Abstract
Background
Long-term effectiveness is an important factor when considering treatment decisions.
Objective
To determine the long-term retention patterns of Canadian inflammatory bowel disease (IBD) and rheumatologic disease (RD) patients, including rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, treated with innovator infliximab (IFX) and to assess the impact of year-over-year cumulative IFX exposure on retention in both patient populations.
Patients and methods
This analysis used a Canadian longitudinal prescription claims database to measure retention on IFX over a period of 5 years. Twelve-month unadjusted odds ratios of retention by time on IFX were calculated for the overall cohort, and within-group comparisons evaluated differences according to age, sex, region, insurance coverage, use of concomitant immunosuppressant therapy, indication (RD cohort only), and previous biologic experience. Between-group analyses compared unadjusted 5-year retention among the same variables. Variables that were independently associated with longer retention on IFX were identified using multivariable regression.
Results
Seven thousand eight hundred and six IBD patients and 2,935 RD patients on stable treatment with IFX were included in the analysis. Sixty-nine percent of IBD patients and 66% of RD patients were retained on IFX after 1 year and 33% and 29%, respectively, were retained after 5 years. Moreover, the probability of being retained on IFX significantly increased with cumulative time on IFX. Independent predictors of 5-year retention included sex, region, and type of insurance coverage among IBD patients and region, type of insurance, prior biologic therapy, and specific indication among RD patients. Patients with IBD were 17% more likely to be retained on IFX over 5 years compared to patients with RD.
Conclusion
Real-world Canadian IBD and RD patients on IFX have good overall long-term treatment retention. Previous duration of IFX treatment predicts better future retention, and this knowledge could help inform treatment decisions when patients have been stable on IFX treatment for varying periods of time.
Introduction
Inflammatory bowel disease (IBD) and rheumatic disease (RD) are chronic disorders characterized by inflammation in the gastrointestinal tract and the joints or other musculoskeletal tissues, respectively.Citation1–Citation3 Treatment goals for both IBD and RD include relief of symptoms, resolution of inflammation, and prevention of disease progression.Citation1–Citation5 Tumor necrosis factor-alpha (TNF-α) inhibitors have demonstrated efficacy and safety for the treatment of both IBD and RD. Infliximab (IFX; Remicade®; Janssen Inc., Toronto, ON, Canada) was one of the first TNF-α inhibitors approved in Canada for the treatment of IBD and RD.
Long-term therapy is critical for successful management of chronic diseases such as IBD and RD.Citation1,Citation4,Citation6 Despite being an effective treatment for most, a high percentage of patients treated with TNF-α inhibitors discontinue therapy, mainly due to perceived lack of efficacy or concern for adverse effects.Citation6,Citation7 Given the chronic nature of IBD and RD, knowledge of the real-world, long-term effectiveness of various treatments is important when considering a management plan. A surrogate measure of long-term effectiveness and tolerability is drug retention (drug survival) or the length of time a patient remains on a drug.Citation8
The primary objective of this analysis was to examine the long-term retention of Canadian IBD and RD patients who had been on stable treatment with IFX for at least 1 year. Additional objectives were to examine the cumulative impact of IFX exposure on retention, to assess differences in retention patterns among various subpopulations of IBD and RD patients, and to identify factors that were independently associated with long-term retention on IFX.
Patients and methods
Sample selection
A population cohort study using the IQVIA™ RxDynamics® (Kirkland, QC, Canada) database was conducted to determine the retention patterns of IBD and RD patients taking IFX. This dataset collects information on over 10 million Canadians with >100 million annual prescriptions from the private sector, as well as patient-level data from two public drug plans (Ontario and Quebec).Citation9 In accordance with our institutional policies, ethics approval and informed consent were not required since this is a prescription claims-level study using anonymized data.
Patients with IBD and RD were chosen for this analysis because these reflect the label indications for IFX in Canada; although IFX is also approved for the treatment of plaque psoriasis, these patients were excluded from the analysis because of the small sample size.
The retention analysis included IBD and RD patients with 1) an initial IFX claim during the selection period of Jan 2008–May 2015; 2) no IFX claims in the 12 months prior to the first IFX claim during the selection period (index claim); 3) ≥1 claim for any drug other than IFX in the 12 months prior to the index claim; and 4) ≥1 drug claim in the 4 months following the last IFX claim or after May 2015. A 1-year look back allowed for the identification of biologic-experienced and biologic-naïve patients. The 4-month look-forward step confirmed that each patient remained active in the database and/or discontinued IFX rather than leaving the database for other possible reasons such as death or change in insurance provider. The IBD and RD indications were inferred based on the specialty of the prescribing physician and the patient’s medication history. For the RD cohort, specific disease indications were deduced according to prescribing patterns, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), or ankylosing spondylitis (AS). Unfortunately, this approach could not distinguish between Crohn’s disease (CD) and ulcerative colitis (UC). The exclusion criteria included 1) no information on age or region; 2) insufficient plan activity; 3) previous exposure to IFX; 4) pharmacy claims with drug cost $0 or units=0; 5) indications other than IBD or RD; and 6) cash-only payment.
Measures
Retention was defined as the time between the first and last observed IFX claims for each patient and was reported at 12-month intervals. Retention results were only reported for patients with sufficient claim history (eg, retention at 3 years included only patients with ≥3 years of claim history). The overall retention for the total IBD and RD cohorts was determined over a 5-year period, and retention based on cumulative IFX exposure was determined year over year.
Statistical analysis
Unadjusted odds ratios were calculated and pairwise comparisons conducted at the 95% CI based on cumulative time on IFX (). Within-group analyses examined 12-month retention by number of years on IFX and compared sub-populations of patients according to age (ie, 0–18, 19–64, or 65+ years), sex (ie, male or female), biologic status (ie, naïve or experienced), use of concomitant therapy (ie, methotrexate, azathioprine, 6-mercaptopurine, or none for the IBD cohort and methotrexate, azathioprine, cyclosporine, leflunomide, sulfasalazine, hydroxychloroquine, prednisone, or none for the RD cohort), geographic region (ie, Western Canada, Ontario, Quebec, or Eastern Canada), insurance type (ie, public or private), and, for the RD cohort only, the specific disease indication (ie, RA, PsA, or AS).
Between-group analyses compared unadjusted 5-year retention among subgroups defined by variables outlined above. Multivariable regression analysis using a binomial model was used to identify independent predictors of longer retention on IFX after controlling for covariates of interest. A separate multivariable regression analysis was conducted to examine differences in 5-year retention on IFX between patients with IBD and those with RD.
For all comparisons, a P-value of <0.05 was considered statistically significant. SAS software version 9.3 was utilized for all analyses (SAS Institute Inc., Cary, NC, USA).
Results
A total of 7,806 patients with IBD and 2,935 patients with RD (n=2,420 RA [82%], n=243 PsA [8%], and n=272 AS [9%]) were included in the analysis. The majority of patients were aged 19–64 years, biologic naïve, privately insured, and residing in Ontario (). The RD cohort had more females and more patients on concomitant therapy, when compared to the IBD cohort.
Table 1 Patient baseline characteristics in the overall IBD cohort (n=7,806) and RD cohort (n=2,935)
After 1 year, 69% of patients with IBD and 66% of those with RD remained on IFX (). Retention rates at 5 years were 33% and 29%, respectively (). Within-group comparisons showed that the probability of being retained on IFX in successive 12-month periods increased significantly (). Patients on IFX for 2–5 years showed significantly higher retention in the subsequent 12 months than patients on IFX for only 1 year (P<0.05 for each comparison), with the highest odds of being retained demonstrated in year 5 compared to year 1. Similar trends were observed across both sexes, among patients aged 19–64 years, in both publicly and privately insured patients, those residing in Ontario, biologic-naïve patients, patients who were treated with or without concomitant immunomodulators, and in patients with RD ( and ).
Figure 1 Overall 5-year retention of IBD and RD patients on IFX.
Abbreviations: IBD, inflammatory bowel disease; IFX, infliximab; RD, rheumatologic disease.
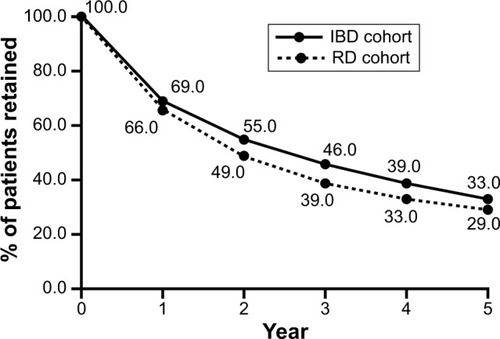
Table 2 Unadjusted OR of being retained based on cumulative IFX exposure in the overall IBD and RD cohorts
All covariates of interest, such as region, biologic status, type of insurance, and indication (RD cohort only), significantly influenced the relative risk (RR) of being retained on IFX for 5 years (). Specifically, the probability of being retained on IFX over 5 years was significantly higher among male IBD patients compared to females (RR=1.18, P=0.0022) and among those residing in Ontario (RR=1.16, P=0.0353) and Eastern Canada (RR=1.36, P=0.005) compared to those in Quebec (). Conversely, the probability of being retained on IFX over 5 years was significantly lower in IBD patients who were reimbursed by private vs public insurance plans (RR=0.68, P<0.0001). In the RD cohort (), the RR of being retained on IFX over 5 years was significantly lower for biologic-experienced patients (RR=0.65, P=0.0001) and privately insured patients (RR=0.57, P<0.0001), whereas it was significantly higher for patients residing in Eastern Canada (RR=1.72, P=0.0015) vs Quebec and for those with AS (RR=1.38, P=0.026) or PsA (RR=1.80, P<0.0001) vs RA.
Figure 2 Relative risk of being retained on IFX at 5 years after controlling for variables of interest in (A) the overall IBD and RD cohort and (B) RD vs IBD patients.
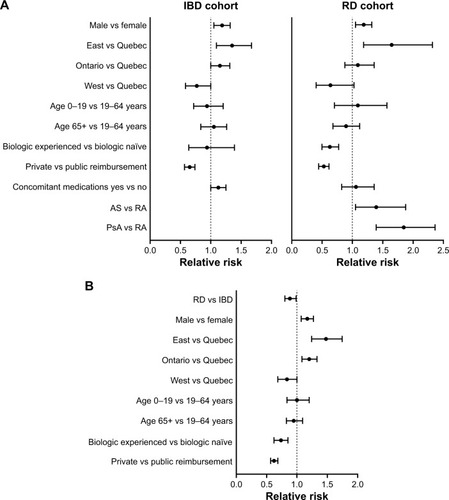
Comparing across indications, patients with IBD were 17% more likely to be retained on IFX over 5 years than patients with RD (RR=1.17, P=0.0039; ). Variables that independently influenced the likelihood of being retained on IFX for 5 years in IBD vs RD patients included sex, region, biologic status, and type of insurance. Specifically, males were 17% more likely to be retained (RR=1.17, P=0.0005), patients residing in Ontario and Eastern Canada were 14% and 44% more likely to be retained, respectively, whereas those residing in Western Canada were 22% less likely to be retained (RR=1.14, P=0.023; RR=1.44, P<0.0001; and RR=0.78, P=0.014, respectively), biologic-experienced patients were 28% less likely to be retained (RR=0.72, P=0.0008), and patients reimbursed by private insurance plans were 35% less likely to be retained (RR=0.65, P<0.0001) on IFX over 5 years compared to patients with RD.
Discussion
This is the first study to examine the long-term retention of a large Canadian cohort in patients with IBD or RD who are on stable IFX treatment in a real-world setting. Using pharmacy-level claims data, the overall retention was approximately two thirds after 1 year of treatment and one third after 5 years. Retention increased with each successive year on treatment. These results suggest that physicians can expect a high rate of retention following the first year of IFX treatment, although the potential contribution of habit strength and sampling bias to these observations cannot be excluded. This is a clinically important observation since continued long-term maintenance therapy in randomized controlled trials has been associated with sustained remission in both IBDCitation1,Citation2 and RD.Citation6
While this is the first study to examine IFX retention rates in a real-world Canadian population, studies published on overall IFX retention in other patient cohorts have reported similar results. Choi et al reported that 58% of Korean patients with CD (n=317) were retained on IFX after 1 year.Citation10 In a Belgian study of 547 patients with IBD (CD, UC, or indeterminate colitis), 63.4% were retained on IFX after 55 months of treatment.Citation11 When initial non-responders were included in the analysis (n=614), retention was 56.5%.Citation11 In a 5-year retrospective study of French CD patients (n=350) treated with either IFX or another TNF-α inhibitor, adalimumab (ADA), as monotherapy, a retention rate of 90.6% was reported after 1 year and 57.9% after 5 years.Citation12 A single-center study conducted in Spain reported 1-year retention rates of 69% and 45% at 5 years in 130 patients with CD treated with ADA (58%) and IFX (42%), respectively; the corresponding retention rates in the smaller subset of patients with UC (n=30) were 48% and 15%, respectively.Citation7
In the RD literature, several studies have compared retention of RD patients on IFX and other available biologics (most commonly, other TNF-α inhibitors such as etanercept [ETA] and ADA).Citation13–Citation16 Favalli et al report a 55% retention rate after 8 years in Italian patients being treated for AS and PsA, with no significant difference in retention among IFX, ADA, and ETA.Citation13 In a small French study, Soubrier et al reported similar retention between IFX and ADA in PsA patients after 5 years (67.2% vs 71.2%, respectively), but a significantly lower retention on ETA (30.8%).Citation14 In the Swedish Biologics Register (ARTIS), the 5-year retention rates of RA patients newly initiated on biologic therapy were 38% for IFX, 50% for ADA, and 55% for ETA.Citation15 In the DANBIO registry, the reported 1-year retention rate for patients who were switched from innovator IFX to a biosimilar (CT-P13) was 84.1% after adjusting for age, sex, diagnosis, use of concomitant methotrexate, presence of comorbidities, and patient’s global score.Citation16 This was similar to the crude and unadjusted 1-year retention rate of 86.2% (P=0.22) from a historical DANBIO cohort of RD patients who had been on innovator IFX for a mean of 6.8 years. The adjusted absolute retention rates were 83.4% for the switch cohort and 86.8% for the historical IFX cohort. The RR of withdrawal was significantly higher for the cohort that switched to CT-P13 than for the historical innovator IFX cohort (hazard ratio 1.31 [95% CI 1.02–1.68]; P=0.03). These observations are of particular interest given the recent introduction of biosimilar IFX to the Canadian market and the interest in nonmedical switching policies based on the experience of some European jurisdictions.Citation17 There are no data at this time on the Canadian long-term retention patterns of patients treated with biosimilar IFX. Moreover, there is some evidence to suggest that the structure and services of patient support programs, which differ among molecules and also between innovator and biosimilar variants, has an impact on patient adherence and persistence.Citation18 Overall, given the high year-over-year retention reported herein, treatment stability on IFX seems to be an important factor to consider before changing biologic treatment for nonmedical reasons.
Several interesting relationships emerged when subpopulations were examined in a multivariable regression analysis. Males with IBD were 18% more likely to be retained on IFX over 5 years than females (RR=1.18, P=0.0022). This may potentially reflect a tendency for young women on IFX to discontinue treatment for reasons of family planning or pregnancy, which reflects the IFX recommendation not to be used during pregnancy and for 6 months after the last IFX dose.Citation19 Further supporting this notion, a survey conducted among 183 Canadian physicians reported that a substantial number would feel uncomfortable recommending the continuation of TNF-α therapy during pregnancy (44% for IFX, 22% for ADA).Citation20
Patients residing in Quebec were less likely to be retained on IFX over 5 years than those in other parts of Canada. Specifically, IBD patients in Eastern Canada and Ontario were 16% and 36% more likely, respectively, to be retained on IFX over 5 years than those in Quebec, and patients with RD in Eastern Canada were 72% more likely to be retained on IFX vs those in Quebec. Since the majority of patients receiving IFX in Canada are infused in clinics that are part of the manufacturer’s infusion network, such differences are unlikely to be related to infusion clinic factors. Variances in regional practices such as time of onset of prescribing a biologic therapy and utilization of dose optimization strategies, as well as regional differences in access to drug and specialized care, might account for the observed differences in retention across Canada. Geographic differences in retention rates could also be confounded by provincial variations in drug reimbursement and access. Indeed, regional differences in retention rates related to practice patterns and access to care have been reported in patients with RD receiving TNF-α inhibitors.Citation16
Privately insured patients were less likely to be retained on IFX over 5 years than publicly insured patients. This could reflect the availability of fewer publicly funded biologic options and more stringent public payer criteria for biologics, which would reduce the chance of switching to an alternative biologic agent compared to private payer patients. This may also be related to differences in the populations covered by the different types of insurance plans. In Canada, private plans generally cover a younger, healthier, working-age population, whereas public plans cover older (65+) and lower-income patients. It is plausible that patient characteristics that were unable to be captured through claims data may differ between publicly and privately insured patient populations and may, therefore, contribute to the observed differences in retention. Socioeconomic status has previously been shown to significantly impact drug retention in patients with RA.Citation21 A recent analysis of IBD patients treated with TNF-α inhibitors in Ontario reported that patients with public insurance had longer wait time to initiation of therapy and worse outcomes (eg, more hospitalizations and emergency visits) than those who were privately insured, even after adjusting for confounders including socioeconomic factors, and disease severity, type, and phenotype.Citation22 This adds further support to the possibility that factors that were not captured in the IQVIA database may be influencing this result.
Surprisingly, there was no difference in retention between biologic-naïve vs biologic-experienced IBD patients, whereas in the RD cohort, there was a 35% higher probability of being retained on IFX over 5 years in biologic-naïve patients compared with patients previously exposed to biologic therapies (RR=0.65, P=0.0001). This suggests that the real-world effectiveness of a first biologic is higher than that of subsequent biologics in patients with RD. There is evidence that response rates in RD patients who remain on their initial TNF-α inhibitor are higher than in those switching to a second or third biologic.Citation23,Citation24
Differences in retention by indication emerged in this analysis. Using a multivariable regression model, RD patients were 17% less likely to be retained on IFX over 5 years compared to IBD patients. Even within the RD cohort, patients with AS and PsA were 38% and 80%, respectively, more likely to be retained on IFX over 5 years than patients with RA. These differences could be explained by the more limited number of treatment options available for the management of IBD, AS, and PsA, compared to RA. Moreover, differences in practice patterns and physician behavior could contribute to the differential retention rates across indications. It is conceivable, though untested, that gastroenterologists may be more reluctant to switch biologics than rheumatologists or dermatologists, and they may make more effort to optimize treatment on an initial TNF-α inhibitor before switching therapies, given the limited menu of treatment options in IBD. Since these differences in practice patterns and patient characteristics could not be controlled for in this prescription claims analysis, future studies examining the impact of region, physician behavior, and insurance coverage on retention in Canadian IBD patients are warranted.
There are several limitations to this study. First, there is inherent bias in using pharmacy claims data for estimation of retention, as not all patients are captured in the analysis (eg, patients who change plans, move, or have treatment interruptions). Second, the dataset does not inform on reasons for treatment discontinuation. Subpopulations by specific diagnoses were only possible for the RD cohort, and these were estimated based on participants’ overall prescription history and not on diagnostic codes. Additionally, information on disease severity and/or comorbidities is not available. The 12-month look-back period for study inclusion might have been insufficient to capture all biologic-experienced patients in the IQVIA database. Finally, pharmacy claims studies are retrospective.
There are also several strengths to this study. First, this is a large national study with prescription data from a robust database that included thousands of patients tracked over a period of several years from both community and academic centers. This allowed analysis of important subpopulations, such as geographic location and with type of insurance coverage. The 4-month follow-up period ensured that retention reflected true discontinuation of treatment, while the 12-month look-back period allows for the comparison of biologic-naïve vs biologic-experienced patients. Despite the retrospective nature of the study, the data provide useful information on what clinicians can expect in terms of long-term retention with IFX in real-world clinical practice and the predictive aspect of cumulative IFX exposure on retention rates. Finally, the overall results reported herein for patients with IBD and RD are consistent with other real-world datasets.Citation7,Citation10,Citation25
Conclusion
Real-world Canadian IBD and RD patients treated with IFX have good year-over-year retention, and longer IFX treatment appears to predict better future retention. The results were robust and consistent across various subpopulations of IBD and RD patients. This current analysis provides a Canadian benchmark of retention that can be expected for IBD and RD patients treated with IFX and offers physicians reassurance that patients are more likely to remain on IFX the longer they have been on treatment. This knowledge could help inform treatment decisions when patients have been stable on IFX treatment for varying periods of time.
Author contributions
All authors met the ICMJE criteria and all those who fulfilled these criteria are listed as authors. PAB, GA, MK, JKM, RP, and JW contributed to research design, data interpretation, revising the article critically, and approving the final version of the manuscript. EME and AMM contributed to research design, data collection and interpretation, revising the article critically, and approving the final version of the manuscript. All authors had full access to data while writing the paper. All authors accept accountability for all aspects of the work including accuracy and integrity.
Acknowledgments
This paper was presented as a poster presentation with interim findings at the following meetings: the European League Against Rheumatism (EULAR) annual meeting in London, England in June 2016 (poster abstract published in Ann Rheum Dis 2016;75(Suppl 2):496–497 and available online at: http://ard.bmj.com/content/75/Suppl_2/496.2); the Canadian Rheumatology Association (CRA) annual meeting in Ottawa, Canada in February 2017 (poster abstract available online at: https://rheum.ca/images/documents/2017_Poster_Presentations_for_JRheum.pdf); the European Crohn’s and Colitis Organization (ECCO) annual meeting in Barcelona, Spain in February 2017 (poster abstract published in J Crohns Colitis 2017;11(Suppl 1) and available online at: https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2017/item/p383-increasing-treatment-time-on-remicadex00ae-infliximab-predicts-subsequent-long-term-retention-in-stable-infliximab-inflammatory-bowel-disease-patients-in-canada-2.html?highlight=WyJtYXJzaGFsbCJd); and the Canadian Digestive Diseases Week (CDDW) annual conference in Banff, Canada in March 2017 (poster abstract available online at: https://www.cag-acg.org/images/cddw/cddw2017_exhibitabstractbook_FINAL.pdf). The authors wish to acknowledge the support of Shoghag Khoudigian-Sinani and Driss Oraichi from IQVIA™ for their support in the design and analysis of this study, as well as Ardeane Healthcare Solutions and medical writer, Christina Clark, for their assistance in the preparation of this paper.
Supplementary materials
Figure S1 Derivation of pair-wise comparisons.
Notes: The equations used to conduct the pair-wise comparisons. Equation 1 was used to calculate the OR. Equation 2 was used to calculate SE which was used in Equation 3 to calculate the 95% CI of the estimated OR. “a” is the number of patients who were retained in the subsequent 12 months, “c” is the number of patients who were retained in the reference year, “b” the number of patients who were not retained in the subsequent 12 months, and “d” is the number of patients who were not retained in the reference year.
Abbreviations: OR, odds ratio; SE, standard error.
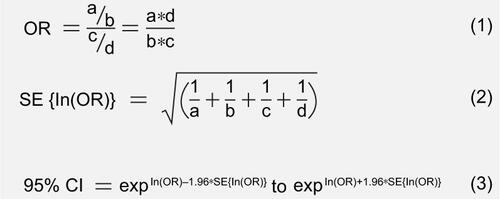
Table S1 Unadjusted OR of being retained based on cumulative IFX exposure in subpopulations of IBD patients (OR [95% CI])
Table S2 Unadjusted OR of being retained based on cumulative IFX exposure in subpopulations of RD patients (OR [95% CI])
Disclosure
This study was funded in full by Janssen Inc. Writing support was provided by Ardeane Healthcare Solutions and funded by Janssen Inc. Philip Baer has received consultant and speaking fees of <$10,000 from Amgen, Novartis, AbbVie, Johnson & Johnson, Takeda, LifeLabs, Paladin, Sanofi-Genzyme, SOBI, Merck, and Roche; consultant fees of >$10,000 from Eli Lilly; and consultant and speaking fees of >$10,000 from Janssen and Pfizer. Guy Aumais has received consultancy/advisory fees from Janssen, AbbVie, Takeda, Shire, Ferring, and Allergan and has received research funding from Pfizer, Janssen, Genentech, Celgene, and Shire. Emanuel M Ewara and A Marilise Marrache are employees of Janssen Inc. Majed Khraishi has received consultant or speaking fees and/or honoraria of <$10,000 from Janssen, Pfizer, and AbbVie. Remo Panaccione has received consultancy and/or speaker and/or advisory fees from AbbVie, Abbott, Amgen, Aptalis, AstraZeneca, Baxter, BMS, Celgene, Centocor, Cubist, Elan, Eisai, Ferring, Gilead, GlaxoSmithKline, Genentech, Janssen, Merck, Pfizer, Prometheus, Robarts, Salix, Samsung, Schering-Plough, Shire, Takeda, and UCB and research funding from AbbVie, Abbott, Ferring, Janssen, and Takeda. John Wade has received consultancies, speaking fees, and/or honoraria of <$10,000 from Amgen, Abbvie, Janssen, Novartis, Roche, BMS, Sanofi, and Celgene. John K Marshall has received speaking and/or consultancy fees from AbbVie, Allergan, AstraZeneca, Boehringer-Ingelheim, Celgene, Celltrion, Ferring, Hospira, Janssen, Lilly, Merck, Pfizer, Pharmascience, Procter & Gamble, Shire, and Takeda. The authors report no other conflicts of interest in this work.
References
- SmolenJSAletahaDMcInnesIBRheumatoid arthritisLancet2016388100552023203827156434
- BraunJSieperJAnkylosing spondylitisLancet200736995701379139017448825
- AnandarajahAPRitchlinCTThe diagnosis and treatment of early psoriatic arthritisNat Rev Rheumatol200951163464119806150
- BresslerBMarshallJKBernsteinCNClinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensusGastroenterology201514851035105825747596
- GomollónFDignassAAnneseV3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical managementJ Crohns Colitis201711132527660341
- BluettJMorganCThurstonLImpact of inadequate adherence on response to subcutaneously administered anti-tumour necrosis factor drugs: results from the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate cohortRheumatology201554349449925213131
- GisbertJPArredondoMChaparroMDrug survival and reasons for discontinuation of anti-TNF therapy in inflammatory bowel disease (IBD) in clinical practicePoster presented at: European Crohn’s and Colitis Organisation Annual Meeting2014Copenhagen, Denmark
- CramerJARoyABurrellAMedication compliance and persistence: terminology and definitionsValue Health2008111444718237359
- IQVIA: Private DrugPlans (PDP), Ontario Public Drug Programs (OPDP), Régie de l’assurance maladiedu Québec (RAMQ) Available from: www.iqvia.comAccessed December 16, 2016
- ChoiCHSongIDKimYHEfficacy and safety of infliximab therapy and predictors of response in Korean patients with Crohn’s disease: a nationwide, multicenter studyYonsei Med J20165761376138527593865
- SchnitzlerFFidderHFerranteMMucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s diseaseInflamm Bowel Dis20091591295130119340881
- Peyrin-BirouletLSalleronJFilippiJAnti-TNF monotherapy for Crohn’s disease: a 13-year multicentre experienceJ Crohns Colitis201610551652426802084
- FavalliEGSelmiCBeccioliniAEight-year retention rate of first-line tumor necrosis factor inhibitors in spondyloarthritis: a multicenter retrospective analysisArthritis Care Res2017696867874
- SoubrierMPereiraBFrayssacTPsoriatic arthritis treated by anti-TNFs: a monocentric trial of 102 cases in Auvergne, FranceClin Exp Rheumatol20163461059106427607233
- NeoviusMArkemaEVOlssonHDrug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximabAnn Rheum Dis201574235436024285495
- GlintborgBSørensenIJLoftAGA nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registryAnn Rheum Dis20177681426143128473425
- BrodszkyVRenczFPéntekMBajiPLakatosPLGulácsiLA budget impact model for biosimilar infliximab in Crohn’s disease in Bulgaria, the Czech Republic, Hungary, Poland, Romania, and SlovakiaExpert Rev Pharmacoecon Outcomes Res201616111912526162458
- MarshallJKBessetteLThorneCImpact of the adalimumab patient support program’s care coach calls on persistence and adherence in Canada: an observational retrospective cohort studyClin Ther201840341542929519714
- Remicade® (infliximab) [product monograph]TorontoON: Janssen Inc2017
- HuangVWChangHJKroekerKIManagement of inflammatory bowel disease during pregnancy and breastfeeding varies widely: a need for further educationCan J Gastroenterol Hepatol20162016619327527725926
- FinckhANetoDIannoneFThe impact of patient heterogeneity and socioeconomic factors on abatacept retention in rheumatoid arthritis across nine European countriesRMD Open201511e00004026509062
- RummanACandiaRSamJJPublic versus private drug insurance and outcomes of patients requiring biologic therapies for inflammatory bowel diseaseCan J Gastroenterol Hepatol20172017736593728239601
- GlintborgBOstergaardMKroghNSClinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor α inhibitor therapy: results from the Danish Nationwide DANBIO RegistryArthritis Rheum20136551213122323460467
- FagerliKMLieEvan der HeijdeDSwitching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD studyAnn Rheum Dis201372111840184423562987
- StarrMKeystoneEFaraawiRPredictors of treatment retention among patients with rheumatoid arthritis or ankylosing spondylitis treated with Remicade® (infliximab) for long-term in Canadian real-worldPoster presented at: American College of Rheumatology Annual MeetingNovember 11–16; 2016Washington, DC
