Abstract
Objective
In patients with rheumatoid arthritis (RA), nonadherence to treatment is often related to patients’ beliefs and concerns regarding their medication. This study aimed to analyze the correlations regarding patients’ medication beliefs, medication adherence, and objective measures of disease activity and safety in patients with RA established on subcutaneous (SC) anti-tumor necrosis factor α (TNFα) therapy.
Methods
This Phase IV, noninterventional, non–drug-specific study enrolled patients with RA being treated with stable-dose SC anti-TNFα (adalimumab, etanercept, golimumab, and certolizumab pegol). At initial visit and 6 and 12 months later, patients completed the Beliefs about Medicines Questionnaire-Specific section, assessing perceptions of personal need for anti-TNFα therapy (anti-TNFα-Necessity) and concerns (anti-TNFα-Concerns), Medication Adherence Rating Scale (MARS), mean Disease Activity Score in 28 joints (DAS28), and other scales. Longitudinal data were analyzed by linear mixed models.
Results
A total of 460 patients were included. At initial visit, anti-TNFα-Necessity beliefs were high (mean ± SD: 4.3 ± 0.55) vs anti-TNFα-Concerns (2.8 ± 0.78). Medication adherence (MARS) was high (4.8 ± 0.39). All scores remained stable over the 1-year follow-up period. Anti-TNFα-Necessity beliefs and anti-TNFα-Concerns were not related to each other, but strongly correlated with medication adherence. While concerns worsened with disease activity, clinical status, and low quality of life, necessity beliefs remained unaffected.
Conclusion
In patients with RA established on stable-dose SC anti-TNFα, anti-TNFα-Necessity beliefs persistently outweighed anti-TNFα-Concerns, but both correlated with adherence. These findings may be of use in subsequent studies looking to predict adherence in patients starting treatment with SC anti-TNFα.
Supplementary materials
Beliefs about Medicines Questionnaire items: BMQ-Specific
The BMQ-Specific consists of two scales assessing the patient’s beliefs about the Necessity (N) of the currently prescribed medications for controlling the disease (BMQ-Necessity) and his/her Concerns (C) about potential adverse consequences of taking them (BMQ-Concerns).
The items are:
My health at present depends on my medicines (N)
Having to take medications worries me (C)
My life would be impossible without my medications (N)
I sometimes worry about the long-term effects of my medications (C)
Without my medications I would be very ill (N)
My medications are a mystery to me (C)
My health in the future will depend on my medications (N)
My medications disrupt my life (C)
I sometimes worry about becoming too dependent on my medications (C)
My medications protect me from becoming worse (N)
These medicines cause me unpleasant adverse events (C).
Respondents indicate their degree of agreement with each statement on a 5-point Likert scale, ranging from 1 = strongly disagree to 5 = strongly agree. Scores obtained for individual items within both scales are summed and divided by the total number of items in the scale to give a scale score of 1–5. Higher scores indicate stronger beliefs.
Medication Adherence Report Scale items
This five-item scale asks the patient to rate the frequency with which he/she engages in each of the five aspects of nonadherent behavior:
Forget to take medications
Modify doses
Stop taking medications during a certain period
Decide to miss a dose
Take less than what is prescribed.
Each item is rated on a 5-point Likert scale, where 1 = always, 2 = often, 3 = sometimes, 4 = rarely, and 5 = never. Scores for each of the five items are summed and divided by five to give a scale score of 1–5, where higher scores indicate higher levels of reported adherence.
Figure S1 BMQ, DAS28, and MARS scores throughout the study (mean ± SD).
Notes: (A) BMQ-Necessity score over 12 months; (B) BMQ-Concerns score over 12 months; (C) BMQ Necessity-Concerns differential over 12 months; (D) DAS28 score over 12 months; (E) MARS total score over 12 months.
Abbreviations: BMQ, Beliefs about Medicines Questionnaire; DAS28, Disease Activity Score (28 joints); MARS, Medication Adherence Rating Scale.
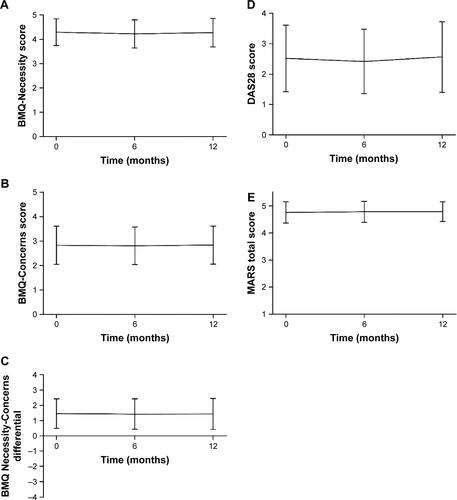
Table S1 Participating study centers
Table S2 Correlations regarding anti-TNFα-Necessity and anti-TNFα-Concerns scores and clinical and quality-of-life parameters at initial visit
Acknowledgments
This study was funded by Pfizer. Medical writing support for this manuscript was provided by Lorna Forse, PhD, and Samantha Forster, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Disclosure
RH was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust. He has undertaken speaker engagements with honoraria with AbbVie, Amgen, Biogen Idec, Gilead Sciences, GlaxoSmithKline, Janssen, Pfizer, Roche, Shire Pharmaceuticals, MSD, Astellas, AstraZeneca, DRSU, Erasmus, and Novartis. He is founder and shareholder of an UCL-business spin-out company (Spoonful of Sugar), providing consultancy on medication-related behavior to health care policy makers, providers, and industry. AA is professor emeritus of biostatistics and adviser at the University Hospital of Liège and was funded by Pfizer for the statistical analysis of this study. CB was employed by Pfizer at the time of the study and owns stock in Pfizer. The authors report no other conflicts of interest in this work.
