Abstract
Background
The objective of this study was to evaluate the impact of pharmaceutical care on the knowledge, adherence, and efficacy of hormone therapy in climacteric women participated in multidisciplinary collaborative clinic, launched by Peking University First Hospital (Beijing, People’s Republic of China) in 2012.
Methods
A total of 296 patients were recruited (intervention group n=150, control group n=146). The patients in the intervention group visited the multidisciplinary collaborative clinic for their initial hormone therapy, receiving individualized pharmaceutical care (PC), whereas the control group visited only the general clinic without PC. The pill count method, knowledge assessment questionnaire (Cronbach’s α=0.80), and the modified Kupperman Index were used to assess the knowledge, adherence, and efficacy at months 3, 6, and 12.
Results
The intervention group, which received PC, showed significantly higher and better knowledge, adherence, and efficacy than the control group, demonstrating the effectiveness of the PC provided. The knowledge scores of the intervention and control groups at months 3, 6, and 12 were (73.12 vs 59.28), (77.63 vs 66.19), and (80.81 vs 66.64); the data for adherence were (90.97% vs 82.17%), (93.21% vs 87.79%), and (95.81% vs 93.38%); and the values of the modified Kupperman Index were (17.15 vs 24.05), (13.22 vs 22.01), and (12.21 vs 23.15), respectively.
Conclusion
PC improved the knowledge, adherence, and efficacy of hormone therapy in climacteric women. Therefore, the multidisciplinary collaborative model investigated in our study should be advocated in other health care institutions for the benefit of more patients. Further large-sample and long-term studies should be conducted to evaluate the effects of PC on patient clinical outcomes, including its impact on the safety and efficacy of long-term use of hormone therapy, as well as the economic benefits.
Introduction
The climacteric period is a physiological stage that women go through in the transition from adulthood to old age. During the process of ovarian aging, menopausal women may experience menstrual disorders, vasomotor dysfunction (eg, hot flashes, night sweats), genitourinary symptoms (eg, vaginal irritation and itching, and incontinence), and neuropsychiatric symptoms (eg, memory loss, depression) caused by sex hormonal changes. Menopause is also considered to be the initial stage of chronic diseases, such as osteoporosis, cardiovascular disease, and Alzheimer’s disease, although no clear evidence exists. Thus, climacteric women should undergo regular, effective disease screening and health education activities based on a multidisciplinary medical care approach.Citation1,Citation2
With the extension of human life expectancy, middle-and older-aged women have formed a huge population. Currently, the women aged 40–60 years account for 11.28% of our population, and the number of women over 50 years in the People’s Republic of China is estimated to increase to more than 280 million by 2030. Improving the quality of life in old age has been listed as one of the 3 major themes of health promotion in the 21st century by the World Health Organization (WHO). Menopause health care aims at health promotion in climacteric women, delaying the occurrence of diseases and laying the foundation for health in old age.Citation3
Hormone therapy (HT) is the most effective treatment for climacteric-related syndrome. The benefits and risks of HT for women have remained controversial for decades. The latest findings of Women’s Health Initiative trials showed that there was no long-term increase in either all-cause or cause-specific mortality among menopausal women during the cumulative 18-year follow-up. These women received HT for up to 5.6 years (combination of estrogen plus progestin HT group) and another received it for up to 7.2 years (estrogen-alone group).Citation4 Rational use of HT is a prerequisite for maximizing the benefits of patient. Pharmacists now have the opportunity to assist patients to use HT correctly by providing individualized pharmaceutical care (PC). The first clinic for climacteric women in the People’s Republic of China was launched at Peking University First Hospital. In that clinic, using a multidisciplinary collaborative model, a physician, pharmacist, dietitian, and nurse can work together to optimize the clinical outcomes of climacteric women. Therefore, the primary objective of this study was to evaluate the impact of PC on the medication adherence, knowledge, and efficacy of HT in climacteric women.
Methods
Setting
This study was conducted from May 2015 to May 2017 at Peking University First Hospital. Patients enrolled during the period from May 2015 to May 2016 were followed up for 1 year, until May 2017. Pharmacists provided individualized medication guidance and counseling, and conducted group sessions in the form of lectures, during which pharmacists introduced the basics and specifics of the rational use of HT. The aim of this study was to assess the impact of PC provided by pharmacist on the knowledge, adherence, and efficacy of HT in climacteric women. The study was approved by the Ethics Committee of Peking University, and all patients signed informed consent forms.
Patients
Inclusion criteria were as follows: aged 40 years or older, diagnosed with climacteric-related syndrome, and recommendation to use HT.
Exclusion criteria were as follows: patients treated with progestin alone, presence of communication and language barriers, and unwillingness to participate in the follow-up.
Procedure
Patients who met the inclusion criteria were enrolled in the intervention group based on their willingness to attend the multidisciplinary collaborative menopause clinic. The patients in the intervention group received standard PC by a trained pharmacist, which included face-to-face counseling, guidance on goals, medication usage, side effects, and precautions. Emphasis was also made on the importance of adherence to HT and other combined medication. The materials used included a booklet about HT, a personalized medication regimen, and a follow-up schedule. The pharmacist spent approximately 25 minutes per intervention patient.
Similarly, according to the patients’ willingness, those who met the criteria for inclusion but attended only the general clinic, without provision of standardized PC, were enrolled in the control group.
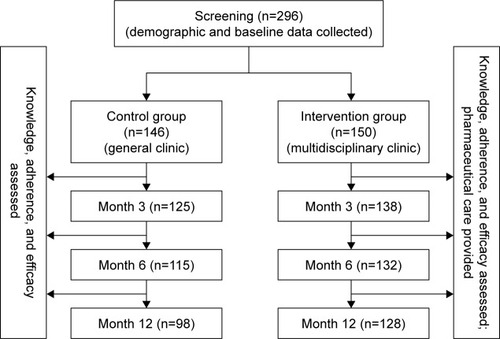
The length of the follow-up period in this study was 12 months. All patients were asked questions by phone by the pharmacist at months 3, 6, and 12. The knowledge, adherence, and efficacy of HT were assessed based on validated scales. The patients in the intervention group also received referral reminders and necessary counseling during the follow-up period ().
Outcomes
The primary outcomes measured were medication adherence, patient’s knowledge about the treatment, and its efficacy.
A knowledge questionnaire (Cronbach’s α=0.80) was used to measure the patient’s adherence and knowledge. The questionnaire involved 37 questions on four aspects, including general information on drugs, adverse drug reactions (ADRs) and precautions, treatment options, and follow-up procedures. The score of the questionnaire was finally converted into percentile.
The pill count method was applied to assess adherence, expressed as the doses actually taken relative to the doses prescribed in the current cycle.Citation5 The patients of the 2 groups returned to the clinic monthly to refill or adjust their prescriptions, and we counted the number of tablets left at each visit. The quality of all dispensed hormonal medications as well as the time and date of the prescription issuance were recorded in the hospital information system (HIS). Then, we used the data retrieved from HIS to calculate patient adherence. The modified Kupperman Index (KI)Citation6 is widely used internationally to evaluate the severity of climacteric-related symptoms. However, taking into account the impact of the various physical, social, regional, and cultural factors, a modified KICitation7 has been validated and is currently used in the People’s Republic of China,Citation8 and thus in our study also. The modified KI consists of 13 items. Adjusted by the weighting factor of each question, the score reaches a total of up to 63 points. The severity of climacteric-related symptoms is represented by the score ranges of 0–6, 7–15, 16–30, and >30 as none, mild, moderate, and severe, respectively.
Statistical analysis
Data were analyzed using SPSS version 22 (IBM Corporation, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD), whereas categorical variables were expressed as an absolute value (number) and frequency (percentage). The continuous variables of the 2 groups in the demographic data were analyzed using independent samples t-test and the categorical data by the χ2 test. Comparison of the impact of PC on knowledge, adherence, and efficacy between the two groups was made by the nonparametric Mann–Whitney U-test when normality assumptions could not be fulfilled. All P-values <0.05 were considered statistically significant.
Results
Patients
A total of 296 patients were recruited in the 2 groups (intervention n=150, control n=146). There was no difference in the baseline characteristics between the control and intervention groups concerning the age of the patients, the duration and severity of symptoms, body mass index (BMI), level of education, and exercise (). Finally, 98 patients in the control group completed the entire follow-up, but 48 patients dropped out due to adverse reactions (17 cases), poor adherence (14 cases), or lost to follow-up (17 cases). On the other hand, 128 patients in the intervention group completed the entire follow-up, and only 22 patients dropped out due to adverse reactions (13 cases), poor adherence (6 cases), or lost to follow-up (3 cases).
Table 1 Baseline characteristics of the control and intervention groups
Knowledge
Compared with the control group, the intervention group had higher knowledge scores, (73.12 vs 59.28), (77.63 vs 66.19), and (80.81 vs 66.64), at months 3, 6, and 12 with statistically significant differences (Mann–Whitney U-test) (), demonstrating the positive impact of PC. The knowledge scores of both groups continued to improve during the follow-up period.
Table 2 Knowledge score of the control and intervention groups during the follow-up period
Adherence
The adherence levels of the 2 groups were assessed by the pill count method. Our results showed that the intervention group had better adherence (90.97% vs 82.17%), (93.21% vs 87.79%), and (95.81% vs 93.38%) than the control group at month 3, 6, and 12 with statistically significant differences (). It is noteworthy that the patients that received PC had better adherence.
Table 3 Adherence of the 2 groups, assessed by the pill count method
Efficacy
The symptoms of both groups improved after receiving HT. The patients in the intervention group had better modified KI scores than those in the control group (a lower score means better efficacy) (17.15 vs 24.05), (13.22 vs 22.01), and (12.21 vs 23.15) at months 3, 6, and 12 (). These results obtained further confirmed that PC can optimize patient outcomes.
Table 4 Modified KI scores in the control and intervention groups
Discussion
As is known, adherence of HT is suboptimal.Citation9–Citation12 The 2 most common reasons are patient’s concerns about cancer and vaginal bleeding.Citation13–Citation16 In our study, the knowledge, adherence, and efficacy of HT in both groups continued to improve over time. However, the patients of the intervention group, who received PC, had better and higher scores with statistically significant differences. Moreover, the dropout rate in the intervention group (22/150) was lower than that in the control group (48/146). Our results confirmed the positive impact of PC on the clinical outcomes of HT in climacteric women. The involvement of the pharmacist helped patients obtain a better understanding and confidence in the initial stage of HT. Additionally, the consultation provided by the pharmacist during the follow-up period further improved adherence, eventually resulting in achieving the desired efficacy.
The results of the present investigation are similar to those of other studies on the impact of PC on the improvement of patient’s knowledge, adherence, and efficacy.Citation17–Citation19 Nevertheless, to the best of our knowledge, our research is the first to explore the impact of PC on hormonal treatment in climacteric women. One publication reported that climacteric women need more access to information on HT as their physicians cannot provide extensive information and education.Citation20 In the current medical environment, physicians are too overloaded to provide comprehensive guidance to patients, especially in the People’s Republic of China. Thus, there are opportunities for pharmacists to collaborate with physicians and other staff to provide multidisciplinary care to climacteric women. Such a favorable interaction will improve their knowledge, adherence, and efficacy of HT, which will increase the benefits to the patients. This model was also acknowledged to save medical human resources and reduce the medical costs.Citation21
This study was not randomized. Because the patients had to pay an additional consultation fee for therapy in a multi-disciplinary care clinic, the grouping of the study was based on the patient’s willingness. The baseline characteristics of the 2 groups were comparable without statistically significant differences. Nonetheless, the dropout rate in the control group was higher than that in the intervention group (32.9% vs 14.7%). Poor adherence and lost to follow-up were the main reasons for dropout in the control group. Therefore, the actual situation of knowledge, adherence, and efficacy in the control group might have been even worse than that found in the results of this study.
Limitations
There are some limitations in our study. First, it was carried out only at one single center, and the sample size was small. Thus, the results cannot be extrapolated to all climacteric women in the People’s Republic of China. Second, the duration of the follow-up was not sufficiently long to observe serious ADR (eg, cancer, thrombosis); hence, we will continue to follow-up to obtain more information. Third, the results cannot rule out the influence of other members of the team. Meanwhile, the regular follow-up of the pharmacist might inevitably have exerted certain positive effects in the control group.
Conclusion
In our study, PC had positive effects on the knowledge, adherence, and efficacy of HT in climacteric women in the People’s Republic of China. This is a preliminary study that provides evidence which can serve as the foundation for more rigorous and comprehensive randomized controlled trials. Further controlled studies with larger samples and longer follow-up periods should be conducted to verify the influence of PC on the safety, efficacy, and cost-effectiveness of HT in climacteric women. A multidisciplinary care model should be advocated in more medical institutions to benefit more patients, especially those with chronic diseases who need long-term management.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShepherdJEEffects of estrogen on cognition mood, and degenerative brain diseasesJ Am Pharm Assoc2001412221228
- RebarRNachtigallLAvisNClinical challenges of perimenopause: consensus opinion of The North American Menopause SocietyMenopause20007151310646698
- Chinese Preventive Medicine Women’s Health Branch, Menopause Health Care GroupGuideline on Menopause Women’s Health (2015)J Pract Gynecol Endocrinol2016322133
- MansonJEAragakiAKRossouwJEMenopausal hormone therapy and long-term all-cause and cause-specific mortality: the women’s health initiative randomized trialsJAMA20173181092793828898378
- SteinerJFProchazkaAVThe assessment of refill compliance using pharmacy records: methods, validity, and applicationsJ Clin Epidemiol199750501051169048695
- KuppermanHSBlattMHWiesbaderHFillerWComparative clinical evaluation of estrogenic preparations by the menopausal and amenorrheal indicesJ Clin Endocrinol Metab195313668870313061588
- CaoZYChinese Obstetrics and GynecologyBeijing, People’s Republic of ChinaPeople’s Medical Publishing House2005
- MinfangTHongfangSChangbingLCorrelation between the modified Kupperman index and the Menopause Rating Scale in Chinese womenPatient Prefer Adherence2013722322923569361
- StumpfPGTroliceMPCompliance problems with hormone replacement therapyObstet Gynecol Clin North Am19942122192267936542
- RyanPJHarrisonRBlakeGMFogelmanICompliance with hormone replacement therapy (HRT) after screening for postmenopausal osteoporosisBr J Obstet Gynaecol199310043994008494850
- TorgersonDJDonaldsonCRussellITReidDMHormone replacement therapy: compliance and cost after screening for osteoporosisEur J Obstet Gynecol Reprod Biol199559157607781863
- CanoACompliance to hormone replacement therapy in menopausal women controlled in a third level academic centerMaturitas1994202–391997715479
- SchiffIRebarRWCramerJAAchieving long-term continuance of menopausal ERT/HRT: consensus opinion of the North American Menopause SocietyMenopause19985269769689198
- RavnikarVACompliance with hormone therapyAm J Obstet Gynecol19871565133213343578453
- FergusonKJHoeghCJohnsonSEstrogen replacement therapy: a survey of women’s knowledge and attitudesArch Intern Med198914911331362912402
- NachtigallLEEnhancing patient compliance with hormonal replacement therapy at menopauseObstet Gynecol1990754380
- BlumlBMMcKenneyJMCzirakyMJPharmaceutical care services and results in project ImPACT: hyperlipidemiaJ Am Pharm Assoc2000402157165
- KripalaniSYaoXHaynesRBInterventions to enhance medication adherence in chronic medical conditions: a systematic reviewArch Intern Med2007167654055017389285
- TaitelMJiangJRudkinKEwingSDuncanIThe impact of pharmacist face-to-face counseling to improve medication adherence among patients initiating statin therapyPatient Prefer Adherence2012632332922563240
- ConnellyMTFerrariNHagenNInuiTSPatient-identified needs for hormone replacement therapy counseling: a qualitative studyAnn Intern Med1999131426526810454947
- BataldenPBDavidoffFWhat is “quality improvement” and how can it transform healthcare?Qual Saf Health Care20071612317301192