Abstract
Objective
The aim of this study was to identify factors that influence treatment adjustments and adoption of a treat-to-target (T2T) strategy in patients with rheumatoid arthritis (RA) in European practices.
Methods
Cross-sectional data were drawn from the Adelphi 2014 RA Disease Specific Programme. Treatment patterns and clinical characteristics were investigated in patients treated with biologic disease-modifying antirheumatic drugs (bDMARDs) vs non-bDMARDs. For the T2T analysis, patients were subdivided into two subsets (RA diagnosis <2 or ≥2 years) and compared according to the approach used (no target = no T2T approach; pragmatic = target different from remission; and aspirational = target set as remission).
Results
Data from 2,536 patients were analyzed (mean age: 52.76 years and mean time since RA diagnosis: 6.05 years). Of the 1,438 patients eligible to receive bDMARDs, 55% did not receive them. Initiation of bDMARDs in a bDMARD-naïve patient was prompted by worsening of the disease. In the RA diagnosis <2 years subset, a T2T approach was not adopted in 58% of the patients, whereas 8% and 34% adopted a pragmatic and aspirational approach, respectively. In the RA diagnosis ≥2 years subset, 45%, 19%, and 36% of the patients adopted a no target, pragmatic, and aspirational approach, respectively. Physician satisfaction with RA control was lower in the RA diagnosis <2 years subset than in the RA diagnosis ≥2 years subset (65% vs 77% satisfied, respectively; P<0.0001).
Conclusion
This analysis shows that the use of bDMARDs remains suboptimal and that a T2T strategy is not universally adopted.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation and deterioration of the joints. RA causes significant loss of functionality and increases morbidity and mortality.Citation1
Treatment for RA involves early initiation of disease-modifying antirheumatic drugs (DMARDs).Citation2,Citation3 Current European League Against Rheumatism (EULAR) recommendations support the use of conventional synthetic DMARDs (csDMARDs), such as methotrexate, as first-line therapy, combined with glucocorticoids for a limited time. Biologic DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) may be combined with a csDMARD if csDMARD therapy fails.Citation3 The wide array of DMARDs available enables optimum disease management by allowing monotherapy or combination therapy, different dose titrations, and treatment switching. The recommendations suggest that treatment assessments should be made every 1–3 months in patients with high-to-moderate disease activity and that therapy adjustments should be made every 3–6 months if the target has not been reached.Citation2
EULAR recommendations state that the primary target for all patients with RA should be sustained remission or low disease activity (LDA), the latter for patients with long-standing or aggressive disease.Citation3 In addition, the American College of Rheumatology (ACR) recommends an aggressive therapy approach in patients with early RA to achieve better outcomes and prevent damage progression.Citation2 Indeed, early RA evaluation and treatment appear to be critical in optimizing the subsequent response.Citation4,Citation5 Better disease control can be achieved by adopting a treat-to-target (T2T) approach, which has been shown to be beneficial in various illnesses.Citation6–Citation10 This strategy is based on setting therapeutic targets that are reached through frequent assessments and drug therapy adjustments in order to optimize treatment. The assessments require measurement of disease activity using validated metrics to enable informed treatment decisions. A T2T approach is widely accepted to improve outcomes in patients with RA,Citation11–Citation13 but has not been universally adopted.Citation14
One of the aims of this study was to identify the factors influencing the introduction of bDMARD treatment in RA patients in a real-world setting, the choice of a specific bDMARD, and switching from one class of bDMARD to another. A further objective was to assess the adoption of a T2T strategy for the treatment of RA and physician satisfaction with different targets in the T2T management approach.
Methods
Study design
This was an analysis of cross-sectional data drawn from the Adelphi 2014 RA Disease Specific Programme (DSP), a point-in-time survey of rheumatologists and their patients with RA. The DSP aims to provide impartial observations of real-world clinical practice from a physician and matched patient viewpoint without consideration of current guidelines, but with the intention of providing a view on contemporary RA management. The survey was conducted in France, Germany, Italy, Spain, and the UK from January to August 2014. Patients provided written informed consent for use of their anonymized and aggregated data for research and publication in scientific journals. For each patient meeting the eligibility criteria, the physicians filled out a patient record form (PRF), which included questions on demographics, symptoms, past and current treatments, compliance, and general patient management. Data were collected in such a way that patients and physicians could not be identified directly; all data were aggregated and de-identified before receipt. Data collection was undertaken in line with the European Pharmaceutical Marketing Research Association (EPhMRA) guidelines and as such it did not require ethics committee approval.Citation15 The survey was performed in full accordance with relevant legislation at the time of data collection, including the Health Insurance Portability and Accountability Act,Citation16 a cornerstone of the EPhMRA guidelines relating to data privacy.
Local fieldwork teams identified and invited rheumatologists to participate in the study upon fulfillment of the inclusion criteria listed subsequently. Compensation was set according to time spent on the study at fair market rates. Each rheumatologist recruited the first eight consecutive patients who consulted them and satisfied the inclusion criteria.
For the analysis of bDMARD use, the clinical and disease characteristics and satisfaction level of patients with moderate-to-severe RA were compared among patients who received a bDMARD vs those who did not (non-bDMARD). For the assessment of adoption of a T2T approach/strategy, as this approach is particularly recommended during the early phase of the disease, the patients were subdivided into two subsets: RA diagnosis <2 years and RA diagnosis ≥2 years. Physicians indicated the T2T goal they adopted as either remission (defined as disease activity score in 28 joints [DAS28]<2.6), LDA (DAS28 <3.2), or other target. Patients were subsequently compared according to the T2T approach used: no target (patients treated without a T2T approach), pragmatic (T2T target set as LDA or something other than remission), or aspirational (T2T goal set as remission).
Physicians and patients
Only rheumatologists who qualified between 1975 and 2010 and who were actively involved in RA management were included in the DSP. In addition, rheumatologists were required to manage more than eight patients with RA in a calendar month. Patients with RA were eligible for inclusion if they were ≥18 years of age and were not involved in clinical trials for RA. In order to be considered eligible for bDMARD therapy, patients had to have received at least one csDMARD before their current treatment, have moderate-to-severe RA at the initiation of their current treatment (as reported by the physician), and remain on that current treatment for ≥6 months to determine effectiveness. In addition, these patients had to have consulted a physician who was authorized to initiate bDMARDs. Assessment of the severity of RA (mild, moderate, or severe) was based on the physician’s own perception of the disease status. For the assessment of the adoption of a T2T strategy, patients with an unknown time since RA diagnosis were excluded.
Statistical analyses
Mean and standard deviation (SD) for numerical outcomes and frequency and percentage of patients within each response for nominal outcomes were reported. Statistical differences were assessed using Mann–Whitney U or Kruskal–Wallis test for numeric outcomes, and chi-squared or Fisher’s exact test for nominal outcomes.
Missing data were not imputed. Any patients with missing values for a particular variable were removed for all analyses where that variable was used, but remained eligible for inclusion in other analyses.
Results
Overall, 307 rheumatologists provided data on a total of 2,536 patients from across Europe (France: n=502; Germany: n=491; Italy: n=501; Spain: n=486; and UK: n=556). The mean (SD) age of the patients was 52.76 (14.32) years and the mean (SD) time since diagnosis of RA was 6.05 (6.81) years (). The majority (71%) of the patients were women, and 63% of the patients had never received bDMARDs. Of the 37% of patients (n=926) who had previously received a bDMARD, 93% of these were currently receiving bDMARD therapy (the remaining 7% had discontinued bDMARD therapy) and 68% had received only one prior bDMARD therapy.
Table 1 Overall patient demographics
For the analysis of bDMARD use, 126 patients were excluded because it could not be determined whether these patients were receiving bDMARD or non-bDMARD treatment at the time of the survey due to missing or contradictory information. Clinical characteristics of the remaining patients are shown in . The patients on bDMARD therapy had a significantly longer time since RA diagnosis compared with the patients who were not on a bDMARD (8.62 vs 6.69 years, respectively; P<0.0001). These patients also had a more severe condition at the initiation of their current treatment as perceived by rheumatologists (52% vs 27% with severe RA, respectively; P<0.0001) and were experiencing more pain at the initiation of their current treatment (mean levels of pain on a 1–10 scale were 7.13 vs 6.44, respectively; P<0.0001; ). The two main reasons why rheumatologists chose to initiate bDMARD therapy in patients receiving bDMARDs were “strong overall efficacy” of bDMARD therapy (71%) and “convincing efficacy shown in clinical trials” (55%; ). When switching between bDMARD therapies occurred, the principal reasons that prompted the patient’s most recent change in bDMARD treatment were “worsening of the condition” (46%) and “loss of response over time” (38%; ). Second-line bDMARD therapies were chosen based on similar reasons as for the first-line bDMARDs, even when switching to a different class of bDMARD. The main reasons that would prompt initiation of a bDMARD in those patients who were still bDMARD-naïve (as stated by the physician) were worsening of the disease (61%) and failure of non-bDMARD therapy (33%) as assessed by the physician (). In 7% of the patients, physicians stated that nothing would prompt bDMARD initiation (ie, bDMARDs were likely contraindicated).
Figure 1 Factors influencing bDMARD treatment start.
Abbreviations: bDMARD, biologic disease-modifying antirheumatic drug; DAS, disease activity score.
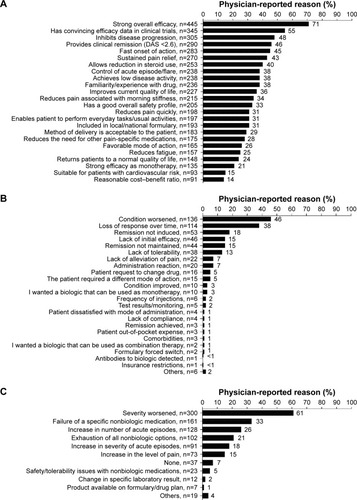
Table 2 Clinical characteristics of patients according to DMARD therapy group
Only patients for whom the time since RA diagnosis was known were included in the assessment of T2T strategy adoption (n=2,381). The baseline characteristics of these patients are shown in . Twenty-four percent of the patients (n=579) had been diagnosed with RA for <2 years and 76% (n=1,802) for ≥2 years. A T2T approach was not adopted (“no target”) in 1,144 patients (regardless of time since RA diagnosis), whereas physicians adopted a pragmatic approach in 395 patients and an aspirational approach in 842 patients. A higher proportion of patients in the RA diagnosis <2 years subset had no target than those in the ≥2 years subset (58% vs 45%, respectively; ), and fewer had a pragmatic one (8% vs 19%, respectively; ). The percentage of patients with an aspirational target was similar in both subsets (34% vs 36%, respectively; ). In the <2 years subset, 7–9% of the patients were currently receiving bDMARDs across all T2T subgroups, whereas in the ≥2 years subset, 38%, 42%, and 52% of the patients received bDMARDs in the no target, pragmatic, and aspirational subgroups, respectively.
Table 3 Baseline characteristics of patients across different T2T subgroups
Physician satisfaction with RA control was lower in the RA diagnosis <2 years subset than in the RA diagnosis ≥2 years subset (65% [377/579] vs 77% [1,387/1,802] satisfied, respectively). In the RA diagnosis <2 years subset, physician-reported satisfaction with RA control differed significantly across T2T subgroups and was highest in the “no target” subgroup (70% vs 64% vs 56% satisfied in no target, pragmatic, and aspirational subgroups, respectively; P=0.0040; ). However, in the RA diagnosis ≥2 years subset, while physician-reported satisfaction with RA control differed significantly across T2T subgroups, it was highest in the aspirational subgroup (79% vs 63% vs 82% satisfied in the no target, pragmatic, and aspirational subgroups, respectively; P<0.0001; ).
Discussion
These data from the Adelphi 2014 RA DSP study provide valuable insights into the factors affecting treatment adjustments in RA management in current European practices. For instance, escalation of treatment from csDMARD to bDMARD seems to be triggered by worsening of the disease rather than high baseline RA activity. Current recommendations in RA management, however, advise that physicians should aim for remission or LDA in order to prevent further damage.Citation2,Citation3 This study suggests that the goals of early intervention and optimal disease control are not satisfactorily achieved in these patients.
A large proportion of patients did not receive any bDMARDs even though they experienced pain and their condition was perceived as “moderate-to-severe” by physicians. The reason for this requires further investigation. When bDMARD therapy was prescribed, first and second bDMARDs were chosen according to the perceived efficacy and safety of the bDMARD derived from clinical trial data and/or the individual physician’s professional experience, which is in agreement with the results from a previous study.Citation17
In our analysis sample, 68% of those patients who had previously received a bDMARD had only ever received one bDMARD therapy (n=926), even if 1,438 patients had moderate-to-severe RA and were considered potentially eligible for bDMARD at the initiation of current treatment. Previous studies have shown that initial treatment with TNF inhibitors can fail due to intolerance, inefficacy, or loss of efficacy, and that prior exposure to TNF inhibitors results in a decline in the proportion of patients responding to subsequent biologic treatment, including a decline of about 10% in ACR categorical response criteria for the same class of bDMARDs.Citation18,Citation19 Such observations may delay a physician’s decision to switch to other bDMARDs. However, switching to another drug of this group may still be beneficial in some patients.Citation18 Indeed, an immediate switch to a second bDMARD within the TNF inhibitor class after failure of the first bDMARD has previously proven to be effective in achieving better disease control in approximately 60% of the nonresponders to first bDMARD.Citation20
In the T2T adoption analysis, a T2T approach was not adopted in almost half of the patients with RA even though previous studies showed that knowledge and implementation of T2T are both high among rheumatologists.Citation21–Citation23 Even when a T2T approach was implemented, it was mostly used in patients with ≥2 years since RA diagnosis rather than in those with early disease (ie, RA diagnosis <2 years). However, a T2T approach is particularly recommended during the early phase of the disease when RA is not yet established and optimum disease control is more readily achieved, thereby preventing joint damage. The reasons for poor adoption of T2T recommendations in 2014Citation24 can only be speculative because this specific question was not investigated. However, potential reasons include physician and/or patient dissatisfaction with therapeutic targets that concentrate solely on the attainment of disease activity states as recently published.Citation25 Clinical time constraints precluding the ability to book frequent patient follow-ups that assess components of composite DASs can also contribute.
In terms of RA control, physicians’ satisfaction was highest in patients who had been diagnosed with RA ≥2 years compared with those with RA diagnosis <2 years. In the RA diagnosis ≥2 years subset, the use of bDMARDs was also higher than in the RA diagnosis <2 years subgroup. This suggests that the lower satisfaction with outcome in early disease may be associated with delayed use of bDMARDs, although other factors could play a role as well.
In terms of limitations, this study was conducted in selected European countries and the results may not be an accurate reflection of other international practices. Furthermore, the sample may not be entirely representative of the practicing population of rheumatologists because the physicians participating in the DSP were those who met minimum workload criteria (regarding the number of RA patients they treat) for recruitment purposes. A certain degree of bias is expected because assessment of current disease activity relies on the physicians’ accuracy in interpreting and reporting information. Therefore, patients of physicians who took part in this study may have characteristics that differ from those of physicians who declined to participate, thereby reducing the generalizability of the findings.
Conclusion
This cross-sectional analysis shows that the use of bDMARDs is suboptimal in patients with RA and that a T2T approach is not universally adopted.
Author contributions
ES and JP contributed to study concept, design, data collection, and interpretation. RW contributed to study concept and design, provided statistical support, and contributed to data interpretation. PCT, RA, JJGR, RC, and PB contributed to interpretation of data for the work. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We wish to thank all patients and rheumatologists who participated in the survey. Medical writing support was provided by Sabrina Giavara, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Disclosure
PCT has received fees from AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Merck, Pfizer, Sandoz, Biogen, and UCB Pharma. RA has received fees from AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Merck, Pfizer, Sandoz, Biogen, and UCB Pharma. JJGR has received fees from AbbVie, Biogen, Bristol-Myers Squibb, Hospira, Janssen, Merck, Pfizer, Regeneron, and UCB Pharma. RC has received fees from AbbVie, MSD, Pfizer, Roche, and UCB Pharma. PB has received fees from MSD, Pfizer, Reckitt Benckiser, and Roche. ES, RW, and JP are employees of Adelphi Real World and were contracted by Pfizer to provide data, input into design of data collection, and statistical support for the development of this study. This study was spon sored by Pfizer. Adelphi Real World was contracted by Pfizer to conduct the survey and provide data, input into design of data collection, and statistical support for the development of this study. Preliminary data from this study were presented as posters at the British Society for Rheumatology (BSR) Annual Meeting, April 26–28, 2016, Glasgow, Scotland, and at the Annual Scientific Meeting of the American College of Rheumatology/Association of Rheumatology Health Professionals (ACR/ARHP); November 6–11, 2015; San Francisco, CA, USA. The authors report no other conflicts of interest in this work.
References
- SmolenJSAletahaDMcinnesIBRheumatoid arthritisLancet2016388100552023203827156434
- SinghJASaagKGBridgesSL2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid ArthritisArthritis Care Res2016681125
- SmolenJSLandewéRBijlsmaJEULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 updateAnn Rheum Dis201776696097728264816
- EspinozaFFabreSPersYMRemission-induction therapies for early rheumatoid arthritis: evidence to date and clinical implicationsTher Adv Musculoskelet Dis20168410711827493689
- MontiSMontecuccoCBugattiSCaporaliRRheumatoid arthritis treatment: the earlier the better to prevent joint damageRMD Open20151Suppl 1e00005726557378
- SmolenJSBreedveldFCBurmesterGRTreating rheumatoid arthritis to target: 2014 update of the recommendations of an international task forceAnn Rheum Dis201675131525969430
- SmolenJSTreat-to-target as an approach in inflammatory arthritisCurr Opin Rheumatol201628329730227027815
- SmolenJSTreat-to-target: rationale and strategiesClin Exp Rheumatol2012304 Suppl 73S2S623073266
- RidkerPMMoving toward new statin guidelines in a post-JUPITER world: principles to considerCurr Atheroscler Rep200911424925619500487
- Diabetes Control and Complications Trial Research GroupEffect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research GroupJ Pediatr199412521771888040759
- GrigorCCapellHStirlingAEffect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trialLancet2004364943026326915262104
- KnevelRSchoelsMHuizingaTWCurrent evidence for a strategic approach to the management of rheumatoid arthritis with disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritisAnn Rheum Dis201069698799420448280
- BakkerMFJacobsJWWelsingPMLow-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trialAnn Intern Med2012156532933922393128
- TugnetNPearceFTosounidouSTo what extent is NICE guidance on the management of rheumatoid arthritis in adults being implemented in clinical practice? A regional surveyClin Med20131314246
- European Pharmaceutical Market Research Association (EphMRA) [webpage on the Internet]Code of Conduct2017 Available from: http://www.ephmra.org/Code-of-Conduct-SupportAccessed June 28, 2017
- GottliebAKormanNJGordonKBGuidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologicsJ Am Acad Dermatol200858585186418423261
- GreenappleRTrends in biologic therapies for rheumatoid arthritis: results from a survey of payers and providersAm Health Drug Benefits201252839224991313
- LloydSBujkiewiczSWailooAJSuttonAJScottDThe effectiveness of anti-TNF-alpha therapies when used sequentially in rheumatoid arthritis patients: a systematic review and meta-analysisRheumatology201049122313232120566736
- Rendas-BaumRWallensteinGVKonczTEvaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-α inhibitorsArthritis Res Ther2011131R2521324169
- SmolenJSBurmesterGRCombeBHead-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE studyLancet2016388100612763277427863807
- CaporaliRContiFCovelliMTreating rheumatoid arthritis to target: an Italian rheumatologists’ survey on the acceptance of the treat-to-target recommendationsClin Exp Rheumatol201432447147624960620
- HaraouiBBensenWBessetteLLe ClercqSThorneCWadeJTreating rheumatoid arthritis to target: a Canadian physician surveyJ Rheumatol201239594995322467920
- HetlandMLJensenDVKroghNSMonitoring patients with rheumatoid arthritis in routine care: experiences from a treat-to-target strategy using the DANBIO registryClin Exp Rheumatol2014325 Suppl 85S141S14624528956
- SmolenJSLandewéRBreedveldFCEULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 updateAnn Rheum Dis201473349250924161836
- TaylorPCAltenRGomez-ReinoJJClinical characteristics and patient-reported outcomes in patients with inadequately controlled rheumatoid arthritis despite ongoing treatmentRMD Open201841e00061529593881
