Abstract
Purpose
To synthesize evidence of the effects and potential effect modifiers of different electronic health (eHealth) interventions to help people quit smoking.
Methods
Four databases (MEDLINE, PsycINFO, Embase, and The Cochrane Library) were searched in March 2017 using terms that included “smoking cessation”, “eHealth/mHealth” and “electronic technology” to find relevant studies. Meta-analysis and meta-regression analyses were performed using Mantel–Haenszel test for fixed-effect risk ratio (RR) and restricted maximum-likelihood technique, respectively. Protocol Registration Number: CRD42017072560.
Results
The review included 108 studies and 110,372 participants. Compared to nonactive control groups (eg, usual care), smoking cessation interventions using web-based and mobile health (mHealth) platform resulted in significantly greater smoking abstinence, RR 2.03 (95% CI 1.7–2.03), and RR 1.71 (95% CI 1.35–2.16), respectively. Similarly, smoking cessation trials using tailored text messages (RR 1.80, 95% CI 1.54–2.10) and web-based information and conjunctive nicotine replacement therapy (RR 1.29, 95% CI 1.17–1.43) may also increase cessation. In contrast, little or no benefit for smoking abstinence was found for computer-assisted interventions (RR 1.31, 95% CI 1.11–1.53). The magnitude of effect sizes from mHealth smoking cessation interventions was likely to be greater if the trial was conducted in the USA or Europe and when the intervention included individually tailored text messages. In contrast, high frequency of texts (daily) was less effective than weekly texts.
Conclusions
There was consistent evidence that web-based and mHealth smoking cessation interventions may increase abstinence moderately. Methodologic quality of trials and the intervention characteristics (tailored vs untailored) are critical effect modifiers among eHealth smoking cessation interventions, especially for web-based and text messaging trials. Future smoking cessation intervention should take advantages of web-based and mHealth engagement to improve prolonged abstinence.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Plain language summary
Which eHealth interventions help people to stop smoking?
Background: Smoking is expected to kill 8 million people per year by 2030. New web-based and mobile phone applications are attractive platforms to help people quit. Electronic health (eHealth)/mobile health (mHealth) approaches (ie, health care practice assisted by electronic processes and communication) are easy to use, affordable, flexible and have a wide reach. We sought to identify which strategies work the best.
Study characteristics: Up to March 2017, this review found 108 trials from over 110,000 participants. We examined those that were web-based including those integrated with medication, mobile-based, SMS texts, computer-assisted and others like “video doctor”.
Key results: In combined results, web-based approaches and those using mobile-based approaches led to higher six-month quit rates than a nonactive comparison (eg, usual care, assessment only). Quit smoking programs with personalized messages were also likely to help. Other computer-assisted programs had lower quit rates and daily messages lower than weekly messages.
Quality of the evidence: The evidence was moderate to low quality. Studies at high risk of bias showed higher quit rates.
Implications for practice: Web-based and mobile health strategies help people quit smoking, although their effects are modest over a short period.
Implications for research: New and emerging interventions should be evaluated. More research should be conducted in low and middle-income countries.
Introduction
Smoking is a modifiable cause of mortality and morbidity. Globally, 5% of all deaths are attributed to smoking and 14% of adults over 30 years old are at risk of premature death from smoking-related diseases.Citation1 It is predicted that tobacco use will be responsible for more than 8 million deaths worldwide per year by 2030 if effective interventions are not implemented.Citation2 A reasonable approach to tackle the global burden of smoking could be to expand the accessibility of cessation programs to all smokers. Electronic health (eHealth) approaches are considered to have many advantages including 1) easy accessibility regardless of time and place; 2) affordability and efficient delivery; 3) wider reach to large populations; and 4) flexibility to enable proactive and tailored messages and allow matching to target user characteristics (for example, by age, sex, education, or quit plan).Citation3 Thus, recent research has suggested that innovative, technology-based strategies can be applied to improve smoking cessation.Citation4
The technology landscape is changing rapidly, with the growth of smartphone ownership globally, reaching approximately one-third of the world’s population. In 2016 there were an estimated 2.16 billion users with access to 70,000 health and fitness apps and 79,000 medical apps via the Apple App Store® and Google Play store®.Citation5 This revolution in technology also offers great opportunities for clinical research and interventions using mobile health, defined as “medical and public health practice supported by mobile devices”.Citation3,Citation6 Mobile phone text messaging services have been piloted and implemented in some tobacco cessation programs.Citation7–Citation11 One limitation of this approach has been a focus on text message reminders for quitting smoking, while other potentially useful tools such as abstinence behavior tracking, peer group chats, and distractions remain unused.Citation12 Perhaps the most serious limitation of mobile health (mHealth) technology assessment to date is the lack of engagement with underserved populations (eg, low-literacy groups, geographical areas with limited internet access, or poorer communities where there are multiple users per device).Citation13
Most of the previous systematic reviews of eHealth interventions for smoking cessation have investigated the effects of specific devices upon smoking abstinence outcomes including internet/web-based cessation program,Citation14–Citation21 text messaging system,Citation22–Citation24 and computer-based program.Citation25 The most recent systematic review pooled results from 67 studies and found a moderate effect on smoking cessation using web-based/internet penetration with behavioral support, compared to an nonactive control group (risk ratio [RR] 1.69, 95% CI 1.30–2.18).Citation20 That review failed to detect the effect of web-based smoking cessation compared to active control group (RR 0.92, 95% CI 0.78–1.09).Citation20 Similarly, another systematic review of 40 internet-based smoking cessation interventions suggested a moderate effect of the smoking cessation intervention using web-based/internet penetration (eg, websites, e-mail, and multimedia component) compared to assessment-only groups (RR 1.48, 95% CI 1.11–2.78) and a significant strong effect of systems that use interactive webpages compared to print materials (RR 2.1, 95% CI 1.42–2.97).Citation19 Computer-delivered interventions were also found to improve knowledge, attitudes, and intentions related to various health behaviors including smoking cessation.Citation25 Although web-based smoking cessation interventions are generally found to be effective in decreasing smoking prevalence in many studies, heterogeneity in effect sizes is a significant concern.Citation26
Previous systematic reviews have focused on identifying and evaluating the mediators of intervention effects that use text messaging to improve smoking cessation.Citation22–Citation24,Citation26 A Cochrane review that pooled data from 12 studies indicated that mobile phone-based smoking interventions resulted in a moderate increase in cessation (RR 1.67).Citation24 A meta-analysis (in 2015), pooled effects shown in 35 studies using text messaging to improve health behaviors including smoking cessation, suggested that interventions, which are not theoretically grounded, have supplementary intervention activities and have 6–12 months duration, and are most effective. In contrast, tailoring, targeting, or personalization of SMS content did not moderate the effect of the interventions.Citation27 A related issue is that there is a dearth of knowledge on how to improve user acceptance of mHealth smoking cessation interventions.Citation12 The most comprehensive systematic review (conducted in 2012) on the efficacy of various eHealth interventions to improve smoking abstinence from 60 studies found that internet and other electronic platforms improved smoking cessation outcomes compared to control group with assessment only or printed materials (RR 1.32 for prolonged abstinence and RR 1.14 for point prevalence abstinence).Citation28 The main limitation of that review, however, was the focus on smoking cessation among adults, with limited evidence for young smokers.
Most intervention researches to date have been restricted to narrow comparisons of a single eHealth platform among young smokers or adult tobacco users while other vulnerable subgroups such as people living with HIV, hospitalized patients, or opioid-dependent people were not included. Second, previous reviews have shown that combining multiple cessation services and tailoring them to the specific smoker is more effective than a single, broad-based intervention (eg, mHealth and internet-based services).Citation12 How-ever, to our knowledge, there has been no comprehensive systematic review comparing direct effects, intervention cost-effectiveness, and potential effect modifiers among published eHealth platform interventions. Third, there is little evidence regarding the level of eHealth penetration on current smoking cessation trajectories. Finally, few studies have examined the effect modifiers that contribute to the prolonged effects of eHealth smoking cessation interventions. Thus, the purpose of this systematic review was to evaluate and compare the effectiveness and identify some potential effect modifiers of the most promising eHealth platforms in smoking cessation contexts including web-based, computer-based, and mobile phone-based programs to help smoking cessation seekers to quit. The effect modifiers were examined in relation to intervention characteristics (eg, tailored/interactive vs untailored/noninteractive), risk of bias, publication date, and study site. This study will extend knowledge of the efficacy and possible effect modifiers based on recent novel evidence and should be beneficial for evidence-based policy and decision making regarding stop-smoking programs.
Method
Protocol and registration
A review protocol was registered on the international prospective register of systematic reviews under number CRD42017072560 (https://www.crd.york.ac.uk/PROS-PERO/display_record.asp?ID=CRD42017072560).
Eligibility and exclusion criteria
Studies were included based upon the following PICO questions:
Population: current smokers in the general population;
Intervention: eHealth smoking cessation interventions were classified into four groups including:
Web-based interventions defined by a unique web-page/portal address that could be accessed online.
Computer-generated programs (alone) were those with a program/application running on PC/laptop foundation. In this method, the program must be downloaded and installed online or offline before using its resources.
Mobile-based interventions included any apps or text messages (SMS) and other communication via Wireless and mobile phone/cell phone technologies.
Other platforms: social media (Facebook, Twitter), Chat rooms, digital games, or specific devices aimed at improving smoking abstinence.
Interventions were considered to be “interactive” if information sharing and interaction with the target participants (based on their personal characteristics) were described in the intervention content. Tailored interventions were defined as individually personalized messaging or content delivery to participants.
Comparison: usual practice or other smoking cessation methods;
Primary outcomes: levels of smoking abstinence;
Included study designs: randomized controlled trial (RCT), quasi-experimental designs, interrupted time series, and controlled before–after studies.
We excluded studies if they 1) did not meet the inclusion criteria; 2) utilized eHealth methods (e-mail, website, and so on) primarily for data collection or sample recruitment; 3) had short-term follow-up (<1 month); and 4) were smokeless tobacco studies. Additionally, studies that focused on eHealth penetration on recruiting participants or smoking prevention without reporting on quitting smoking behaviors were excluded.
Search strategy
A systematic search was performed in March 2017 on four electronic databases including MEDLINE, PsycINFO, Embase, and Cochrane Library from inception. lists the “text words” searches. We used multiple combinations of search terms and Boolean operators related to smoking, type of device, eHealth, and intervention. The search strategy was modified for each database by experienced experts and researchers. Additionally, reference lists of articles retrieved, and prior relevant systematic reviews were also scanned to determine the additional eligible documents. Table S1 describes the included studies. Open Grey and Google Scholar were searched to find any additional relevant reference, which might not be captured by the selected database searches.
Table 1 “Textword” searches
Study selection
All references were exported to EndNote version X7 and duplicates were removed. Titles and abstracts were independently screened by two authors, HD and LN. The studies that did not meet the eligibility criteria were removed. Full texts of relevant information or those that required further clarifications were retrieved for the final assessment of data collection. A third review author (BT) made a final decision when two reviewers had differing opinions.
Data collection process
A structured data extraction form was first piloted by two reviewers (HD and LN) and then applied to all studies. Extracted data consisted of 1) description of participants, 2) study design, 3) timing, 4) sample size, 5) length of intervention and follow-up, 6) types of eHealth (web-based, computers, mobile phone-based, quit-line, and so on), 7) primary outcomes, 8) a brief description of experiment, 9) study findings and bias assessment, and 10) effect modifiers (eg, setting, intervention characteristics, year of publication, and risk of bias). Details of the conceptual frameworks were extracted if they were described in sufficient detail. The smoking cessation outcome was also extracted. If the published paper did not provide sufficient information, we searched for its protocol or contacted the authors for further detail.
After completion of the data extraction, the results of the two reviewers (HD and LN) were compared, discussed, and any disagreements were resolved before producing the final data. Senior researchers were consulted to address any disagreements. The meta-analysis was carried out using Review Manager (RevMan) Analyses Ver. 5.3.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline was used to describe the flow of the searching and screening.Citation29
Data items
A summary matrix was created with the data extracted from all the included studies. This matrix listed all variables for which data were sought including PICO, length of follow-up, significant behavioral outcomes, theoretical framework, author recommendation, and bias appraisal.
Risk of bias in individual studies
The included studies were assessed for risk of bias using the Quality Assessment Tool for Quantitative Studies, developed by the Effective Public Health Practice Project (EPHPP).Citation30 The EPHPP score ranged from 6 to 24 and global ratings were based on the number of weak ratings (no WEAK rating – strong “quality”, one WEAK rating – moderate “quality”, and more than two WEAK ratings – weak “quality”). Two independent reviewers assessed six potential biases including selection bias, study design, presence or absence of blinding, confounders, data collection bias, and attrition bias. Any disagreements between the two reviewers were discussed together and if necessary with coauthors for verification.
Summary measures
This review measured the difference in smoking abstinence prevalence by type of eHealth platform, population, and time points. Smoking point prevalence of abstinence at final follow-up (self-assessed abstinence or/with biochemically validated abstinence) was recorded as the primary outcome for treatment effect. Effectiveness was measured by 7-day point abstinence or prolonged abstinence interchangeably because these indicators are strongly related to each other, and it has been shown that the use of either does not affect the results.Citation31 Further, we note that 7-day point abstinence prevalence is the most common measure in tobacco cessation intervention studies. Based on recommendations from the literature, we considered abstinence to be short-term if the intervention used was <6 months of follow-up, and longer-term abstinence if the intervention reported was ≥6 months of follow-up.Citation32,Citation33 The meta-analysis was performed based on the 7-day point prevalence abstinence (PPA) or prolonged abstinence indicator, stratified by follow-up time-points and effect modifier characteristics of the control group (). To address missing data, withdrawal and dropout cases were considered to be smokers, using results from intention-to-treat-analysis based on Cochrane Tobacco Group’s guideline.Citation34
Table 2 Subgroups of effect modifiers employed in the meta-analysis and meta-regression
Synthesis of results
RR and 95% CI were used to measure the difference in smoking abstinence prevalence between control and intervention groups for each included study. A meta-analysis was conducted using the Mantel–Haenszel fixed-effect approach to pool RRs. The I2 statistic was applied to assess the presence of substantial statistical heterogeneity with three cutoff points (LOW – ICitation2 <25%, MODERATE – ICitation2 from 25% to 75%, and HIGH – ICitation2 <75%).Citation35 The visual figure of funnel plots in RevMan5 was employed to assess any potential publication bias and no evidence of publication bias in included studies was found because the included studies distributed symmetrically around the mean. Each primary outcome was Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessed and concluding statements were drawn based on the importance of the evidence to identify what the difference means (eg, may provide an effect [poorer cessation outcome] vs may increase cessation [better cessation outcome]).Citation36
To examine further explanations of the high heterogeneity between studies, multi-meta-regression analyses were performed using restricted maximum-likelihood technique. The covariates, which are hypothesized to be effect modifiers, included 1) intervention characteristics (eg, interactive, tailored, frequent, and theory-based intervention); 2) risk of bias (EPHPP); 3) year of publication; and 4) country of study, stratified by three categories of eHealth platforms (ie, web-based, computer-assisted, and mHealth).
Results
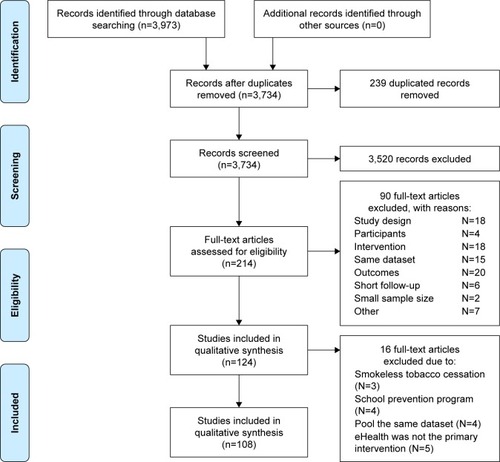
Search outcomes
shows the flow of studies. Initially, 3,973 potentially relevant records were identified. After deduplication (n=239) and screening titles and abstract, 3,520 records were excluded. For the remaining 214 records, full-text papers were retrieved and assessed for eligibility. Only 124 papers met the inclusion criteria following further appraisal using the EPHPP tool. An additional 16 studies were excluded due to the focus on smokeless tobacco cessation,Citation37–Citation39 school tobacco control program,Citation40–Citation43 duplicate publication of study results,Citation37,Citation44–Citation46 and eHealth intervention used for participant recruitment.Citation47–Citation51 In total, 108 studies met all selection criteria with 110,372 participants included in the review. High consensus (90%) was achieved between two reviewers.
Study characteristics
summarizes the main characteristics of the included studies in the review. All but one of the included studies were published in the 21st century (from 2000).
Table 3 General characteristics of included studies (n=91)
Participants
Adult smokers were the main target population (n=64), followed by youth (n=18) and other subpopulations with special needs such as people living with HIV, COPD, or pregnant women. Most of the studies were implemented in the USA (n=58), Europe (n=41), and Asia and Australia regions (n=9). No studies were found in Africa.
eHealth interventions
This review captured 67 studies that used web-based programs, followed by wireless and mobile phone-based programs (n=24), and computer-assisted intervention (n=13). Social media, virtual chat room, or other electronic aids were investigated in four studies only.
Comparison
The included studies were dominated by two arms (n=82), following by three arms (n=20), four arms (n=4), and five arms (n=2).eHealth smoking cessation interventions were compared to nonactive control groups including usual care, self-help materials, assessment only (n=63); active control groups (eg, quit-line, face-to-face counseling) (n=13), and other types of eHealth interventions (n=30). Only two studies, the improvement of sleep or physical activity, were compared with non-smoking-cessation groups.
Outcome
Most of the studies measured abstinence from 6 to 12 months of follow-up (n=48), and some measured longer follow-up at more than 12 months (n=28). The remaining studies (n=32) measured short-term outcomes (from 1 to less than 6 months).
Risk of bias within studies
provides the risk of bias appraisal by individual studies, using the EPHPP tool. The average quality score among 108 included studies was 8.5 (described in Table S1). Attrition bias for noncompliance (less than 60%) was the major bias, accounting for 27% among 30 studies. Meanwhile, high risk of inadequate blinding was found in 22 studies due to the fact that participants were aware of treatment allocation while blinding of assessors was not reported clearly. Selection bias might have occurred in 18 studies since <60% of recruited respondents agreed to participate in the intervention. Data collection method was rated unclear/moderate bias in 27 studies due to self-reported assessment without biochemical confirmation of smoking abstinence. Overall, 18% of included studies (n=17) were deemed to be of weak quality (with two weak points). In contrast, the remaining part was of moderate and strong quality (41% vs 39%, respectively).
Effectiveness of internet smoking cessation interventions
Web-based compared to nonactive control
The forest plot () shows a positive effect of web-based smoking cessation interventions compared to control groups. Twenty web-based trials reported higher abstinence prevalence compared to participants in the nonactive control groups who received minimal or no intervention, such as assessment only or printed materials for adult smokers,Citation52–Citation69 youth,Citation70–Citation78 and other smokers with special needs such as pregnant women,Citation79 people with COPD,Citation80 heart disease,Citation81 cancer,Citation82 or HIV/AIDS.Citation83,Citation84 When 23 studies about the prevalence of smoking abstinence were pooled together, the interactive, tailored, and web-based programs demonstrated increased cessation effects for 6-month follow-up interventions (RR 2.03, 95% CI 1.73–2.38), I2=27%, and a moderate increase of smoking cessation in 11 studies with less than 6 months of follow-up (RR 1.21, 95% CI 1.08–1.36, ICitation2=20%). In contrast, no meaningful effect on quitting cigarettes was found after pooling eleven internet studies at 12 months of follow-up (RR 1.06, 95% CI 0.98–1.16, ICitation2=47%).
Interactive tailored web-based compared to an active control group
Five of 18 studies found significant long-term effects of the tailored proactive web-based interventions compared to active control activities such as a brief generic text advice,Citation85 a noninteractive, nontailored internet,Citation86 and a state-of-the-art tailored web-based tobacco interventions.Citation87 Two studies detected a short-term effect at both 1 and 3 months when measuring the effect of the proactive website (Opptur/www.slutta.no)Citation88 or a video-validated abstinence via financial incentives based on the Mōtiv8 website.Citation89 Compared to an active control group, the web-based program tailored to participants’ characteristics indicated little or no increase of cessation effect (RR 1.09, 95% CI 1.02–1.17, I2=0%).
Web-based compared to other eHealth control groups
Eight studies compared the effects of online smoking cessation programs with other eHealth modes for adult smokers.Citation85,Citation90–Citation96 Fraser (2014)Citation90 concluded that website information without e-mail messaging had significantly increased smoking abstinence because messaging may undermine website utilization.Citation90 McKay (2008) measured the abstinence difference between a website using a cognitive-behavioral therapy approach and a control group receiving physical activity advice, but no significant difference was detected at either 3 or 6 months of follow-up.Citation91 Other RCTs compared the effects of interactive smoking cessation websites and multiple types of methods such as e-mail reminders or virtual groups,Citation93,Citation94 video messages vs SMS advice,Citation85,Citation95 and proactive telephone counseling.Citation96 It was concluded that participants who used the video-based website were more likely to be abstinent than people receiving tailored feedback via plain SMS. Meanwhile, Swan (2010) indicated that compared to web-based counseling, proactive telephone-based counseling has a significant short-term effect at 3 months, but no substantial benefit at 6 months.Citation96 When putting together the smoking abstinence prevalence from eight RCTs, web-based interventions had no additional effect on 7-day abstinence prevalence compared to other eHealth platforms (e-mail, video, and chat room) with RR 1.03, 95% CI 0.94–1.13, P=0.55. Statistical heterogeneity was moderate (I2=37%).
Web-based integrated with pharmacotherapy
Results from 11 studies offered nicotine replacement therapy (NRT) via webpage showed moderate effect (RR 1.29, 95% CI 1.17–1.43, P<0.001). Based on the GRADE guideline, these interventions probably increase cessation slightly due to statistical heterogeneity (I2=42%, P<0.001). Of the 11 studies, six had pharmacotherapy components that were associated with significant improvement in abstinence in webpage interventions vs control conditions. Brendyen (2008) emphasized that SMS and webpages with both e-mail and interactive voice response improved treatment outcomes (eg, repeated point abstinence, NRT adherence, and self-efficacy) compared to those who received only self-help material and NRT.Citation53 Meanwhile, there was greater evidence of effectiveness when a tobacco tactics website was combined with nurse counseling via phone and NRT, when compared with eHealth smoking cessation intervention without human contact (eg, quit-line group,Citation97 nicotine mini-lozenges supported by a website,Citation90 a smoke-free”website for people living with HIV/AIDS,Citation98 e-treatment software with mobile text messages,Citation99 web-based smoking-cessation with tailored achievement storiesCitation100). In contrast, some RCTs were unable to show substantial effects of webpage smoking treatment plus NRT for HIV-positive smokers.Citation101–Citation103 Similarly, no significant treatment difference was detected in the internet-home-based program for adolescents compared to a clinic-based, individual counseling.Citation104
Effectiveness of mHealth smoking cessation intervention
Mobile-based smoking cessation programs were conducted in eight studies for adult smokers,Citation7–Citation11,Citation105–Citation107 nine studies for young smokers,Citation108–Citation116 and five interventions (seven reports) for HIV-positive patients or pregnant smokers.Citation117–Citation123 Among 22 mHealth interventions, the significant effect on smoking abstinence was confirmed in five studies among adults,Citation7,Citation9,Citation105–Citation107 six studies among young smokers,Citation109,Citation111–Citation114,Citation116 and three smoking interventions for pregnant woman and HIV-positive patients.Citation118,Citation119,Citation123 However, due to high heterogeneity (I2>81%), the effect of the study sample was not pooled by the meta-analysis.
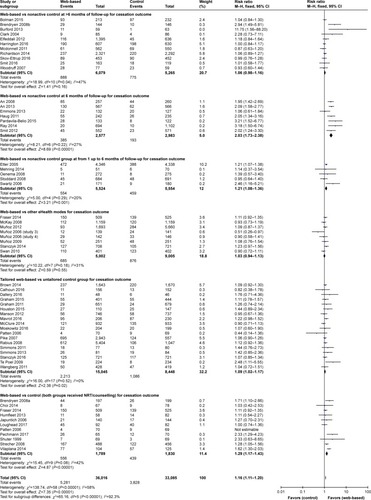
Regarding mobile apps, only three studies examined the impact of mobile apps on improving smoking cessation. However, due to methodologic weaknesses, such as small sample size (eleven participants),Citation124 no control group and short-term follow-up (1 week),Citation125,Citation126 three studies were excluded in the review. demonstrates the benefits of mobile-based smoking cessation intervention compared with various types of control groups.
Figure 4 Forest plot of mHealth intervention effects by characteristics of control group.
mHealth interventions vs nonactive controls
Text messaging-supported cessation interventions were examined in eight studies, with comparisons to nonactive controls who received usual care or a quitting brochure only. Seven studies did not detect statistically significant differences between the conditions,Citation10,Citation11,Citation114,Citation118,Citation119 even where there were fully automated tailored SMS,Citation111 or mobile phone-delivered counseling combined with varenicline.Citation121 Ferguson found that the text messages may support improved use of pharmacotherapy. It is also possible that the reverse is also true if medications promote the beneficial effects of a behavioral strategy.Citation10 A statistically significant benefit of proactive counseling phone calls was detected by Vidrine (2015) in an effort to improve smoking abstinence through changing self-efficacy among HIV-positive patients.Citation123 After including eight text messaging studies in the meta-analysis, the RR illustrated an important increase of cessation effect of text-messaging and cell-phone intervention, compared to nonactive control group (RR 1.71, 95% CI 1.35–2.16, I2=5%).
High-frequency SMS vs low-frequency control group
Four studies compared effects on smoking abstinence between text-message conditions (multiple SMS intervention vs single SMS per week) to explore the impact of SMS intensity on behavior change. Augustson (2016) developed an intervention that delivered smoking cessation advice via three messages daily, compared to the control group that received once-weekly SMS containing smoking health effects information only.Citation8 Bramley (2005) utilized personalized SMS daily vs SMS monthly among the Maori population in New Zealand.Citation108 Müssener (2016) designed a 12-week NEXit core program, which had 157 text messages compared to one text per 2 weeks in the control group. Only the NEXit core program detected a short-term effect of the intervention, whereas no significant effects were found in the other studies. An mHealth application used a camera phone to prompt participants to conduct exhaled carbon monoxide tests, in combination with a brief advice via mobile phone and transdermal nicotine. Participants in the intervention group received a prize for a negative exhaled carbon monoxide test outcome.Citation105 However, no significant effect was found at 6 months of follow-up (OR =0.99, 95% CI 0.98–1.01). Due to high heterogeneity (I2=93%), the meta-analysis was not employed to pool the effects of these trials.
mHealth vs other eHealth platforms (webpage/social media/computer-based program)
Four studies evaluated the text messages program compared to website or computer-supported program. Abroms (2014) developed a Text2Quit program delivering automated, interactive text messages based on social cognitive theory with the supports of e-mails and a website, compared to a smoking cessation website (Smokefree.gov). This trial revealed a strong effect of the intervention with RR =2.22 (95% CI 1.16–4.26).Citation7 Similarly, a 3-month mobile phone-based counseling intervention plus hotline for people living with HIV, compared with an audio computer-supported self-interview and quit advice in the USA, found that the treatment effect was maximized at 3 months (OR=4.3, P<0.001) and decline to 2.41 (P=0.49) at 6 and 12 months.Citation117 In contrast, no significant difference was found between a text messaging program (onQ), which provided a daily SMS tailored to the participant’s progress toward quitting, vs a static website in Australia.Citation106,Citation127 After pooling, the mHealth effect was confirmed with RR =1.35, 95% CI 1.04–1.75. This group of three studies had no observable heterogeneity (I2=0%). This result suggested that the mHealth intervention via text messaging may increase cessation slightly compared to a website-only intervention.
Tailored SMS vs untailored SMS
Six studies compared the tailored SMS to general SMS. Three mHealth studies applied a text messaging quitting program called txt2stop that included motivational SMS and behavioral change encouragement plus a £20 prepaid voucher, while the control group received a general SMS on other health topics. Rodgers (2005) initiated a trial in New Zealand and Free (2005) piloted and adapted the trial for young smokers (>16 years) in the UK, along with a quit buddy and SMS regarding craving, before conducting a full trial in 2011. Roger (2005) found that the quit rate at 26-week follow-up was statistically greater in the intervention (7.5%) compared to the control group (4.6%), RR =1.64 (95% CI 1.12–2.42).Citation113 The study by Free (2001) among 200 smokers found a short-term effect of smoking abstinence at 4-week follow up (RR=2.08) but not a statistically significant difference at 6 months of follow-up (RR=1.28).Citation109 When the full trial was carried out among 5,800 smokers, participants in the intervention group received personalized SMS advice while the control group was delivered one untailored SMS/2 weeks. That study found a 6-month sustained benefit of the intervention on 7-day PPA and continuous abstinence (RR=1.32, 95% CI 1.19–1.47).Citation110
Further evidence of a substantial effect of tailored text messages (based on quit stage) compared to text messages unrelated to smoking cessation in a control group was found in a trial conducted by Bock et al.Citation9 When integrated with a video, the tailored text messages were not significantly beneficial to smokers after 6 months of follow-up, compared to a control group that received general health multimedia messages.Citation115 A short-term improvement in smoking abstinence at 4 weeks of follow-up was found in a 6-week messaging program in the USA (OR 3.33, 95% CI 1.48–7.45) but when measured at 3 months, the effect was not statistically significant.Citation116 The pooled effect of six studies demonstrated that tailored SMS may increase cessation (RR=1.8, 95% CI 1.54–2.1), but this result should be interpreted with caution due to significantly moderate heterogeneity among studies (I2=66%, P=0.01).
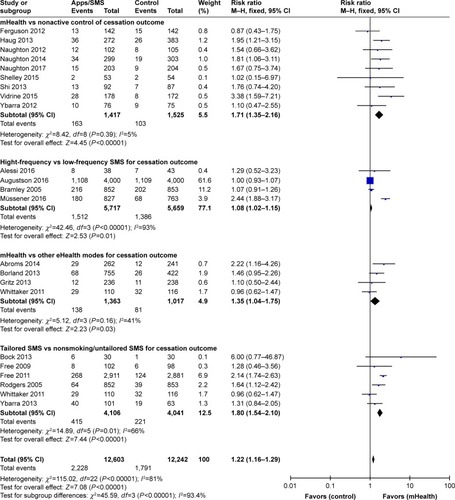
Effectiveness of computer-assisted smoking cessation intervention
illustrates that nine computer-assisted studies were conducted among adult smokersCitation128–Citation136 while only four studies were undertaken among young smokers (n=2),Citation137,Citation138 HIV patients, and pregnant women (n=2).Citation139,Citation140 Among adult smokers, six studies showed improved smoking abstinence.Citation129–Citation133,Citation135 The pooled effect of nine intervention resulted in greater smoking abstinence prevalence compared to control groups (RR=1.19, 95% CI 1.09–1.29, P<0.001) and low heterogeneity (ICitation2=0%). Similar results were found after pooling effects of two studies targeting patients (eg, HIV-positive patients, chronic patients) and pregnant women (RR=1.66, 95% CI 1.06–2.62, P=0.03, ICitation2=0%). In contrast, two computer-based interventions among young smokers did not yield a statistically significant effect (RR=1.39, 95% CI 0.88–2.19, P=0.15, ICitation2=0%).
Figure 5 Forest plot of computer-assisted intervention effects by characteristics of control group.
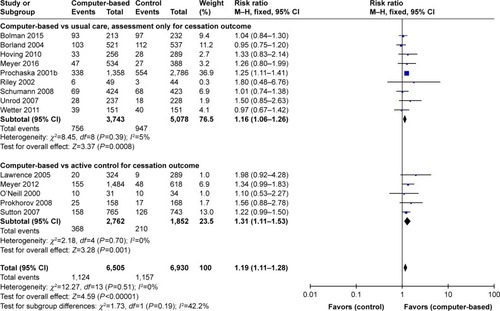
Among six studies using computer-assisted smoking cessation interventions compared to nonactive control groups,Citation129,Citation131,Citation133,Citation134,Citation136,Citation141 only two studies using individualized and interactive expert system computer reports,Citation132 and tailored computer-generated advice reports sent to smokers at strategically suitable times found greater 6-month treatment outcomes compared to control conditions.Citation128,Citation132 The pooled effect of eight studies revealed little or no increase of cessation (RR=1.16, 95% CI 1.06–1.26, P=0.0007, I2=7%). The stronger efficacy of computer-assisted intervention was demonstrated in the pooled effect of five studiesCitation130,Citation135,Citation137–Citation139 (RR=1.31, 95% CI 1.11–1.53, P=0.001, ICitation2=0%). However, all five individual studies did not find statistically significant difference between a computer-assisted program and active control groups receiving brief counseling,Citation130,Citation138 or self-help material,Citation139 or telephone counseling.Citation135 The total effect of computer-generated technology was pooled, and based on the results of 14 studies, the magnitude of effect was RR=1.21 (95% CI 1.11–1.31) with low heterogeneity (ICitation2=0%).
Effectiveness of other eHealth intervention
The efficacy of integrated video features was investigated in three studies using a “video doctor” for pregnant women,Citation141 a tailored video-based website,Citation66 and video messages for adults.Citation85 Due to the high heterogeneity of studies (I2=76%), the effect of the varieties of eHealth technology was not pooled via meta-analysis. summarizes the effect magnitude of the three eHealth interventions, subgrouped by different comparison groups.
Table 4 Summary of findings on effects of eHealth smoking cessation interventions, based on GRADE guidelines
Core effect modifiers
Results from meta-regression in show heterogeneity and identify sources of effect modifiers in the meta-analysis. The log RR was estimated to decrease by 0.146 per unit increase in the EPHPP ranking. In other words, studies that have high risk of bias are more likely to overestimate the effect of web-based smoking cessation interventions. On the other hand, for mobile-based smoking cessation, the treatment effect is moderated by the trial characteristics (eg, tailored content, frequent SMS) and country where the trial was conducted. Interestingly, the log RR was estimated to increase by 0.45 and decrease by 0.224 when quitting SMS was tailored to participants’ characteristics and daily SMS delivery, compared to untailored and weekly SMS, respectively.
Table 5 Core effect modifier using meta-regression, stratified by eHealth platforms
Discussion
This systematic review examined the efficacy of four eHealth behavioral interventions (internet-based, mobile-based, computer-assisted, and other electronic aids) in helping people to quit smoking. Unfortunately, most of the evidence for beneficial effects is limited to follow-up at 6 months or less, with few demonstrations of longer-term abstinence. This review also explored specific attributes of eHealth and other interventions that can contribute to successful smoking abstinence. Most of the studies were conducted in affluent countries such as the USA, Canada, Europe, Australia, China, and Japan where communications technology has been widely available over several decades. There is a lack of evidence from middle- and low-income countries in Asia and Africa, where there is limited access to coordinated eHealth efforts. Future eHealth smoking cessation interventions should be conducted in developing countries, focus on disadvantaged groups, and measure long-term outcomes for a more comprehensive evaluation of the effectiveness of technology-based interventions.
The duration of follow-up is a critical consideration. This review highlights the preponderance of studies of short-term abstinence (from 1 month to less than 6 months of follow-up) of web-based interventions and text messaging systems. Only two studies obtained substantially longer effects at 1 year of follow-up. Those interventions included a video-based computer-tailored program compared to a text-based version with brief generic text advice,Citation85 and an internet and mobile phone-based version compared to printed material only.Citation53 This is consistent with previous systematic reviews and confirms the promising impacts of technology-based interventions in behavioral change especially to sustain smoking cessation.Citation18,Citation22,Citation23,Citation28,Citation142,Citation143 Further investigations should be done to address the prolonged effect of the interventions.
After pooling findings from 67 internet smoking cessation studies, the strongest evidence of efficacy is for smoking cessation webpages compared to nonactive control conditions (no intervention, print materials only, assessment only) at 6 months of follow-up, and for joint pharmacotherapy plus web-based intervention. Previous reviews support the use of pharmacotherapy in combination with behavioral support, to improve cessation rates.Citation21,Citation144 Interactive and tailored web-based interventions have moderate effects on smoking abstinence compared to nonactive controls at 6 months or longer.Citation20 This finding supports the efforts of health care providers and policymakers who seek to establish web-based smoking cessation interventions conjunctive with NRT. They may be especially helpful smokers in vulnerable groups, such as patients with HIV, COPD, and cancer and those undergoing treatment for substance use disorders.
When compared to active control groups or other eHealth modes, we could not find evidence of the effectiveness of internet-based tobacco cessation programs. This is consistent with an earlier Cochrane review, which concluded that eHealth smoking cessation interventions (eg, websites or text messages) may produce little or no increase in quitting compared to an active control group.Citation20,Citation21 Among 12 eHealth smoking cessation interventions conducted with vulnerable patient groups, including five web-based,Citation80,Citation81,Citation118,Citation119,Citation123 three text-messaging systems,Citation84,Citation98 and two computer-assisted programs,Citation139,Citation140 the subgroup analysis suggested that the web-based and tailored text messaging supports may increase cessation while computer-assisted interventions alone have little impact on smoking abstinence. This suggests that tobacco cessation interventions via mobile phone and other wireless devices probably offer the best uptake.
The effect size of web-based and mobile-based smoking intervention is moderated by methodologic quality. The efficacy of text message smoking cessation trials was affected by the location of study, which is the best in the USA, Canada, Europe, Australia, China, and Japan, and characteristics of interventions (eg, personalized message and frequency of SMS received). The present review suggests that stop-smoking interventions via websites and SMS with a weak quality of methodology are likely to overestimate the smoking cessation outcomes. It is possible to hypothesize that participants are less likely to disclose their smoking status if there is only self-report measurement without bioverification (as typically exists for internet smoking cessation programs). Additionally, according to the present synthesis, it is clear that trials are challenged by the difficulty of establishing double-blinding in web-based environments. The participants may be aware of the allocated intervention trial conditions. If so, the effect size of eHealth interventions might be overestimated. Finally, a critical barrier for establishing effectiveness of online interventions is attrition bias, because the penalized imputation method is highly conservative (eg, missing cases are assumed to be continuing smokers). This problem was shown by Blankers et al (2016), where the estimated effects of internet-based smoking cessation interventions are more likely to overestimate outcomes in the conditions that have the higher retention rates.Citation145
Among 24 mobile phone-based studies, 21 interventions utilized SMS-based reminders for smoking cessation and the three other studies provided counseling via cell phones for HIV-positive patients.Citation117,Citation122,Citation123 Interestingly, all three mHealth programs for patients living with HIV/AIDS showed significant improvement in smoking abstinence at 3 months. Pooled, tailored text message interventions have a moderate effect on improving health behaviors. The strongest effect in mHealth experiments was contributed by nine studies that used tailored SMS. This emphasizes the benefits of tailoring the content of messages.Citation20,Citation24,Citation146,Citation147 To maximize the effect of individually tailored messages, mHealth smoking cessation intervention should pay attention to selecting a negatively framed health message (eg, severe smoking harm), usually appropriate for those who have a high degree of nicotine dependence, or positively framed health message, targeting smokers who have lower nicotine dependence (eg, emphasizing well-being after quitting).Citation148
This review found that effect sizes from mobile-based smoking cessation interventions were moderated by the frequency of texts. Four studies investigated the impact of SMS frequency and no significant difference was detected when comparing high-frequency vs low-frequency SMS groups. This is consistent with previous reviews that found that the effect of health behavior change interventions in general and smoking cessation programs, in particular, might not be influenced by the message frequency.Citation24,Citation27,Citation149 Quantity of text messages may not be critical in determining smoking cessation outcomes,Citation147 but rather the effects may vary in relation to personal preferences, as some smokers like to receive fewer SMS per day.Citation150 Some participants may feel annoyed by multiple reminders per day, leading to negative results. Some studies have noted the importance of texting during critical times at high risk of cravings for smoking (eg, morning after wake-up), rather than sending texts randomly throughout the day.Citation151
The role of health care providers is crucial for technology-based interventions such as mobile text messages or e-mails from clinicians,Citation99 or computer-generated expert letters,Citation84 or online resources supported by nursesCitation72 to assist high-risk smokers to reduce and cease tobacco consumption. Programs provided largely by nurses,Citation152 dentists, or physicians help to effectively foster patients’ initial attempts to quit and help them consider using preferred smoking cessation technology platforms.Citation153–Citation155 For quit program developers it is often challenging to engage clinicians who are busy and not familiar with advanced technology. Clinicians may prefer face-to-face counseling that is coupled with eHealth interventions, with the goal of establishing rapport and improving communication between patients and practitioners.
Limitations
This review has limitations. First, with the rapid growth of technology, it was not possible for us to cover all studies and eHealth interventions worldwide. The English language criterion may also have restricted capture of international studies. Second, it was difficult to compare the overall effects across multiple studies because they calculated outcomes using different indicators (self-reported 7-day or 30-day PPA with or without biochemical validation) at different time points (24 hours, 7 days, 3 months, 6 months, and so on). The significant differences in study design and outcome indicators lead to high heterogeneity, and difficulty in pooling data on the effectiveness of some eHealth subgroups. Finally, this study did not take into account the contributions of user experience (eg, user satisfaction, perceived acceptability), which might moderate the effect size of eHealth/mHealth smoking cessation interventions.
Conclusions
This review found that smoking cessation interventions using web-based and mHealth approaches may increase cessation, but with moderate effects over short term periods. There is a clear need for more research in low- and middle-income countries. Considering all of the evidence together, the findings support the use of interactive, tailored, web-based and mobile phone-based smoking cessation interventions. Although the effect sizes are not high, even interventions that influence a minority of smokers to quit are worthwhile, as the cost per person is generally low. Policymakers should encourage the use of eHealth interventions by health professionals, and provide resources to ensure that expertise, intervention materials, and funded programs are authentic and sensitive to the characteristics and needs of target populations.
Disclosure
The authors report no conflicts of interest in this work.
References
- SorianoJBAbajobirAAAbateKHGlobal, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015Lancet Respir Med20175969170628822787
- World Health OrganizationWHO report on the global tobacco epidemic 2009: implementing smoke-free environments2009 Available from: http://www.who.int/tobacco/en/Accessed December 6, 2017
- KrepsGLNeuhauserLNew directions in eHealth communication: opportunities and challengesPatient Educ Couns201078332933620202779
- AhernDKKreslakeJMPhalenJMBockBWhat is eHealth (6): perspectives on the evolution of eHealth researchJ Med Internet Res200681e416585029
- VogelMMECombsSEKesselKAmHealth and application technology supporting clinical trials: today’s limitations and future perspective of smartRCTsFront Oncol2017733728348978
- KayMSantosJTakaneMmHealth New horizons for health through mobile technologies: based on the findings of the second global survey on eHealth2011
- AbromsLCBoalALSimmensSJMendelJAWindsorRAA ran-domized trial of Text2Quit: a text messaging program for smoking cessationAm J Prev Med201447324225024913220
- AugustsonEEngelgauMMZhangSText to quit China: an mHealth smoking cessation trialAm J Health Promot201631321722526730560
- BockBHeronKJenningsEA text message delivered smoking cessation intervention: the initial trial of TXT-2-Quit: randomized controlled trialJMIR Mhealth Uhealth201312e1725098502
- FergusonSWaltersJThe effect of mobile phone text messages for quitting smoking in motivated smokers: a randomised controlled trialEur Respir J201444Suppl 58P4454
- YbarraMBağci BosiATKorchmarosJEmriSA text messaging-based smoking cessation program for adult smokers: randomized controlled trialJ Med Internet Res2012146e17223271159
- GhoraiKAkterSKhatunFRayPmHealth for smoking cessation programs: a systematic reviewJ Pers Med20144341242325563359
- CashenMSDykesPGerberBeHealth technology and Internet resources: barriers for vulnerable populationsJ Cardiovasc Nurs200419320921415191264
- ParkEDrakeESystematic review: Internet-based program for youth smoking prevention and cessationJ Nurs Scholarsh2015471435025130325
- HuttonHEWilsonLMApelbergBJA systematic review of randomized controlled trials: web-based interventions for smoking cessation among adolescents, college students, and adultsNicotine Tob Res201113422723821350042
- BalharaYSVermaRA review of web based interventions for managing tobacco useIndian J Psychol Med201436322623525035543
- JayasekaraRInternet-based interventions for smoking cessationInt J Evid Based Healthc201192195195
- ShahabLMcewenAOnline support for smoking cessation: a systematic review of the literatureAddiction2009104111792180419832783
- GrahamACarpenterKChaSSystematic review and meta-analysis of Internet interventions for smoking cessation among adultsSubst Abuse Rehabil20167556927274333
- TaylorGMJDaliliMNSemwalMInternet-based interventions for smoking cessationCochrane Database Syst Rev2017472CD007078
- CivljakMSteadLFHartmann-BoyceJSheikhACarJInternet-based interventions for smoking cessationCochrane Database Syst Rev20137Cd007078
- HeadKJNoarSMIannarinoNTGrant HarringtonNEfficacy of text messaging-based interventions for health promotion: a meta-analysisSoc Sci Med201397414824161087
- JardimCMobile phone-based interventions for smoking cessationSao Paulo Med J20101282106107
- WhittakerRMcrobbieHBullenCRodgersAGuYCochrane Tobacco Addiction GroupMobile phone-based interventions for smoking cessationCochrane Database Syst Rev201617Suppl 1153
- PortnoyDBScott-SheldonLAJJohnsonBTCareyMPComputer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials, 1988–2007Prev Med200847131618403003
- HaugSSannemannJMeyerCJohnUInternet and mobile phone interventions to decrease alcohol consumption and to support smoking cessation in adolescents: a reviewGesundheitswesen201274316017721387218
- ArmanascoAAMillerYDFjeldsoeBSMarshallALPreventive health behavior change text message interventions: a meta-analysisAm J Prev Med201752339140228073656
- ChenY-FMadanJWeltonNEffectiveness and cost-effectiveness of computer and other electronic aids for smoking cessation: a systematic review and network meta-analysisHealth Technol Assess201216381205
- MoherDLiberatiATetzlaffJAltmanDGThe PRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA StatementPLoS Med200967e100009719621072
- Armijo-OlivoSStilesCRHagenNABiondoPDCummingsGGAssessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological researchJ Eval Clin Pract2012181121820698919
- HughesJRCarpenterMJNaudSDo point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic reviewNicotine Tob Res201012775676220504946
- CheungKLde RuijterDHiligsmannMExploring consensus on how to measure smoking cessation. A Delphi studyBMC Public Health201717189029162043
- HughesJRKeelyJNaudSShape of the relapse curve and long-term abstinence among untreated smokersAddiction2004991293814678060
- GuptaSIntention-to-treat concept: a reviewPerspect Clin Res20112310911221897887
- FletcherJWhat is heterogeneity and is it important?BMJ20073347584949617218716
- BalshemHHelfandMSchünemannHJGRADE guidelines: 3. Rating the quality of evidenceJ Clin Epidemiol201164440140621208779
- DanaherBGLichtensteinEMckayHGSeeleyJRUse of non-assigned smoking cessation programs among participants of a Web-based randomized controlled trialJ Med Internet Res2009112e2619632976
- SeversonHGordonJDanaherBAkersLChewFree.com: evaluation of a web-based cessation program for smokeless tobacco usersNicotine Tob Res200810238139118236303
- SeversonHHAkersLAndrewsJALichtensteinEJeromeAEvaluating two self-help interventions for smokeless tobacco cessationAddict Behav200025346547010890303
- AveyardPSherrattEAlmondJThe change-in-stage and updated smoking status results from a cluster-randomized trial of smoking prevention and cessation using the transtheoretical model among British adolescentsPrev Med200133431332411570836
- FlayBRHansenWBJohnsonCAImplementation effectiveness trial of a social influences smoking prevention program using schools and televisionHealth Educ Res198724385400
- GordonJSMahabee-GittensEMAndrewsJAChristiansenSMByronDJA randomized clinical trial of a web-based tobacco cessation education programPediatrics20131312e455e46223319529
- HollisJFPolenMRWhitlockEPTeen reach: outcomes from a randomized, controlled trial of a tobacco reduction program for teens seen in primary medical carePediatrics2005115498198915805374
- LenertLMunozRFPerezJEBansodAAutomated e-mail messaging as a tool for improving quit rates in an internet smoking cessation interventionJ Am Med Inform Assoc200411423524015064291
- ProchaskaJOVelicerWFFavaJLCounselor and stimulus control enhancements of a stage-matched expert system intervention for smokers in a managed care settingPrev Med2001321233211162323
- SeidmanDFWestmaasJLGoldbandSRandomized controlled trial of an interactive internet smoking cessation program with long-term follow-upAnn Behav Med2010391486020177844
- HahnEJRayensMKWarnickTAA controlled trial of a quit and win contestAm J Health Promot200520211712616295703
- LinkeSERutledgeTMyersMGIntermittent exercise in response to cigarette cravings in the context of an Internet-based smoking cessation programMental Health and Physical Activity201251859223956792
- PengWBSchoechDEvaluation of a web-phone intervention system in changing smoking behavior – a randomized controlled trialJ Technol Hum Serv2013313248268
- ReinwandDACrutzenRKienhuisASTalhoutRde VriesHWebsite use and effects of online information about tobacco additives among the Dutch general population: a randomized controlled trialJ Med Internet Res2017193e6028292739
- ShiffmanSPatyJARohayJMdi MarinoMEGitchellJThe efficacy of computer-tailored smoking cessation material as a supplement to nicotine polacrilex gum therapyArch Intern Med2000160111675168110847261
- BrendryenHDrozdFKraftPA digital smoking cessation program delivered through internet and cell phone without nicotine replacement (happy ending): randomized controlled trialJ Med Internet Res2008a105e5119087949
- BrendryenHKraftPHappy ending: a randomized controlled trial of a digital multi-media smoking cessation interventionAddiction2008b1033478484 discussion 485–47618269367
- ElfeddaliIBolmanCCandelMJJMWiersRWde VriesHPreventing smoking relapse via web-based computer-tailored feedback: a randomized controlled trialJ Med Internet Res2012144e109e10222903145
- HarringtonKFKimY-IlChenMWeb-based intervention for transitioning smokers from inpatient to outpatient careAm J Prev Med201651462062927647062
- McdonnellDDKazinetsGLeeH-JMoskowitzJMAn internet-based smoking cessation program for Korean Americans: results from a randomized controlled trialNicotine Tob Res201113533634321330285
- RayMNFunkhouserEWilliamsJHSmoking-cessation e-referrals: a national dental practice-based research network randomized controlled trialAm J Prev Med201446215816524439349
- BrownJMichieSGeraghtyAWInternet-based intervention for smoking cessation (StopAdvisor) in people with low and high socioeconomic status: a randomised controlled trialLancet Respir Med2014212997100625262458
- SmitESde VriesHHovingCEffectiveness of a Web-based multiple tailored smoking cessation program: a randomized controlled trial among Dutch adult smokersJ Med Internet Res2012143e8222687887
- StoddardJLAugustsonEMMoserRPEffect of adding a virtual community (bulletin board) to smokefree.gov: randomized controlled trialJ Med Internet Res2008105e5319097974
- SmitESCandelMJJMHovingCde VriesHResults of the PAS Study: a randomized controlled trial evaluating the effectiveness of a web-based multiple tailored smoking cessation program combined with tailored counseling by practice nursesHealth Commun20163191165117326934538
- EtterJ-FComparing the efficacy of two internet-based, computer-tailored smoking cessation programs: a randomized trialJ Med Internet Res200571e215829474
- OenemaABrugJDijkstraAde WeerdtIde VriesHEfficacy and use of an internet-delivered computer-tailored lifestyle intervention, targeting saturated fat intake, physical activity and smoking cessation: a randomized controlled trialAnn Behav Med200835212513518363076
- BolmanCEggersSMvan OschLTe PoelFCandelMde VriesHIs action planning helpful for smoking cessation? Assessing the effects of action planning in a web-based computer-tailored interventionSubst Use Misuse201550101249126026440754
- BrownJMichieSGeraghtyAWAInternet-based intervention for smoking cessation (StopAdvisor) in people with low and high socioeconomic status: a randomised controlled trialLancet Respir Med2014212997100625262458
- SwartzLHGNoellJWSchroederSWAryDVA randomised control study of a fully automated internet based smoking cessation programmeTob Control200615171216436397
- CameronDEptonTNormanPA theory-based online health behaviour intervention for new university students (U@Uni:LifeGuide): results from a repeat randomized controlled trialTrials201516155526643917
- BurfordOJiwaMCarterOParsonsRHendrieDInternet-based photoaging within Australian pharmacies to promote smoking cessation: randomized controlled trialJ Med Internet Res2013153e6423531984
- CalhounPSDattaSOlsenMComparative effectiveness of an Internet-based smoking cessation intervention versus clinic-based specialty care for veteransJ Subst Abuse Treat201669192727568506
- LcAKlattCPerryCLThe RealU online cessation intervention for college smokers: a randomized controlled trialPrev Med200847219419918565577
- MehringMHaagMLindeKWagenpfeilSSchneiderAEffects of a guided web-based smoking cessation program with telephone counseling: a cluster randomized controlled trialJ Med Internet Res2014169e21825253539
- Pardavila-BelioMIGarcía-VivarCPimentaAMCanga-ArmayorAPueyo-GarriguesSCanga-ArmayorNIntervention study for smoking cessation in Spanish college students: pragmatic randomized controlled trialAddiction2015110101676168326053958
- Skov-EttrupLSDalumPBechMTolstrupJSThe effectiveness of telephone counselling and internet- and text-message-based support for smoking cessation: results from a randomized controlled trialAddiction201611171257126626748541
- WoodruffSIConwayTLEdwardsCCElliottSPCrittendenJEvaluation of an Internet virtual world chat room for adolescent smoking cessationAddict Behav20073291769178617250972
- BanninkRBroerenSJoosten-van ZwanenburgEvan AsEvan de Looij-JansenPRaatHEffectiveness of a web-based tailored intervention (E-health4Uth) and consultation to promote adolescents’ health: randomized controlled trialJ Med Internet Res2014165e14324878521
- CobbNKJacobsMAWileytoPValenteTGrahamALDiffusion of an evidence-based smoking cessation intervention through Face-book: a randomized controlled trialAm J Public Health201610661130113527077358
- MañanesGVallejoMAUsage and effectiveness of a fully automated, open-access, Spanish Web-based smoking cessation program: randomized controlled trialJ Med Internet Res2014164e11124760951
- MavrotCStuckiISagerFEtterJ-FEfficacy of an Internet-based, individually tailored smoking cessation program: a randomized-controlled trialJ Telemed Telecare201723552152827365318
- HerbecABrownJTomborIMichieSWestRPilot randomized controlled trial of an internet-based smoking cessation intervention for pregnant smokers (‘MumsQuit)’Drug Alcohol Depend201414013013624811202
- Voncken-BrewsterVTangeHde VriesHNagykaldiZWinkensBvan der WeijdenTA randomized controlled trial evaluating the effectiveness of a web-based, computer-tailored self-management intervention for people with or at risk for COPDInt J Chron Obstruct Pulmon Dis2015101061107326089656
- ZulligLLSandersLLShawRJMccantFDanusSBosworthHBA randomised controlled trial of providing personalised cardiovascular risk information to modify health behaviourJ Telemed Telecare201420314715224647384
- EmmonsKMPuleoESprunck-HarrildKPartnership for health-2, a web-based versus print smoking cessation intervention for childhood and young adult cancer survivors: randomized comparative effectiveness studyJ Med Internet Res20131511e21824195867
- ClarkMMCoxLSJettJREffectiveness of smoking cessation self-help materials in a lung cancer screening populationLung Cancer2004441132115013579
- HaugSMeyerCJohnUEfficacy of an internet program for smoking cessation during and after inpatient rehabilitation treatment: a quasi-randomized controlled trialAddict Behav201136121369137221907496
- StanczykNEde VriesHCandelMJJMMurisJWMBolmanCAWEffectiveness of video- versus text-based computer-tailored smoking cessation interventions among smokers after one yearPrev Med201682425026577867
- GrahamALPapandonatosGDCobbCOInternet and telephone treatment for smoking cessation: mediators and moderators of short-term abstinenceNicotine Tob Res201517329930825156528
- HoustonTKSadasivamRSAllisonJJEvaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation studyImplementat Sci2015101154
- WangbergSCNilsenOAntypasKGramITEffect of tailoring in an internet-based intervention for smoking cessation: randomized controlled trialJ Med Internet Res2011134e12122169631
- DalleryJRaiffBRKimSJMarschLAStitzerMGrabinskiMJNationwide access to an internet-based contingency management intervention to promote smoking cessation: a randomized controlled trialAddiction20161125875883
- FraserDKobinskyKSmithSSKramerJTheobaldWEBakerTBFive population-based interventions for smoking cessation: a MOST trialTransl Behav Med20144438239025584087
- MckayHGDanaherBGSeeleyJRLichtensteinEGauJMComparing two web-based smoking cessation programs: randomized controlled trialJ Med Internet Res2008105e4019017582
- MuñozRFAguileraASchuellerSMLeykinYPérez-StableEJFrom online randomized controlled trials to participant preference studies: morphing the San Francisco Stop Smoking site into a worldwide smoking cessation resourceJ Med Internet Res2012143e6422739225
- MuñozRFBarreraAZDelucchiKPenillaCTorresLDPérez-StableEJInternational Spanish/English Internet smoking cessation trial yields 20% abstinence rates at 1 yearNicotine Tob Res20091191025103419640833
- MuñozRLenertLDelucchiKToward evidence-based Internet interventions: a Spanish/English Web site for international smoking cessation trialsNicotine Tob Res200681778716497602
- StanczykNBolmanCvan AdrichemMCandelMMurisJde VriesHComparison of text and video computer-tailored interventions for smoking cessation: randomized controlled trialJ Med Internet Res2014163e6924589938
- SwanGEMcclureJBJackLMBehavioral counseling and varenicline treatment for smoking cessationAm J Prev Med201038548249020409497
- ChoiSHWaltjeAHRonisDLWeb-enhanced tobacco tactics with telephone support versus 1-800-QUIT-NOW telephone line intervention for operating engineers: randomized controlled trialJ Med Internet Res20141611e25525447467
- ShuterJMoralesDAConsidine-DunnSEAnLCStantonCAFeasibility and preliminary efficacy of a web-based smoking cessation intervention for HIV-infected smokersJ Acquir Immune Defic Syndr2014671596625118794
- VilaplanaJSolsonaFAbellaFCuadradoJAlvesRMateoJS-PC: an e-treatment application for management of smoke-quitting patientsComput Methods Programs Biomed20141151334524742965
- StrecherVJMcclureJBAlexanderGLWeb-based smoking-cessation programs – results of a randomized trialAm J Prev Med200834537338118407003
- JapuntichSZehnerMSmithSSmoking cessation via the Internet: a randomized clinical trial of an Internet intervention as adjuvant treatment in a smoking cessation interventionNicotine Tob Res2006815967
- LougheadJFalconeMWileytoEPCan brain games help smokers quit?: results of a randomized clinical trialDrug Alcohol Depend201616811211827635998
- HumfleetGLHallSMDelucchiKLDilleyJWA randomized clinical trial of smoking cessation treatments provided in HIV clinical care settingsNicotine Tob Res201315814361445 Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/438/CN-00983438/frame.html23430708
- PattenCACroghanITMeisTMRandomized clinical trial of an Internet-based versus brief office intervention for adolescent smoking cessationPatient Educ Couns2006641–324925816616449
- AlessiSMRashCJPetryNMA randomized trial of adjunct mHealth abstinence reinforcement with transdermal nicotine and counseling for smoking cessationNicotine Tob Res2016193290298
- BorlandRBalmfordJBendaPPopulation-level effects of automated smoking cessation help programs: a randomized controlled trialAddiction2013108361862822994457
- NaughtonFJamisonJBoaseSRandomized controlled trial to assess the short-term effectiveness of tailored web- and text-based facilitation of smoking cessation in primary care (iQuit in practice)Addiction201410971184119324661312
- BramleyDRiddellTWhittakerRSmoking cessation using mobile phone text messaging is as effective in Maori as non-MaoriN Z Med J20051181216U149415937529
- FreeCKnightRRobertsonSSmoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trialLancet20113789785495521722952
- FreeCWhittakerRKnightRAbramskyTRodgersARobertsIGTxt2stop: a pilot randomised controlled trial of mobile phone-based smoking cessation supportTob Control2009182889119318534
- HaugSSchaubMPVenzinVMeyerCJohnUEfficacy of a text message-based smoking cessation intervention for young people: a cluster randomized controlled trialJ Med Internet Res2013158e17123956024
- MüssenerUBendtsenMKarlssonNWhiteIRMccambridgeJBendtsenPEffectiveness of short message service text-based smoking cessation intervention among university students: a randomized clinical trialJAMA Intern Med2016176332132826903176
- RodgersACorbettTBramleyDDo u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messagingTob Control200514425526116046689
- ShiHJJiangXXYuCYZhangYUse of mobile phone text messaging to deliver an individualized smoking behaviour intervention in Chinese adolescentsJ Telemed Telecare201319528228724163238
- WhittakerRDoreyEBramleyDA theory-based video messaging mobile phone intervention for smoking cessation: randomized controlled trialJ Med Internet Res2011131e10e7221371991
- YbarraMLHoltropJSPrescottTLRahbarMHStrongDPilot RCT results of stop my smoking USA: a text messaging-based smoking cessation program for young adultsNicotine Tob Res20131581388139923348969
- GritzERDanyshHEFletcherFELong-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDSClin Infect Dis201357460861523704120
- NaughtonFCooperSFosterKLarge multicentre pilot randomized controlled trial testing a low-cost, tailored, self-help smoking cessation text message intervention for pregnant smokers (MiQuit)Addiction201711271238124928239919
- NaughtonFPrevostATGilbertHSuttonSRandomized controlled trial evaluation of a tailored leaflet and SMS text message self-help intervention for pregnant smokers (MiQuit)Nicotine Tob Res201214556957722311960
- PollakKILynaPBilheimerAA pilot study testing SMS text delivered scheduled gradual reduction to pregnant smokersNicotine Tob Res201315101773177623569007
- ShelleyDTsengTYGonzalezMCorrelates of adherence to varenicline among HIV+ smokersNicotine Tob Res201517896897426180221
- VidrineDJArduinoRCLazevABGritzERA randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDSAIDS200620225326016511419
- VidrineDJKypriotakisGLiLMediators of a smoking cessation intervention for persons living with HIV/AIDSDrug Alcohol Depend2015147768025542824
- HicksTAThomasSPWilsonSMCalhounPSKuhnERBeckhamJCA preliminary investigation of a relapse prevention mobile application to maintain smoking abstinence among individuals with posttraumatic stress disorderJ Dual Diagn20171311627739936
- DanMGrabinskiMJRaiffBRSmartphone-based contingency management for smoking cessation with smokers diagnosed with attention-deficit/hyperactivity disorderTransl Issues Psychol Sci201622116127
- BrickerJBMullKEKientzJARandomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapyDrug Alcohol Depend2014143879425085225
- BalmfordJBorlandRBendaPHowardSFactors associated with use of automated smoking cessation interventions: findings from the eQuit studyHealth Educ Res201328228829923107931
- BorlandRBalmfordJHuntDThe effectiveness of personally tailored computer-generated advice letters for smoking cessationAddiction200499336937714982550
- HovingCMuddeANDijkFde VriesHEffectiveness of a smoking cessation intervention in Dutch pharmacies and general practicesHealth Educ201011011729
- MeyerCUlbrichtSGrossBAdoption, reach and effectiveness of computer-based, practitioner delivered and combined smoking interventions in general medical practices: a three-arm cluster randomized trialDrug Alcohol Depend20121211–212413221924563
- MeyerCUlbrichtSHaugSMotivating smokers to quit using computer-generated letters that target either reduction or cessation: a population-based randomized controlled trial among smokers who do not intend to quitDrug Alcohol Depend201616617718627449274
- ProchaskaJOVelicerWFFavaJLRossiJSTsohJYEvaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessationAddict Behav200126458360211456079
- RileyWJeromeABeharAWeilJComputer and manual self-help behavioral strategies for smoking reduction: initial feasibility and one-year follow-upNicotine Tob Res20024Suppl 2S183S18812573179
- SchumannAJohnUBaumeisterSEUlbrichtSRumpfHJMeyerCComputer-tailored smoking cessation intervention in a general population setting in Germany: outcome of a randomized controlled trialNicotine Tob Res200810237137918236302
- SuttonSGilbertHEffectiveness of individually tailored smoking cessation advice letters as an adjunct to telephone counselling and generic self-help materials: randomized controlled trialAddiction20071026994100017523995
- WetterDWMcclureJBCofta-WoerpelLA randomized clinical trial of a palmtop computer-delivered treatment for smoking relapse prevention among womenPsychol Addict Behav201125236537121500879
- O’NeillHKGillispieMASlobinKStages of change and smoking cessation: a computer-administered intervention program for young adultsAm J Health Promot2000152939611194701
- ProkhorovAVYostTMullin-JonesM“Look at your health”: outcomes associated with a computer-assisted smoking cessation counseling intervention for community college studentsAddict Behav200833675777118280668
- LawrenceTAveyardPChengKKGriffinCJohnsonCCroghanEDoes stage-based smoking cessation advice in pregnancy result in long-term quitters? 18-month postpartum follow-up of a randomized controlled trialAddiction2005100110711615598198
- UnrodMSmithMSpringBDepueJReddWWinkelGRandomized controlled trial of a computer-based, tailored intervention to increase smoking cessation counseling by primary care physiciansJ Gen Intern Med200722447848417372796
- TsohJYKohnMAGerbertBPromoting smoking cessation in pregnancy with Video Doctor plus provider cueing: a randomized trialActa Obstet Gynecol Scand201089451552320196678
- BrownJA review of the evidence on technology-based interventions for the treatment of tobacco dependence in college healthWorldviews Evid Based Nurs201310315016223421669
- WhittakerRMcrobbieHBullenCBorlandRRodgersAGuYMobile phone-based interventions for smoking cessationCochrane Database Syst Rev201211Cd00661123152238
- Hartmann-BoyceJSteadLFCahillKLancasterTEfficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviewsAddiction2013108101711172123834141
- BlankersMSmitESvan der PolPde VriesHHovingCvan LaarMThe missing=smoking assumption: a fallacy in internet-based smoking cessation trials?Nicotine Tob Res2016181253325744969
- Skov-EttrupLSRinggaardLWDalumPFlensborg-MadsenTThygesenLCTolstrupJSComparing tailored and untailored text messages for smoking cessation: a randomized controlled trial among adolescent and young adult smokersHealth Educ Res201429219520524399268
- KongGEllsDMCamengaDRKrishnan-SarinSText messaging-based smoking cessation intervention: a narrative reviewAddict Behav201439590791724462528
- MoormanMvan den PutteBThe influence of message framing, intention to quit smoking, and nicotine dependence on the persuasiveness of smoking cessation messagesAddict Behav200833101267127518584971
- OrrJAKingRJMobile phone SMS messages can enhance healthy behaviour: a meta-analysis of randomised controlled trialsHealth Psychol Rev20159439741625739668
- BockBCHeronKEJenningsEGMageeJCMorrowKMUser preferences for a text message-based smoking cessation interventionHealth Educ Behav201340215215923086555
- JamisonJNaughtonFGilbertHSuttonSDelivering smoking cessation support by mobile phone text message: what information do smokers want? A focus group studyJ Appl Biobehav Res2013181123
- KazemzadehZManzariZSPouresmailZNursing interventions for smoking cessation in hospitalized patients: a systematic reviewInt Nurs Rev201764226327527933638
- WHOThe role of health professionals in tobacco controlFrance2005 Available from: http://www.who.int/tobacco/resources/publications/wntd/2005/bookletfinal_20april.pdfAccessed February 12, 2018
- GreenhalghEStillmanSFordC7.10 Role of health professionals and social servicesScolloMaW MHTobacco in Australia: Facts and issuesMelbourneCancer Council Victoria2016
- EapenZJPetersonEDCan mobile health applications facilitate meaningful behavior change?: Time for answersJAMA2015314121236123726393844