Abstract
Purpose
Proper adherence and persistence to medications are crucial for better quality of life and improved outcomes in rheumatoid arthritis (RA), psoriasis (PsO), and psoriatic arthritis (PsA). We systematically describe current adherence and persistence patterns for RA, PsO, and PsA, with a focus on biologics and identifying factors associated with adherence and persistence.
Patients and methods
Using various databases, a systematic literature review of US-based studies published from 2000 to 2015 on medication adherence and persistence to biologics and associated factors was conducted among patients with RA, PsO, and PsA.
Results
Using the medication possession ratio or the percentage of days covered >80%, RA and PsO adherence rates for etanercept, adalimumab, and infliximab ranged from 16% to 73%, 21% to 70%, and 38% to 81%, respectively. Using the criteria of a ≥45-day gap, RA persistence rates for etanercept, adalimumab, and infliximab ranged from 46% to 89%, 42% to 94%, and 41% to 76%, respectively. In PsO, persistence rates for etanercept and adalimumab ranged from 34% to 50% and 50% to 62%, respectively. Similar persistence rates were observed in PsA. Experienced biologics users showed better adherence and persistence. Younger age, female gender, higher out-of-pocket costs, greater disease severity, and more comorbidities were associated with lower adherence and persistence rates. Qualitative surveys revealed that nonpersistence was partly due to perceived ineffectiveness and safety/tolerability concerns.
Conclusion
Biologic adherence and persistence rates in RA, PsO, and PsA in the United States were low, with significant opportunity for improvement. Various factors – including decrease in disease severity; reduction of comorbidities; lower out-of-pocket costs; refilling at specialty pharmacies; and awareness of drug effectiveness, safety, and tolerability – can inform targeted approaches to improve these rates.
Introduction
Rheumatoid arthritis (RA), psoriasis (PsO), and psoriatic arthritis (PsA) are chronic inflammatory diseases that affect patients’ social, emotional, and physical well-being.Citation1–Citation4 With a diagnosis rate of 41 per 100,000 people worldwide, RA affects approximately 0.5% (1.3 million) of adults in the United States,Citation5 with women approximately 2.5 times more likely to develop RA than men.Citation6 According to the World Psoriasis Day Consortium, 2%–3% of the population (125 million people worldwide) experience PsOCitation7 and 18%–42% of those with PsO also have PsA.Citation7 In the USA, in 2013, an estimated 7.4 million adults had PsO.Citation8
Multiple therapies can treat RA, PsO, and PsA improving in health-related quality of life, relieving symptoms, slowing disease progression for RA and PsA, and providing greater clearance of PsO. However, proper adherence and persistence are crucial to realize these outcomes.
Adherence is the extent to which a patient takes a medication as prescribed by his or her health care professional and is usually reported as medication possession ratio (MPR) or percentage of days covered (PDC).Citation1,Citation9 Medication persistence is generally defined as the duration of time from initiation of therapy to discontinuation and is usually reported as number of days of continuous therapy use.Citation9 Persistence can also be reported as persistence rate, or the percentage of patients continuing with treatment for a defined duration without a predefined gap in treatment.Citation9 Adherence and persistence are problematic in RA, PsO, and PsA.Citation1,Citation2,Citation10–Citation12 Nonadherence and nonpersistence lead to suboptimal patient outcomes and place a substantial burden on the health care system.Citation13 Health care costs attributable to nonadherence annually in the United States are estimated to be $100–$300 billion per year.Citation14
A systematic literature review was performed to evaluate medication adherence and persistence reported among patients with RA, PsO, or PsA who are treated with bio-logics, and identify factors associated with adherence and persistence. There are existing systematic literature reviews of adherence and persistence rates among the individual diseases for multiple treatment types.Citation1,Citation2,Citation4,Citation15–Citation19 The current systematic literature review adds to the available literature by evaluating the consistency in the rates of adherence and persistence across patients with RA, PsO, or PsA that has not been previously reported.
Patients and methods
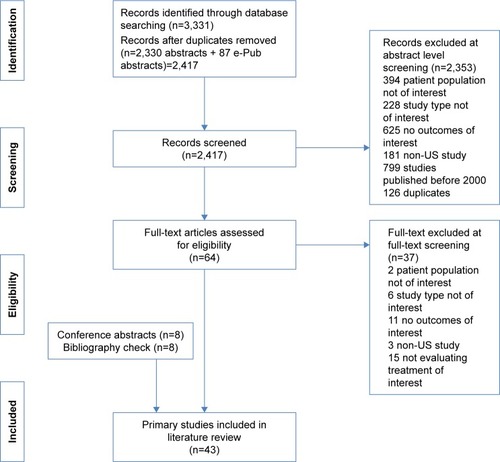
A systematic literature review of medication adherence and persistence to biologics among patients with RA, PsO, or PsA was performed through electronic databases (through December 28, 2015) and e-publications ahead of print (through January 12, 2016) in PubMed and then supplemented with searches of the MEDLINE, Embase, PsycINFO, and Cochrane databases, as well as conference proceedings from the American College of Rheumatology, the American Academy of Dermatology, and the International Society for Pharmacoeconomics and Outcomes Research from 2013 to 2015 (search terms provided in Supplementary materials). Search results were evaluated in a stepwise fashion (), with two independent reviewers screening all abstracts and then full-text articles. A third reviewer provided facilitation of reviewer discussion/disagreement. For inclusion, studies must have met the following criteria: 1) been published between 2000 and 2016; 2) utilized an observational study design (ie, prospective cohorts, retrospective cohorts, cross-sectional studies, and case–control studies except where the cases and controls were prespecified to be adherent/persistent and nonadherent/nonpersistent groups); 3) been based in the United States; 4) evaluated adults aged ≥18 years receiving biologic treatment for RA, PsO, or PsA; and 5) provided rates of medication adherence and/or persistence.
Additional information on reasons or factors related to nonadherence or nonpersistence included in these studies was also collated. Data from studies meeting inclusion criteria were extracted into a standardized extraction template in Microsoft Excel by a single reviewer and were validated by a second reviewer. Any studies excluded after review of the full-text article were tabulated with the reason for exclusion. A critical assessment of the included observational studies was conducted based on the questionnaire developed for the effective health program for the Agency for Healthcare Research and Quality.Citation20
Results
Included studies
Forty-three primary studies were included in this systematic literature review (). The majority of the studies utilized a retrospective design; a few are cross-sectional studies, and two are prospective studies ( and ). Study participants were assessed from a variety of sources but largely represented populations from claims databases or inpatient/outpatient hospital settings. Most studies reported adherence and/or persistence patterns over 1 year, with some studies using survival methods including Kaplan–Meier plots to report longer term patterns up to 5 years. The sample sizes of the included studies vary widely, ranging from 45 patientsCitation35 to 15,834 patients,Citation41 with most including more than 100 patients. Adherence and/or persistence in RA was examined in 26 studies,Citation21–Citation32,Citation41 PsO in 12 studies,Citation34–Citation40,Citation42,Citation44,Citation55–Citation57 and PsA in five studies.Citation32,Citation41,Citation42,Citation58,Citation59 A few studies evaluated a mixed group of patients or data in more than one of the disease states of interest. In addition, some studies examined adherence only, some evaluated persistence only, and others reported both adherence and persistence. In the critical assessment, domains such as study limitations, directness of linkage to the evaluated intervention, consistency, precision, and reporting bias were evaluated. Overall, the studies addressed many of these elements; however, many of the questions related to bias that may stem from differences between groups being compared were not relevant because a majority of the studies were noncomparative in nature. A majority of the studies were considered to be of moderate to high quality for the domains that were applicable ().
Table 1 Medication adherence study characteristics and results
Table 2 Medication persistence study characteristics and results
Table 3 Risk of bias assessmentTable Footnotea
Rates and factors associated with adherence
A total of 20 studies evaluated adherence rates in RA (14 studies),Citation21–Citation34 PsO (six studies),Citation35–Citation40 or PsA (one study)Citation32 patient populations, with one studyCitation32 evaluating both RA and PsA patients (). Of these studies, nine analyzed data from private or commercial databases, six from government databases (Medicare/Medicaid/Veterans Affairs), two from registry data, and three from inpatient/outpatient hospital records (). When reported, follow-up periods were relatively consistent, ranging from 0.5 to 1 year. Individual studies measured adherence in various ways.
Rheumatoid arthritis
In RA, eight studies reported adherence using the standardized threshold of ≥80% for the PDC, the MPR, or the compliance ratio (number of therapy administrations or filled prescriptions divided by the expected number) methods over a 1-year time period.Citation21–Citation27,Citation54 Adherence rates varied widely and were generally low. The median adherence rate for etanercept was 63% (range, 16%–73%) and was reported in seven studies.Citation21,Citation23–Citation26,Citation28,Citation34 The median adherence rate for adalimumab was also 63% (range, 21%–70%) and was reported in four studies.Citation23,Citation24,Citation26,Citation27 Only three studies reported adherence rates for infliximab using the ≥80% MPR or PDC criteria (38%, 43%, and 81% over 1 year).Citation23,Citation25,Citation28 Although limited, in studies where multiple medications were studied simultaneously, adherence rates were greater for infliximab than for adalimumab or etanercept.Citation23,Citation28 For example, after matching for various baseline covariates, Oladapo et alCitation23 reported a higher adherence rate for infliximab (38%) users compared to adalimumab (21%) and etanercept (16%) users. Likewise, Harley et alCitation28 reported a higher compliance ratio for infliximab users (81%) than for etanercept (68%) and methotrexate (64%) users. Harley et alCitation28 further corroborated the high adherence among infliximab users, reporting that etanercept (odds ratio [OR] 0.462; 95% CI 0.290–0.736) and methotrexate (OR 0.385; 95% CI 0.245–0.604) users were less likely to comply with their medications compared to infliximab users. Also using the criteria of ≥80% MPR or PDC, patients taking golimumab had a higher adherence rate (81%; N=261) than those taking adalimumab (70%; N=1,532) or etanercept (61%; N=2,099).Citation24 Two studies assessed biologic–methotrexate combination therapy, and adherence rates were 26% and 28% over 1 year – higher than adherence to triple therapy combination of methotrexate, hydroxychloroquine, and sulfasalazine (11% and 18%).Citation22,Citation54
Nine RA studies reported significant patient factors associated with better adherence using logistic regression analysis.Citation21,Citation24–Citation26,Citation28–Citation30,Citation54,Citation60 Patient factors, including older age,Citation21,Citation24,Citation25 male gender,Citation24,Citation29 other races (not including African Americans or Hispanics) compared with Whites,Citation25 and Whites compared with African Americans,Citation21 were identified as more likely to be adherent.
Borah et alCitation26 reported that experienced biologic users were more adherent than new users for both adalimumab and etanercept treatment groups (mean MPR, 70% vs 63% and 73% vs 65%, respectively) and that experienced adalimumab users had 25% higher odds of nonadherence than experienced etanercept users (OR 1.25; 95% CI 1.05–1.49). In another study, higher adherence (monotherapy: mean MPR) was observed for biologic monotherapies (90% infliximab, 83% etanercept, 85% adalimumab) compared to biologic–methotrexate combination therapies (all mean MPRs <75%) or with methotrexate monotherapy (80%).Citation30
Another study found that patients with RA taking biologics who filled prescriptions through specialty pharmacies had higher adherence than those receiving medication from retail pharmacies.Citation31 Over 3 years (2006, 2007, and 2008), the mean adjusted adherence rates for specialty pharmacy and retail pharmacies patients were 63% vs 50%, 68% vs 51%, and 61% vs 44%, respectively.
Psoriasis
Six studies reported adherence rates on PsO ().Citation35–Citation40 Ustekinumab is the most frequently studied. Defining adherence as a PDC ≥80% over 1 year among 2,707 patients with PsO, Doshi et alCitation36 found adherence to be the greatest with infliximab (49%) users, followed by ustekinumab (43%), adalimumab (41%), and etanercept (29%) users. Each of the other studies defined adherence differently (eg, never missing medication or injection, frequency of a missed dose, or adherence to recommended dosing schedule) and had fewer patient samples. Consequently, a wide range of adherence rates was observed ().
Three PsO studies assessed factors and reasons associated with medication nonadherence.Citation37–Citation39 A higher Charlson Comorbidity Index score is associated with decreased biologic adherence.Citation37 In addition, being adherent to other prescribed PsO medications, including topical, phototherapy, or systemic drugs such as methotrexate, prednisone, acitretin, cyclosporine, and isotretinoin, was associated with better adherence to biologics.Citation37 Older age, male gender, concomitant therapy, and low comorbid anxiety were associated with better adherence pattern.Citation38 Based on a web-based survey, common reasons for nonadherence included the need to reschedule existing injection appointments (7.4% adalimumab; 3.2% ustekinumab), the unaffordability of therapies (18.5% adalimumab; 22.6% ustekinumab), forgetfulness (44.4% adalimumab; 3.2% ustekinumab), and other medical problems (not defined; 25.9% adalimumab; 16.1% ustekinumab).Citation39
Psoriatic arthritis
Using the Truven Health MarketScan Commercial Claims database and defining medication adherence as proportion of adherent refills, one retrospective study of 325 patients with PsA reported a 76% adherence rate for golimumab users ().Citation32
Rates and factors associated with persistence
A total of 23 studies evaluated medication persistence rates in RA (17 studies), PsO (six studies), and PsA (four studies) patient populations, with four studiesCitation41,Citation42,Citation44,Citation54 evaluating more than one disease state for their patient population. Persistence was defined variably across studies ().
Rheumatoid arthritis
In the studies identified, persistence was usually measured as the time that patients continued treatment without a gap, discontinuation, or switching to another therapy over the specified time period. In most studies, the follow-up duration was 1 year and the gaps were ≥30, 45, or 90 days.
Similar to studies of adherence, a wide variation in persistence rates was observed, and the rates were generally low. However, unlike studies examining adherence, no biologic stood out as having higher persistence rates. Using the criteria of a gap of ≥45 days over a 1-year time period, the median persistence rate was 61% (range, 46%–89%) from four studies that evaluated etanercept.Citation42–Citation45 For adalimumab and infliximab, the median rates were 57.5% (range, 42%–94%) and 63% (range, 41%–76%), respectively.Citation42–Citation45 Abatacept and golimumab rates were reported in three studies,Citation42–Citation44 with median rates of 52% (range, 41%–62%) and 29.5% (range, 20%–55%), respectively. Rates were 40% (range, 29%–50%) for rituximabCitation42,Citation44 and 33% for certolizumab.Citation43
Three studies that used the same criteria (≥45-day gap over a 1-year time period) presented the persistence rates by new or experienced biologic users, reporting consistently higher rates for experienced biologic users.Citation42,Citation44,Citation45 However, one study of etanercept, adalimumab, and infliximab users over a 1- and 2-year time period reported that first-time switchers (nonestablished users) and second-time switchers had lower persistence rates, corroborated by higher likelihood of drug discontinuation (OR 1.42; 95% CI 1.22–1.67; P<0.001 and OR 1.35; 95% CI 1.03–1.76; P=0.028, respectively) compared to new biologic users.Citation46 Several studies have compared biologic–methotrexate combinations with various conventional disease-modifying antirheumatic drug (cDMARD) regimens (eg, triple therapy combination of methotrexate, hydroxychloroquine, and sulfasalazine). Generally, persistence in the biologic combination groups was higher than in cDMARD combination patients.Citation22 In comparing various biologic–methotrexate combinations, the mean persistence rate in the overall population over 1 year was 74.6%, with higher rates for infliximab combination (78%) compared to etanercept (72.8%) and adalimumab (70.8%) combination.Citation47 Grijalva et alCitation30 also reported that episodes of adalimumab–methotrexate combinations had a higher likelihood of persistence compared to methotrexate-only episodes (hazard ratio [HR] 0.63; 95% CI 0.48–0.84).
As reported in three studies, patients taking infliximab tended to be more persistent compared to those taking etanercept or adalimumab,Citation45,Citation46,Citation48 and another two studies reported that patients taking etanercept were likely more persistent than those taking adalimumab.Citation26,Citation30 However, there are notable exceptions where adalimumab–methotrexate combinations have greater persistence than infliximab–methotrexate or etanercept–methotrexate combinations,Citation30 with some studies finding no differences in 1- or 5-year persistence rates between tumor necrosis factor (TNF) inhibitors.Citation25,Citation42,Citation44,Citation49,Citation50
Several modifiable and nonmodifiable factors have been associated with persistence either positively or negatively among patients with RA. Nonmodifiable factors identified include age, gender, and race, whereas potentially modifiable factors identified include disease activity, comorbidities, out-of-pocket costs, combination therapy with methotrexate, cumulative use of methotrexate, and therapy management programs (disease therapy management and specialty pharmacy).Citation29,Citation30,Citation46,Citation50,Citation51,Citation61 Older patients tended to be persistent to infliximab (HR 0.99; 95% CI 0.98–1.00; P=0.002); female patients (HR 1.24; 95% CI 1.01–1.51; P=0.040) were more likely to discontinue etanercept; and White patients were more persistent than other races on etanercept.Citation50 Markenson et alCitation50 and Agarwal et alCitation51 reported that patients with higher disease activity (Clinical Disease Activity Index >22: HR 1.35; 95% CI 1.08–1.69; P=0.009; and RA Disease Activity Index scores: HR 1.13; 95% CI 1.05–1.22) and physician global assessment scores (HR 1.27; 95% CI 1.18–1.38) are more likely to discontinue TNF inhibitor use. Higher Charlson Comorbidity Index scores (HR 1.07; P=0.002) and higher weekly out-of-pocket costs (>$50) (HR 1.58; P<0.001) increased the likelihood of nonpersistence to biologic therapy.Citation29 In addition, cumulative use of methotrexate was associated with a decreased risk of TNF inhibitor discontinuation, as combined use of biologic and methotrexate was associated with a decreased risk of discontinuation compared to biologic monotherapy.Citation30,Citation46,Citation51,Citation61
One prospective study evaluated medication persistence to injectable RA medications (etanercept, adalimumab, anakinra, abatacept, infliximab, and rituximab) for patients participating in a disease therapy management program compared to nonparticipants receiving the same medication from specialty or community pharmacies over 0.67 years.Citation33 The persistence rate (>30-day gap or switching) was 77% for disease therapy management intent-to-treat patients, 86.8% for disease therapy management completers, 73% for specialty pharmacy patients, and 52% for community pharmacy patients.Citation33
Other factors reported as reasons for low persistence were lack of effectiveness, continued disease activity, toxicity, and health insurance coverage/cost.Citation52,Citation53
Psoriasis
Six studies reported persistence patterns of biologics over a 1-year time period in PsO.Citation34,Citation42,Citation44,Citation55–Citation57 Three of these use a ≥45-day gap period. Median persistence rates for etanercept and adalimumab were 47% (range, 34%–50%) and 57% (range, 50%–62%), respectively.Citation42,Citation44 Similar rates are observed when the definition was based on a ≥60-day gap.Citation55 In a retrospective study of MarketScan data, ustekinumab persistence (71%) was higher than that for adalimumab (53%) or etanercept (19%); however, it should be noted that this study utilizes a gap criteria that depend on the biologic prescribed (a gap definition of ≥30 days to ≥130 days).Citation56 In a separate study, ustekinumab persistence was high (81%), with a gap definition of ≥130 days.Citation57
Clinical variables such as experience with drug and dosing may impact persistence.Citation42,Citation57 One study reported persistence for infliximab users as 55% in new users and 75% in experienced users.Citation42 Patients who initiated on the 90 mg dose of ustekinumab showed lower persistence compared to those who initiated on the 45 mg dose.Citation57 Reasons for stop/switch are similar across biologics, with lack of efficacy being the most frequent reason for nonpersistence.
Psoriatic arthritis
Four studies evaluated persistence rates in patients with PsA.Citation41,Citation42,Citation58,Citation59 Overall, persistence for all biologics, described as no treatment gap or switching over 1 year of treatment, was 61%. Using a ≥45-day gap criterion, persistence rates for etanercept, adalimumab, and infliximab were 51%, 56%, and 74%, respectively, in new users and 58%, 56%, and 79%, respectively, in established users.Citation42 Similar rates for adalimumab and etanercept users were observed in another study using ≥60-day gap criteria to describe persistence.Citation41 Median duration of persistence with monotherapy and biologic–methotrexate combination therapies was 2.4 and 2.4 years, respectively, for adalimumab; 3.9 and 1.6 years, respectively, for etanercept; and 1.4 years for infliximab monotherapy (no data reported for methotrexate–infliximab combination).Citation58
Two studies reported factors associated with biologic persistence in PsA.Citation58,Citation62 Shorter persistence is associated with a history of prior treatment with methotrexate, a history of coronary artery disease, a higher body mass index, and worse scores on most measures of baseline disability and disease activity.Citation58 Ustekinumab was associated with a lower HR of discontinuing treatment (switching or stopping) compared to infliximab, adalimumab, and etanercept.Citation62
Discussion
Since the inception of biologic therapies for the treatment of RA, patients with the disease have experienced an increase in health-related quality of life, relief of symptoms, and decrease in disease progression; however, proper adherence is the key to these positive outcomes because a lack of consistency to any such treatment regimen puts patients at a higher risk for disease reoccurrence and treatment failure.Citation2 Successful treatment with biologics depends on ensuring long-term medication adherence; however, adherence rates differ between types of biologics and treatment schedules.Citation36,Citation53 In turn, this has an impact on the overall treatment effect of the available therapies.
This systematic literature review included a wide range of US studies describing adherence and persistence to biologics for patients with RA, PsO, or PsA. Most studies assessed patients with RA, with fewer studies available on patients with PsO and PsA. Various measures of adherence were used. Most commonly, MPR and PDC were used for reporting adherence, with an 80% cutoff predominantly used as a measure of successful adherence to treatment. Persistence was also defined differently across the studies but was most commonly reported as time from initial start (fill or refill) to treatment gap of ≥30, 45, or 90 days or to discontinuation. Variation in measures of adherence and persistence contributed to a wide range of adherence and persistence rates observed across studies. Etanercept, adalimumab, and infliximab were the most commonly studied biologics in the RA literature, and ustekinumab was the most common in the PsO literature. There was a notable lack of adherence data for patients with PsA.
Overall, adherence and persistence were variable and low across the three disease states. Limited data were available to support a comparison of adherence for the same biologics in RA as compared with PsO, although some suggest a difference in adherence to the same biologics between disease states. The reasons for observed differences are unknown. Adherence and persistence rates of approximately 50%–60% across the disease states were observed for commonly used therapies such as etanercept, adalimumab, and infliximab, although the observed ranges varied widely. Reasons that may contribute to the wide variation in observed adherence and persistence rates include differences in study populations, source of data, drug administration preference, and approaches used to measure adherence and persistence across the studies. Therefore, comparisons of rates between different drugs can be justifiable when drawn from the same study utilizing a comparable patient population and approach. For example, a few studies reported that infliximab users in RA were more adherent compared to adalimumab or etanercept users.Citation23,Citation25,Citation28,Citation30 This might be because of the wider interval schedule and intravenous route of infliximab administration in an outpatient setting compared to self-administered subcutaneous injections for etanercept and adalimumab. In a qualitative survey of patients’ preferences, patients with RA preferred intravenous routes of administration (34.5%) to subcutaneous administration (25.3%), whereas patients with PsA preferred subcutaneous injections (45%) to intravenous infusions (9.7%).Citation60
Many factors were associated with nonadherence or nonpersistence. Although some are demographic or socioeconomic in nature, others were related to patients’ choices or their understanding of treatment pathways. Nonadherence among patients with RA and PsO and nonpersistence among patients with RA were associated with younger age, female gender, non-White race, and refilling prescriptions at retail pharmacies. Interestingly, biologic–methotrexate combination therapy was associated with lower adherence but also with higher persistence among patients with RA when compared to biologic monotherapy. Increased persistence among patients with RA using biologic–methotrexate combination therapy was in agreement with a recent study by Zhang et al,Citation63 which showed that biologic–methotrexate combination therapy users are 30% less likely to be nonpersistent compared to biologic users. Although experience with biologics and the type of therapy affected adherence among patients with RA, they were also notable factors affecting persistence among patients with PsO. High out-of-pocket cost, greater disease activity, and a high number of comorbidities were also deterrents to adherence or persistence. Qualitative surveys revealed that patients were not persistent because of perceived ineffectiveness and safety/tolerability concerns. In a survey, 59% of patients with PsA were not receiving any therapy or were receiving only a topical therapy, with 46% of them believing that the therapies were worse than the disease.Citation3
Strengths of this systematic literature review on adherence and persistence of biologic users in three chronic inflammatory diseases include the variety of data sources used to inform this study, the different patient populations/study designs, and the summarization of observational studies representing real-world experiences. Based on the most current available evidence, consistently low adherence and persistence rates for biologics were observed in all three disease states studied. In addition, the qualitative surveys from patients provided insight into the areas of concerns from the patients’ perspectives. Inherent limitations to this systematic literature review include inconsistency in methodology across published studies and varied dosing schedules of different biologic drugs. These factors limit the ability to pool results for a quantitative summary and complicate standardization and generalizability of the findings. Additionally, because the data summarized here are secondary data based on the available literature, biases inherent in the studies may be present.
Conclusion
Despite the efficacy that biologics have on the outcomes of RA, PsO, and PsA patients, adherence and persistence rates to these medications were low, presenting significant opportunity for improvement. Various factors that may influence medication adherence or persistence patterns – including lower disease severity/activity; fewer comorbidities; lower out-of-pocket costs; refilling at specialty pharmacies; participation in disease management programs; and awareness of drug effectiveness, safety, and tolerability – point to areas where targeted improvements could be focused. These data should serve to guide future research to identify and implement innovative approaches that measure and reduce nonadherence or nonpersistence and improve treatment outcomes in RA, PsO, and PsA patients.
Acknowledgments
We are grateful to Michael Friedman for providing thorough data integrity review of the manuscript. The study was funded by Eli Lilly and Company. Abstract and poster presented at AMCP Nexus 2016 (https://www.jmcp.org/doi/pdf/10.18553/jmcp.2016.22.issue-10-a).
Supplementary materials
Search strategy.
e-Pub search in PubMed, January 12, 2016
(((((((“Arthritis, Rheumatoid”[Mesh:noexp]) OR (“Rheumatoid Nodule”[Mesh:noexp]) OR (“Psoriasis”[Mesh:noexp]) OR (“Arthritis, Psoriatic”[Mesh:noexp]) OR (rheumatoid nodule*[tiab]) OR (arthritis rheumat*[tiab]) OR (RA[tiab]) OR (psoria*[tiab]) OR (psoriatic arthrit*[tiab]))))) AND ((((“Medication Adherence”[Mesh:noexp]) OR (“Patient Compliance”[Mesh:noexp]) OR (“Treat-ment Refusal” [Mesh: noexp]) O R (“Patient Dropouts”[Mesh:noexp]) OR (adher*[tiab]) OR (complian*[tiab]) OR (persist*[tiab]) OR (cooperat*[tiab]) OR (co-operat*[tiab]) OR (treatment refusal[tiab]) OR (patient dropout*[tiab]) OR (nonadher*[tiab]) OR (non-adher*[tiab]) OR (non-persist*[tiab]) OR (nonpersist*[tiab]) OR (noncomplian*[tiab]) OR (non-complian*[tiab]) OR (uncooperat*[tiab]) OR (unco-operat*[tiab]) OR (patient perspective*[tiab]) OR (patient attitude*[tiab]) OR (patient experience*[tiab]) OR (continuation rate*[tiab]) OR (assessment adher*[tiab]) OR (assessment complian*[tiab]) OR (assessment persist*[tiab]) OR (assessment nonadher*[tiab]) OR (assessment non-adher*[tiab]) OR (assessment noncomplian*[tiab]) OR (assessment non-complian*[tiab]) OR (assessment nonpersist*[tiab]) OR (assessment non-persist*[tiab])))))) AND ((publisher[sb] NOT pubstatusnihms[All Fields] NOT pubstatuspmcsd[All Fields] NOT pmcbook[All Fields]) OR pubstatusaheadofprint[All Fields])
Search Result: 87
Embase 1974 to 2015 December 28, 2015 using OvidSp
Ovid Medline (R) in-process and other non-indexed citations and Ovid and Medline (R) 1946 – December 28, 2015 using OvidSp
Cochrane, December 28, 2015 (CENTRAL only)
Disclosure
MJM, WNM, CL, TM, RTB, and ABA are employees and stockholders of Eli Lilly and Company. VT, CB, NJ, and SC are employees of ICON plc, which received funding from Eli Lilly and Company to conduct the study. SRF is an employee of the Wake Forest University School of Medicine and has received research, speaking, and consulting support from AbbVie, Celgene, Janssen, Lilly, and Novartis. The authors report no other conflicts of interest in this work.
References
- DevauxSCastelaAArchierEAdherence to topical treatment in psoriasis: a systematic literature reviewJ Eur Acad Dermatol Venereol201226Suppl 3616722512682
- FidderHHSingendonkMMvan der HaveMOldenburgBvan OijenMGLow rates of adherence for tumor necrosis factor-α inhibitors in Crohn’s disease and rheumatoid arthritis: results of a systematic reviewWorld J Gastroenterol201319274344435023885145
- HelliwellPCoatesLChandranVQualifying unmet needs and improving standards of care in psoriatic arthritisArthritis Care Res2014661217591766
- RathbunAMReedGWHarroldLRThe temporal relationship between depression and rheumatoid arthritis disease activity, treatment persistence and response: a systematic reviewRheumatology201352101785179423236191
- HelmickCGFelsonDTLawrenceRCEstimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part IArthritis Rheum2008581152518163481
- VandeverLRheumatoid arthritis by the numbers: facts, statistics, and you Available from: http://www.healthline.com/health/rheumatoid-arthritis/facts-statistics-infographicAccessed April 19, 2017
- Arthritis FoundationHow common is psoriatic arthritis in people with psoriasis? Available from: http://blog.arthritis.org/psoriatic-arthritis/psoriatic-arthritis-psoriasis/Accessed April 19, 2017
- RachakondaTDSchuppCWArmstrongAWPsoriasis prevalence among adults in the United StatesJ Am Acad Dermatol201470351251624388724
- CramerJARoyABurrellAFairchildCJFuldeoreMJOllendorfDAWongPKMedication compliance and persistence: terminology and definitionsValue Health2008111444718237359
- CramerJRosenheckRKirkGKrolWKrystalJVA Naltrexone Study Group 425Medication compliance feedback and monitoring in a clinical trial: predictors and outcomesValue Health20036556657314627063
- HaynesRBMcdonaldHGargAXMontaguePInterventions for helping patients to follow prescriptions for medicationsCochrane Database Syst Rev20022279
- HaynesRBMcdonaldHPGargAXHelping patients follow prescribed treatment: clinical applicationsJAMA2002288222880288312472330
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005353548749716079372
- IugaAOMcguireMJAdherence and health care costsRisk Manag Healthc Policy20147354424591853
- BlumMAKooDDoshiJAMeasurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic reviewClin Ther201133790191321715007
- HarroldLRAndradeSEMedication adherence of patients with selected rheumatic conditions: a systematic review of the literatureSemin Arthritis Rheum200938539640218336875
- PasmaAvan’t SpijkerAHazesJMBusschbachJJLuimeJJFactors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic reviewSemin Arthritis Rheum2013431182823352247
- ThorneloeRJBundyCGriffithsCEAshcroftDMCordingleyLAdherence to medication in patients with psoriasis: a systematic literature reviewBr J Dermatol20131681203122963128
- VangeliEBakhshiSBakerAA systematic review of factors associated with non-adherence to treatment for immune-mediated inflammatory diseasesAdv Ther20153211983102826547912
- ViswanathanMBerkmanNDDrydenDMHartlingLAssessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item BankRockville, MDAgency for Healthcare Research and Quality2013
- ChuLHKawatkarAAGabrielSEMedication adherence and attrition to biologic treatment in rheumatoid arthritis patientsClin Ther201537366066625618317
- ErhardtDSauerBTengCCClinical practice experience in rheumatoid arthritis patients treated with triple therapy and methotrexate-tumor necrosis factor inhibition differs from that of randomized controlled trialsArthritis Rheumatol201567Suppl 103197
- OladapoABarnerJCLawsonKANovakSRascatiKLRichardsKMHarrisonDJMedication effectiveness with the use of tumor necrosis factor inhibitors among Texas Medicaid patients diagnosed with rheumatoid arthritisJ Manag Care Spec Pharm201420765766724967519
- TkaczJEllisLBolgeSCMeyerRBradyBLRuetschCUtilization and adherence patterns of subcutaneously administered anti-tumor necrosis factor treatment among rheumatoid arthritis patientsClin Ther201436573774724661783
- LiPBlumMAvon FeldtJHennessySDoshiJAAdherence, discontinuation, and switching of biologic therapies in medicaid enrollees with rheumatoid arthritisValue Health201013680581221054657
- BorahBJHuangXZarotskyVGlobeDTrends in RA patients’ adherence to subcutaneous anti-TNF therapies and costsCurr Med Res Opin20092561365137719425902
- GrijalvaCGKaltenbachLArbogastPGMitchelEFGriffinMRAdherence to disease-modifying antirheumatic drugs and the effects of exposure misclassification on the risk of hospital admissionArthritis Care Res2010625730734
- HarleyCRFrytakJRTandonNTreatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexateAm J Manag Care200396 SupplS136S14314577718
- CurkendallSPatelVGleesonMCampbellRSZagariMDuboisRCompliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter?Arthritis Rheum200859101519152618821651
- GrijalvaCGChungCPArbogastPGSteinCMMitchelEFGriffinMRAssessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritisMed Care20074510 Suppl 2S66S7617909386
- BarlowJFFarisRJWangWVerbruggeRRGaravagliaSBAubertREImpact of specialty pharmacy on treatment costs for rheumatoid arthritisAm J Pharm Benefits201246SP49SP56
- EllisLBolgeSRicePGolimumab utilization patterns and refill adherence in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitisValue Health2014173A49A50
- StocklKMShinJSLewHCZakharyanAHaradaASSolowBKCurtisBSOutcomes of a rheumatoid arthritis disease therapy management program focusing on medication adherenceJ Manag Care Pharm201016859360420866164
- BonafedeMJohnsonBHTangDHShahNHarrisonDJCollierDHEtanercept-methotrexate combination therapy initiators have greater adherence and persistence than triple therapy initiators with rheumatoid arthritisArthritis Care Res2015671216561663
- SandovalLFHuangKEFeldmanSRAdherence to ustekinumab in psoriasis patientsJ Drugs Dermatol201312101090109224085042
- DoshiJATakeshitaJPintoLBiologic adherence among psoriasis patients in the US Medicare populationValue Health2015183A186
- BhosleMJFeldmanSRCamachoFTTimothy WhitmireJNahataMCBalkrishnanRMedication adherence and health care costs associated with biologics in Medicaid-enrolled patients with psoriasisJ Dermatolog Treat200617529430117092860
- LiYZhouHCaiBKahlerKHTianHGabrielSArconaSGroup-based trajectory modeling to assess adherence to biologics among patients with psoriasisClinicoecon Outcomes Res2014619720824748809
- GorenACarterCLeeSPatient reported health outcomes and non-adherence in psoriasis patients receiving adalimumab or ustekinumab for moderate to severe plaque psoriasisJ Dermatolog Treat2016271435026088404
- KamangarFIsipLBhutaniTHow psoriasis patients perceive, obtain, and use biologic agents: survey from an academic medical centerJ Dermatolog Treat2013241132422007699
- ShimAPhamHFairmanKAssociation of medication persistency with route of administration and patient cost-sharing: analysis of commonly used biologicsValue Health2015187A650
- SauerBCTengCCHeTTreatment patterns and annual biologic costs in US veterans with rheumatic conditions or psoriasisJ Med Econ2016191344326337538
- GuTShahNDeshpandeGTangDHEisenbergDHarrisonDJPersistence with first-line biologics used in rheumatoid arthritis in a US managed care populationValue Health2015183A168
- HoweAEyckLTDufourRShahNHarrisonDJTreatment patterns and annual drug costs of biologic therapies across indications from the Humana commercial databaseJ Manag Care Spec Pharm201420121236124425443517
- FisherMDWatsonCFoxKMChenYWGandraSRDosing patterns of three tumor necrosis factor blockers among patients with rheumatoid arthritis in a large United States managed care populationCurr Med Res Opin201329556156823489410
- GreenbergJDReedGDecktorDA comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registryAnn Rheum Dis20127171134114222294625
- TangBRahmanMWatersHCCallegariPTreatment persistence with adalimumab, etanercept, or infliximab in combination with methotrexate and the effects on health care costs in patients with rheumatoid arthritisClin Ther20083071375138418691998
- YaziciYKrasnokutskySBarnesJPHinesPLWangJRosenblattLChanging patterns of tumor necrosis factor inhibitor use in 9074 patients with rheumatoid arthritisJ Rheumatol200936590791319332636
- CannonGWDuvallSLHaroldsenCLPersistence and dose escalation of tumor necrosis factor inhibitors in US veterans with rheumatoid arthritisJ Rheumatol201441101935194325128516
- MarkensonJAGibofskyAPalmerWRKeystoneECSchiffMHFengJBaumgartnerSWPersistence with anti-tumor necrosis factor therapies in patients with rheumatoid arthritis: observations from the RADIUS registryJ Rheumatol20113871273128121572150
- AgarwalSKGlassRJShadickNAPredictors of discontinuation of tumor necrosis factor inhibitors in patients with rheumatoid arthritisJ Rheumatol20083591737174418634159
- AgarwalSKMaierALChibnikLBPattern of infliximab utili-zation in rheumatoid arthritis patients at an academic medical centerArthritis Rheum200553687287816342095
- BolgeSCGorenATandonNReasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: a patient perspectivePatient Prefer Adherence2015912113125653505
- BonafedeMJohnsonBHFoxKMWatsonCGandraSRTreatment patterns with etanercept and adalimumab for psoriatic diseases in a real-world settingJ Dermatolog Treat201324536937323441722
- ChastekBFoxKMWatsonCKricorianGGandraSRPsoriasis treatment patterns with etanercept and adalimumab in a United States health plan populationJ Dermatolog Treat2013241253322668321
- FeldmanSRZhaoYNavaratnamPFriedmanHSLuJTranMHPatterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasisJ Manag Care Spec Pharm201521320120925726029
- CaoZCarterCWilsonKLSchenkelBDosingUUstekinumab dosing, persistence, and discontinuation patterns in patients with moderate-to-severe psoriasisJ Dermatolog Treat201526211312024552612
- MeasePJCollierDHSaundersKCLiGKremerJMGreenbergJDComparative effectiveness of biologic monotherapy versus combination therapy for patients with psoriatic arthritis: results from the Corrona registryRMD Open201511e00018126819748
- ChastekBFoxKMWatsonCGandraSREtanercept and adalimumab treatment patterns in psoriatic arthritis patients enrolled in a commercial health planAdv Ther201229869169722903239
- KwiatkowskiAGrisantiLGrisantiJHatemJPatient preferences regarding route of biologic administration in an inflammatory arthritis cohortArthritis Rheumatol201567Suppl 102326
- ZhangJXieFDelzellESPersistence on biologics is associated with concomitant methotrexate use among rheumatoid arthritis patientArthritis Rheum201365S990S991
- MenterAPappKKruegerGGPersistence of biologic therapy in psoriatic disease: results from the psoriasis longitudinal assessment and registryArthritis Rheum201466S693
- ZhangJXieFDelzellEImpact of biologic agents with and without concomitant methotrexate and at reduced doses in older rheumatoid arthritis patientsArthritis Care Res2015675624632