment.
Introduction
Pulmonary arterial hypertension (PAH) is a rare, chronic and progressive disease characterized by increased pulmonary vascular resistance which ultimately leads to right ventricular failure, profound functional limitations, and death.Citation1–Citation4 The New York Heart Association (NYHA) and World Health Organization (WHO) define four functional classes (FC) of pulmonary hypertension that are used to classify severity in PAH, with Class I representing patients with no limitation of physical activity through to Class IV in which patients are unable to carry out any physical activity without symptoms.Citation5,Citation6 Median survival in untreated idiopathic disease is less than 3 yearsCitation7 and, despite therapeutic advances, mortality remains high.Citation8
The pathophysiology of PAH is not yet completely understood. Three key pathogenic pathways have been identified and can be targeted with pharmaceuticals: the endothelin receptor antagonists (ERA) acting on the endothelin pathway, the phosphodiesterase type 5 inhibitors (PDE5i) and a soluble guanylate cyclase stimulator acting on the nitric oxide pathway, and several drugs acting on the prostacyclin (PGI2) pathway.Citation9 Given the progressive and ultimately terminal nature of PAH, the overall treatment goal is to reduce disease progression and achieve a low risk status (eg, WHO FC I or II and no progression of symptoms).Citation6 Treatment guidelines recommend that for patients who fail to achieve an adequate clinical response with initial monotherapy, sequential double or triple combination therapy is utilized.Citation6 Growing evidence points to the benefits of combining different treatments to target multiple pathways simultaneously.Citation1,Citation10–Citation12
Epoprostenol, the first therapy for PAH acting on the PGI2 pathway, was approved in the United States in 1995 (and a year later in Europe) for use as a continuous intravenous infusion.Citation1 Agents acting on the PGI2 pathway are, however, underused in clinical practice,Citation8,Citation13 being generally reserved for higher risk patients presenting in NYHA/WHO FC IV or with rapidly progressing symptoms.Citation2 This is primarily on account of their mode of administration, which may be intravenous via a central venous catheter. Furthermore, the practical and personal implications of such treatments, and the subsequent impact on the patients’ health-related quality of life (HRQL), should not be overlooked.Citation14,Citation15 Other treatments acting on this pathway available in Europe include the prostacyclin analogs treprostinil (continuous intravenous or subcutaneous infusion) and iloprost (intravenous or inhaled), and the IP receptor agonist selexipag (oral).Citation13,Citation15
Epoprostenol and treprostinil, when administered by continuous intravenous infusion, have been associated with potentially fatal adverse events such as catheter-related sepsis.Citation8,Citation16–Citation18 While treprostinil can also be administered subcutaneously, this has been associated with intolerable infusion site pain in some patients.Citation19 Iloprost can be administered as a continuous intravenous infusion or as an inhaled treatment using a nebulizer device. This also comes with some potential difficulties for patients because the nebulizers need to be taken every 2–3 hours, which is time consuming.Citation20
Overall, the burden of PGI2 pathway treatments and the systemic side effects may contribute to the reluctance of patients to accept these therapies or make it difficult for patients to adhere to the treatment schedule. To overcome these barriers, oral therapies have been developed.Citation21,Citation22
As new treatments become available, it is important to assess their cost-effectiveness in relation to other existing treatments in order to demonstrate their value to health technology assessment bodies, payers, the wider clinical audience, and decision makers.Citation23–Citation27 This can be done as part of cost-utility analyses (CUAs).Citation26,Citation27 In a CUA, the quality of life (QoL) component is measured by utilities, which represent strength of preference for a particular health state, and are measured on a scale of 0 to 1, with 0 representing the state of being dead and 1 representing being in full health.Citation27,Citation28
In order to incorporate the strength of preference for prostacyclin analogs, it is necessary to use utilities that represent the different modes of administration of these treatments. While utilities exist that take into account the PAH disease burden by FC,Citation6 there are currently no published utility values relating to the unique mode of administration of PAH drugs. Thus, the aim of this research was to elicit robust utility values associated with four health states corresponding to different modes of oral and non-oral treatment administration of drugs acting on the prostacyclin pathway in PAH.
Methods
Health state development
Health state descriptions were drafted based on review of the literature and interviews with three clinical experts. Two clinical experts were pulmonary hypertension clinical nurse specialists who work directly with PAH patients in the UK and Ireland. The additional clinical expert was an internal employee who previously worked as a pediatric cardiologist and now works as a medical affairs specialist at Actelion. The interviews followed semistructured interview guides and focused on patients’ experiences with PAH as a disease, as well as with different PAH treatments. All clinical experts then later reviewed and provided comments on the draft health states in order to validate the content. Minor revisions to the health states were made based on these comments. Health state content was also informed and validated based on review of the literature, published clinical guidelines,Citation10 and treatment instructions for use. Literature searching targeted published research related to symptoms and impacts of PAH,Citation29–Citation32 and also included research conducted directly with patients and carers.Citation13,Citation20
Each of the health states contained an identical description of the disease, symptoms, and impacts of PAH but differed in the description of the treatment mode of administration. Health states were intended to be read and valued by the general public, and therefore contained language that could be understood by adults without any clinical background. The health states were labeled with arbitrary symbols and did not include a disease label in order to avoid any possible distortion in health state evaluation on account of preconceived conceptions of the disease.Citation33 In addition to the health states, a support document was developed that included pictures and further descriptions of the treatment modes of administration. This was designed to help participants fully understand what the different treatment devices involve in terms of preparation, drug administration, device cleaning, and patient care. This document was developed primarily using treatment instructions for use and was also reviewed by the clinical experts.
Pilot interviews and final health states
The draft health states and the support document were piloted with 10 members of the UK general public to ensure the content was clear and would be understood during valuation. Participants were asked to complete an in-person time trade-off (TTO) exercise (detailed in the “Health state valuations” section), followed by cognitive interview questions about the TTO exercise and study materials (including each health state). The interviews took place in two waves (n=5 per wave) to allow for the identification and cognitive testing of any revisions to the vignettes and support document indicated from the first wave of interviews. The health states and support document were found to be understood by all of the participants and therefore deemed suitable for use in the valuation exercise, with only minor formatting issues identified by piloting (ie, font size and text alignment) addressed before being finalized.
The final health states are shown in , and an example is shown in . The four health states comprised identical descriptions of PAH as a disease, the main symptoms, and impacts, but differed in treatment description (twice daily oral drug, nebulization, continuous subcutaneous infusion, or continuous intravenous infusion with a permanent catheter).
Table 1 Final health states
Table 2 Example health state
Participants
Pilot interviews were conducted with 10 members of the UK general public based in London by two experienced field interviewers. Participants were recruited by a member of the ICON project team using an advertisement posted on community noticeboards and online. Inclusion criteria were aged 18 years and over; currently resident in the UK; able to read and speak English and able to communicate well with study staff; able to understand the valuation exercise as judged by the interviewer; and willing to provide informed consent. Pilot interviews were audio recorded with permission from the participants for reference purposes only. Participants could refuse permission for audio recording without penalty, and could still participate in the interview. Interviews took place in private meeting rooms at times convenient to the participants. All participants provided written informed consent to participate in the interviews.
Final health state valuation was conducted with 150 members of the UK general public based in Bristol, London, Marlow, and Sheffield. Participants were recruited using an advertisement posted on community noticeboards and online by six experienced field interviewers. Inclusion criteria were the same as for the pilot interviews. Interviews took place in private meeting rooms at times convenient to the participants. All participants provided written informed consent to participate in the valuation and interviews were not recorded.
Health state valuation
Following consent, participants completed a sociodemographic questionnaire, used for the purposes of sample description, and the EQ-5D-5L. The EQ-5D-5L instrument is a self-administered generic preference-weighted QoL measure that can be used to determine each individual’s current health state utility.Citation34 The instrument comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression), each with five levels. A single utility score can be derived from the instrument using a published UK algorithm.Citation34 The instrument also includes a 100-point visual analog scale (VAS) from 0 (worst health imaginable) to 100 (best health imaginable), which asks participants to rate their own health on the day they participated in the study.
A rating exercise using the health states was then completed to familiarize the participants with the individual descriptions. Participants were presented with one health state at a time in a random order and asked to rate each of the health state vignettes. This exercise comprised the placing of cards describing each of the health states on an enlarged 100-point VAS, where the anchors of 0 and 100 were worst and best imaginable health, respectively. Two additional health states describing being in full health and being dead were also rated.
A TTO exercise was then conducted to elicit utility values for each health state. The TTO method, in deriving utility values based upon participants’ responses to decision scenarios, was specifically developed for use in health care.Citation35 Health states were presented to the participants in a random order and the values elicited were noted by the interviewer. The TTO exercise used a board with two hypothetical life choices representing number of years in full health and number of years in a health state, up to a total time of 10 years. Respondents were presented with a series of two choices, and asked to choose their preference between living in the health state for 10 years or living in a state of full health. Time in full health was then varied in 6-month increments until the participant was indifferent between the two choices. The utility is calculated by dividing the time in the full health state by the time in the presented health state, so that the formula can be described as: Utility = years in full health/years in health state. For this study, a maximum total life time horizon of 10 years was chosen in line with the UK measurement and valuation of health studyCitation36 and because it is most commonly used in TTO studies in other chronic diseases.Citation37,Citation38
The lead-time (LT-TTO) approach was utilized for any health state considered by a participant in the TTO exercise to be worse than dead (ie, where on the initial TTO exercise the participant preferred to be dead than to live for 10 years in a health state). The LT-TTO methodology presented participants with a scenario where they could choose between a life comprising full health for a maximum of 10 years followed by immediate death (where the amount of time spent in full health varies), or a life comprising living in full health for 10 years followed by living in the health state for 10 years, and then immediate death. When a participant became indifferent between the two choices, a utility value for that health state was calculated as follows: Utility = (years in full health – years of lead-time)/years in health state.Citation39
Analysis
Basic descriptive statistics (mean [standard deviation], N [%]) were used to describe the sociodemographic characteristics of the study sample, including their EQ-5D-5L scores, VAS scores and utility values of health states, as well as to compare the study sample values to EQ-5D-5L and UK published norms.Citation40 Mixed and general linear models were used to compare VAS and utility scores between health states, with post hoc tests (eg, Dunnett’s test with the oral health state taken as reference) used to compare VAS ratings and utility scores between health states. Univariable and multivariable mixed effects regression models were used to assess relationships between sociodemographic variables and VAS ratings and utility values, with results presented as unadjusted and adjusted regression coefficients (β) with standard error (SE). Throughout, 95% confidence intervals (CIs) were used to express uncertainty in the data, with statistical significance taken at the 5% level (p<0.05).
Ethics approval
This study was approved by Salus IRB on April 27, 2017 (protocol #0179–0042).
Consent for publication
The information presented in this manuscript has been sufficiently anonymized, and so it is not possible to obtain consent for publication from the participants of this study.
Results
Sample description
A total of 150 members of the general public valued the health states (mean age 37.2 years; 62.7% female). The sample characteristics and EQ-5D-5L results of those completing the health state valuation exercises are shown in .
Table 3 Sociodemographic and EQ-5D-5L results
The study sample was compared with the UK general population.Citation40–Citation43 There were some differences with the study sample having higher numbers of females (62.7% compared with 50.8%) and a lower median age (33.5 years compared with 40 years). The ethnicity of the sample was more diverse, with 71.3% reporting as White ethnicity compared with 85.9% in the general population. A lower proportion of the participants were employed with a higher proportion being students and single than the general population, which is likely to reflect the younger age of this sample. The mean state of health, as measured by the VAS, was slightly lower in the study sample than UK norms (81.5 vs 82.8).
Health state valuations
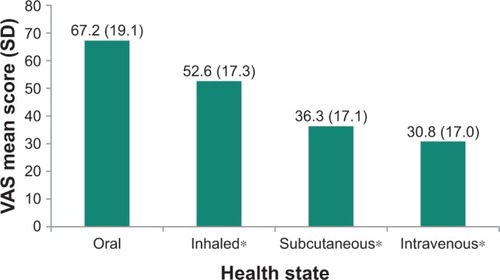
The mean observed TTO and VAS utility scores for the PAH health states are presented in and , respectively. The oral treatment administration health state received the highest mean ratings in the VAS exercise (67.2 [19.1]) and the highest mean TTO utility value (0.85 [0.16]). By contrast, intravenous treatment administration received the lowest VAS rating (30.8 [17.0]) and TTO utility value (0.54 [0.32]). The VAS and TTO scores differed statistically significantly between the health states (both p<0.001).
Figure 1 Mean TTO utility scores for each PAH health state.
Abbreviations: PAH, pulmonary arterial hypertension; SD, standard deviation; TTO, time trade-off.
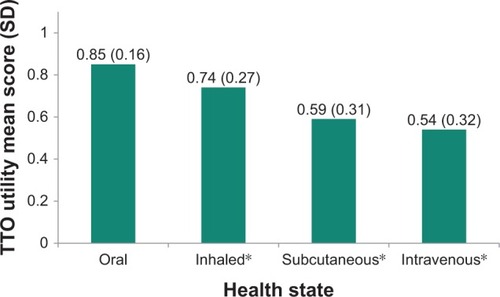
Figure 2 Mean VAS utility scores for each PAH health state.
Abbreviations: PAH, pulmonary arterial hypertension; SD, standard deviation; VAS, visual analog scale.
The disutility of each health state rating was computed by subtracting the mean rating of the oral treatment administration health state from the mean rating of each of the other health states (). The greatest mean disutility value was for the intravenous treatment administration health state at −0.31 (0.29), with the least disutility value for inhaled treatment administration at −0.11 (0.20).
Figure 3 Mean disutilities of TTO utility scores for each non-oral vs oral PAH health state.
Abbreviations: PAH, pulmonary arterial hypertension; SD, standard deviation; TTO, time trade-off.
In terms of TTO utility scores, the univariable mixed model analyses showed that there were two sociodemographic factors with significant associations: age group and having child dependents (). In terms of age group, those in the highest age group (43–74 years) had higher mean scores than those in the youngest age group (18–28 years) (mean=0.11, p=0.04). In terms of child dependents, those with dependents had higher mean scores (mean=0.12, p=0.01). However, after adjusting for the effects of all other factors in multiple variable mixed effects regression models, only the effect of having child dependents remained significant, with these participants giving higher mean scores (mean=0.10, p=0.04). There were no significant associations, in terms of TTO utility scores, for the other sociodemographic factors (sex, ethnicity, employment status, education, marital status). The univariable mixed model analyses also indicated that there was one sociodemographic factor with a significant association with VAS scores: having child dependents. Participants with child dependents had higher mean scores than those without (mean=6.83, p=0.014). There were no significant associations, in terms of VAS scores, for the other sociodemographic factors (sex, age group, ethnicity, employment status, education, marital status, religious status).
Table 4 Results from the univariable and multivariable mixed effects regression models of TTO utility scores, with p-values significant at p<0.05 indicated in bold
Discussion
The aim of this study was to elicit robust utility values associated with health states corresponding to different modes of oral and non-oral treatment administration of drugs acting on the prostacyclin pathway in PAH. The results show that the oral treatment administration health state was the most preferred, with intravenous administration being the least preferred. The differences between the utilities indicate that oral mode of treatment administration is perceived to be associated with a significantly better state of health than the three other modes of treatment administration. In addition, the values show the order of preference following the oral state to be inhaled, followed by subcutaneous, with intravenous administration being the least preferred.
Evaluation of the relationships between population demographic characteristics and utility scores was completed using mixed model analyses, which showed that participants with children had higher mean utility scores, indicating that having child dependents could influence the amount participants are willing to trade. They also had higher mean VAS scores than those without children. This observation is in line with findings from a study by van Nooten et al, in which respondents with children traded off fewer years than those without when valuing health states of varying severity.Citation44 In this study, factors associated with TTO responses were explored, with participants with children indicating that when completing the TTO exercise they were thinking about reaching a particular time or life event, typically related to children and grandchildren (such as seeing them grow up, being at their children’s wedding, living long enough for children to become independent), whereas those without children gave reasons related to having a family.Citation44 The findings of the current study suggest that more research should be done to explore the various factors that may influence TTO responses, and in particular whether or not characteristics such as having dependent children need to be taken into account when estimating utilities.
Although there are no studies in the literature that provide utilities and disutilities for treatment modes of administration related to PAH, attempts were made to compare the disutility values derived from this study with the wider literature associated with treatment modes of administration in other diseases. Regarding subcutaneous mode of administration utilities, a study comparing iron chelation therapy (deferasirox) administered via subcutaneous infusion with once-daily oral medication reported a mean disutility value from oral to subcutaneous of −0.23,Citation45 similar to but slightly less negative than the −0.26 found in this study. This is consistent with the properties of the two treatments, with both causing infusion site pain but with deferasirox being administered 8−12 hours per day, 5−7 days a week,Citation45 while the subcutaneous treatment for PAH (treprostinil) is administered continuously. Additionally, it was considered that our results could arguably be compared to published utilities for insulin pumps used in the treatment of diabetes.Citation46 However, there are two main factors that make this comparison unsuitable. First, the pump for continuous subcutaneous infusion used to treat PAH, unlike in insulin treatment for diabetes, cannot be removed for a period of time.Citation47 Second, and perhaps more importantly, infusion site pain is more frequent and much more intense than with the continuous infusion of insulin.Citation48
Regarding comparison to intravenous infusion in other diseases, a study was identified that provided a disutility for once-daily intravenous infusion of ganciclovir used as therapy for AIDS-related cytomegalovirus retinitis.Citation49 The study reported a mean disutility value of −0.22, which is different from the −0.31 value elicited in this study. However, it is reasonable to conclude that these disutilities are not directly comparable as receiving an intravenous infusion once daily is not equivalent to continuous intravenous infusion in terms of overall treatment burden. Regarding comparability of intravenous treatments, the most comparable treatment modality to intravenous prostacyclin would be the use of left ventricular assist devices (LVADs) as they require continuous use and intravenous lines, similar to the intravenous treatment for PAH.Citation50 However, there are no existing utilities in the literature for LVADs.
Regarding published disutility values for inhaled vs oral treatment administration, it is not possible reasonably to compare the inhaled treatment related to PAH to inhaled treatments used to treat other chronic conditions such as asthma or type 2 diabetes. This is because the preparation, inhalation, and cleaning processes for PAH inhaled treatments require substantially more time (approximately 15 minutes every 2–3 hours, 6–9 times per day) and rigor (washing and drying all parts of the nebulizer device at the end of each day, as well as boiling some parts of the device once per week) than other forms of inhaled medicines.Citation51
This study elicited utilities using TTO methodology, which is well suited to isolate utilities associated with treatment administration, and has been used in other similar study designs.Citation45,Citation49,Citation52–Citation55 However, while the study yielded logical results, with differences between the utilities being in the expected direction, there are some limitations with the study methods that should be highlighted and addressed. The robustness of utilities associated with hypothetical health states is limited by the accuracy of the health states, meaning utilities obtained from participants responding to hypothetical health states might be different from those obtained directly from patients. In this study, the general population was used to value the health states in order better to approximate the societal viewpoint as suggested in the guidance from some health technology assessment bodies.Citation23–Citation25 This methodology could be replicated and used with PAH patients in future research if required. In this study, the health state descriptions of injectable and subcutaneous were developed to represent external pumps that would typically and commonly be used in clinical practice. There are a small number of expert PAH centers that use implantable pumps to administer intravenous treprostinil. These could potentially reduce some of the risks or impacts related with the devices described in this study.Citation56,Citation57 However, implantable pumps require a general anesthetic to implant and are not suitable for all patients. Therefore, vignettes specifically to describe administration with an implantable pump were not developed for this study.
Another study limitation is related to the study sample. Although efforts were made to balance the sample in terms of demographic factors such as age, sex, and ethnic or racial background, the sample was not intended to be nationally representative. Comparisons between the sample and the UK general population found that the participants of this study were younger on average, had a higher percentage of females, and were more ethnically diverse. Similarly, the requirement of attending an in-person interview could have unwittingly biased the sample towards being healthier than the general population. However, there is no reason to believe that the values elicited in this study would be consistently different from values from a nationally representative sample.
Conclusion
This study provides the first set of utility values for modes of administration of PAH drugs acting on the prostacyclin pathway. The results of this study suggest that there are quantifiable HRQL differences perceived between different modes of administration of these drugs.
Data availability
Only SL, CEK, and HAD had access to patient identifying data. Interview data is identifiable only by an ID number and is stored separately from personally identifiable information (eg, consent documents). No additional unpublished data were collected during the study.
Author contributions
EWD and AB contributed to study design, data analysis and interpretation, and drafting or revising of the manuscript. SL, CEK, and HAD contributed to study design, data collection, data analysis and interpretation, and drafting or revising of the manuscript. WGS contributed to study design and drafting or revising of the manuscript. All authors approved the final version of the manuscript for publication, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was funded by Actelion Pharmaceuticals Ltd. This study was conducted by ICON plc under a consulting agreement with Actelion Pharmaceuticals Ltd. The authors would like to acknowledge the participants of the study and the field-based TTO interviewers who were sub-contracted to ICON plc (Sophie Coates, Nell Ellison, Zoe Given-Wilson, Rosie McColl, Eleanor Parker, Nicola Tutt, and Christine Wilson). We would also like to thank Rainer Zimmermann (Actelion Pharmaceuticals Ltd.) and Diane Moran (Mater Misericordiae University Hospital) for clinical input and critical review of the health states and support document; Rachel Ballinger (ICON plc) for contribution to the development of the health states and support document; Sarah Corden (Medi-Ink Ltd.) for editorial assistance in preparing the manuscript; and Alexandra Kitt (Actelion Pharmaceuticals Ltd.) for assistance with literature searching and final manuscript review. The abstract of this paper was presented at the British Thoracic Society Winter Meeting 2017 (6–8 December 2017) as a spoken presentation. The presentation’s abstract was published in Thorax, volume 72, supplement 3 (https://doi.org/10.1136/thoraxjnl-2017-210983.55).
Disclosure
HAD, CEK, and SL are employees of ICON plc. AB and EWD are employees of Actelion Pharmaceuticals Ltd. AB owns stock or options. WGS has received honorarium for speaking and consultancy from Actelion Pharmaceuticals, Bayer AG, GlaxoSmithKline, and United Therapeutics. The authors report no other conflicts of interest in this work.
References
- LajoieACBonnetSProvencherSCombination therapy in pulmonary arterial hypertension: recent accomplishments and future challengesPulm Circ20177231232528597774
- McLaughlinVVMcGoonMDPulmonary arterial hypertensionCirculation2006114131417143117000921
- ShafazandSGoldsteinMKDoyleRLHlatkyMAGouldMKHealth-related quality of life in patients with pulmonary arterial hypertensionChest200412651452145915539712
- FlatteryMPPinsonJMSavageLSalyerJLiving with pulmonary artery hypertension: patients’ experiencesHeart Lung20053429910715761454
- RichSA new classification of pulmonary hypertensionAdv Pulm Hypertens20021136
- GalièNHumbertMVachieryJL2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT)Eur Heart J20153716711926320113
- D’AlonzoGEBarstRJAyresSMSurvival in patients with primary pulmonary hypertension. Results from national prospective registryAnn Intern Med199111553433491863023
- FarberHWMillerDPPomsADFive-year outcomes of patients enrolled in the REVEAL RegistryChest201514841043105426066077
- HumbertMGhofraniHAThe molecular targets of approved treatments for pulmonary arterial hypertensionThorax2016711738326219978
- GalièNBarberàJAFrostAEInitial use of ambrisentan plus tadalafil in pulmonary arterial hypertensionN Engl J Med2015373983484426308684
- SitbonOGaineSBeyond a single pathway: combination therapy in pulmonary arterial hypertensionEur Respir Rev20162514240841727903663
- PulidoTAdzerikhoIChannickRNMacitentan and morbidity and mortality in pulmonary arterial hypertensionN Engl J Med2013369980981823984728
- LangIMGaineSPRecent advances in targeting the prostacyclin pathway in pulmonary arterial hypertensionEur Respir Rev20152413863064126621977
- GuillevinLArmstrongIAldrighettiRUnderstanding the impact of pulmonary arterial hypertension on patients’ and carers’ livesEur Respir Rev20132213053554224293469
- European Pulmonary Hypertension AssociationThe impact of pulmonary arterial hypertension (PAH) on the lives patients and carers: results from an international survey2012 Available from: http://www.phaeurope.org/wp-content/uploads/International-PAH-patient-and-Carer-Survey-Report-FINAL1.pdfAccessed October 1, 2017
- MubarakKKA review of prostaglandin analogs in the management of patients with pulmonary arterial hypertensionRespir Med2010104192119683911
- HumbertMSanchezOFartoukhMShort-term and long-term epoprostenol (prostacyclin) therapy in pulmonary hypertension secondary to connective tissue diseases: results of a pilot studyEur Respir J19991361351135610445611
- KallenAJLedermanEBalajiABloodstream infections in patients given treatment with intravenous prostanoidsInfect Control Hosp Epidemiol200829434234918462147
- SimonneauGBarstRJGalieNTreprostinil Study GroupContinuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trialAm J Respir Crit Care Med2002165680080411897647
- Food and Drug Administration’s (FDA) Patient-Focused Drug Development InitiativeThe Voice of the Patient: Pulmonary Arterial Hypertension5132014 Available from: https://www.fda.gov/downloads/forindustry/userfees/prescriptiondruguserfee/ucm429382.pdfAccessed October 1, 2017
- UptraviSummary of product characteristics62017 Available from: https://www.medicines.org.uk/emc/medicine/31963Accessed October 1, 2017
- Uptravi® (selexipag) tablets [prescribing information]SwitzerlandActelion Pharmaceuticals Ltd2015 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207947s000lbl.pdfAccessed October 1, 2017
- Canadian Agency for Drugs and Technologies (CADT)Guidelines for the Economic Evaluation of Health Technologies: Canada4th edOttawaCanadian Agency for Drugs and Technologies2017
- Pharmaceutical Benefits Advisory CommitteeGuidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (version 5.0)CanberraAustralian Government Department of Health and Ageing2016
- NICE (National Institute for Health and Care Excellence)Process and methods guides, Guide to the methods of technology appraisalLondonNational Institute for Health and Care Excellence2013
- DrummondMFSculpherMJClaxtonKMethods for the Economic Evaluation of Health Care ProgrammesNew YorkOxford University Press2015
- BrazierJRatcliffeJSalomanJTsuchiyaAMeasuring and Valuing Health Benefits for Economic EvaluationNew YorkOxford University Press2017
- FeenyDPreference-based measures: utility and quality-adjusted life yearsFayersPHaysRAssessing Quality of Life in Clinical Trials22nd edNew YorkOxford University Press2011405431
- BenzaRLMillerDPBarstRJBadeschDBFrostAEMcGoonMDAn evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL RegistryChest2012142244845622281797
- WapnerJMaturaLAAn update on pulmonary arterial hypertensionJ Nurse Pract201511555155925954140
- McLaughlinVVShahSJSouzaRHumbertMManagement of pulmonary arterial hypertensionJ Am Coll Cardiol201565181976199725953750
- McLaughlinVVArcherSLBadeschDBAmerican College of Cardiology Foundation Task Force on Expert Consensus DocumentsAmerican Heart AssociationAmerican College of Chest PhysiciansAmerican Thoracic Society, IncPulmonary Hypertension AssociationACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on expert consensus documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension AssociationJ Am Coll Cardiol200953171573161919389575
- RowenDBrazierJTsuchiyaAYoungTIbbotsonRIt’s all in the name, or is it? The impact of labeling on health state valuesMed Decis Making2012321314021685376
- van ReenenMJanssenBEQ-5D-5L User Guide. Version 2.142015 Available from: http://www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/EQ-5D-5L_UserGuide_2015.pdfAccessed February 7, 2017
- TorranceGWThomasWHSackettDLA utility maximization model for evaluation of health care programsHealth Serv Res1972721181335044699
- OppeMDevlinNJvan HoutBKrabbePFde CharroFA program of methodological research to arrive at the new international EQ-5D-5L valuation protocolValue Health201417444545324969006
- BallingerRSMaceyJLloydAJBrazierJUtilities associated with the number of days on parenteral support in the treatment of short bowel syndromeValue Health2016197A513
- KosmasCEShinglerSLSamantaKHealth state utilities for chronic lymphocytic leukemia: importance of prolonging progression-free survivalLeuk Lymphoma20155651320132625213185
- AttemaAEVersteeghMMOppeMBrouwerWBStolkEALead time TTO: leading to better health state valuations?Health Econ201322437639222396243
- KindPDolanPGudexCWilliamsAVariations in population health status: results from a United Kingdom national questionnaire surveyBMJ199831671337367419529408
- Office for National StatisticsOverview of the United Kingdom1152015 Available from: http://www.ons.gov.uk/ons/dcp171776_422383.pdfAccessed July 27, 2017
- Office for National Statistics2011 Census: Key Statistics for England and Wales, March 2011 [serial on the Internet]32011 Available from: http://www.ons.gov.uk/ons/dcp171778_290685.pdfAccessed July 27, 2017
- Office for National Statistics [webpage on the Internet]Statistical Bulletin: UK Labour Market72017 Available from: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/bulletins/uklabourmarket/july2017Accessed July 31, 2017
- van NootenFEvan ExelNJKoolmanXBrouwerWB“Married with children” the influence of significant others in TTO exercisesHealth Qual Life Outcomes20151319426135391
- OsborneRHLourençoRDDaltonAQuality of life related to oral versus subcutaneous iron chelation: a time trade-off studyValue Health200710645145617970927
- ChancellorJAballéaSLawrenceAPreferences of patients with diabetes mellitus for inhaled versus injectable insulin regimensPharmacoeconomics200826321723418282016
- AleppoG webpage on the InternetInsulin Pump: What to Know Before You DisconnectEndocrineWeb Available from: https://www.endocrineweb.com/guides/how-disconnect-pump-plus-tips-traveling-pump-using-pump-schoolAccessed October 19, 2017
- WhiteRJLevinYWessmanKHeiningerAFrutigerKSubcutaneous treprostinil is well tolerated with infrequent site changes and analgesicsPulm Circ20133361162124618545
- JohnsonESSullivanSDMozaffariELangleyPCBodsworthNJA utility assessment of oral and intravenous ganciclovir for the maintenance treatment of AIDS-related cytomegalovirus retinitisPharmacoeconomics199610662362910164062
- CleggAJScottDALovemanEThe clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluationHealth Technol Assess20059451132
- British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN) [webpage on the Internet]British Guidelines on the Management of Asthma92016 Available from: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/Accessed October 20, 2017
- KarnonJTolleyKOyeeJJewittKOssaDAkehurstRCost-utility analysis of deferasirox compared to standard therapy with desferrioxamine for patients requiring iron chelation therapy in the United KingdomCurr Med Res Opin20082461609162118439348
- MatzaLSSapraSJDillonJFHealth state utilities associated with attributes of treatments for hepatitis CEur J Health Econ20151691005101825481796
- MatzaLSCongZChungKUtilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastasesPatient Prefer Adherence2013785586524039408
- OsborneRHDaltonAHertelJSchroverRSmithDKHealth-related quality of life advantage of long-acting injectable antipsychotic treatment for schizophrenia: a time trade-off studyHealth Qual Life Outcomes20121013522472127
- KurzynaMMałaczyńska-RajpoldKKotejaAAn implantable pump Lenus pro® in the treatment of pulmonary arterial hypertension with intravenous treprostinilBMC Pulm Med201717116229195500
- BourgeRCWaxmanABGomberg-MaitlandMTreprostinil administered to treat pulmonary arterial hypertension using a fully implantable programmable intravascular delivery system: results of the DelIVery for PAH trialChest20161501273427396777
