Abstract
Purpose
Depression is a widespread mental disorder which can be treated effectively. However, low adherence to antidepressants is very common. The study of medication adherence in depression (MAPDep study) assesses the effectiveness and cost-effectiveness of a multicomponent strategy to enhance adherence toward medications in patients with depression.
Intervention
The intervention is a multicomponent one consisting of an educational program for psychiatrists and/or a collaborative care program for patients and relatives, plus a reminder system that works through the use of an already available high-quality medication reminder application.
Study design
MAPDep study is an open, multicenter, four-arm cluster randomized controlled trial. The clusters are mental health units where psychiatrists are invited to participate. The clusters are randomly allocated to one of the three interventions or to usual care (control arm). Patients (18–65 years of age) diagnosed with depressive disorder, those taking antidepressant medication for an existing diagnosis of depression, and mobile phone users are selected. In group 1, only patients and relatives receive intervention; in group 2, only psychiatrists receive intervention; and in group 3, patients/relatives and psychiatrists receive intervention. The primary outcome is adherence to the antidepressant drug. The calculated sample size is 400 patients. To examine changes across time, generalized linear mixed model with repeated measures will be used. A cost-effectiveness analysis will be conducted. The effectiveness measure is quality-adjusted life years. Deterministic sensitivity analyses are planned.
Conclusion
MAPDep study aims to assess a multicomponent strategy to improve adherence toward medications in patients with depression, based not only on clinical effectiveness but also on cost-effectiveness. This methodology will enhance the transferability of the expected results beyond mental health services (patients and psychiatrists) to health care policy decision making.
Clinical trial identifier
NCT03668457.
Introduction
Depression is a common mental disorder. Globally, more than 300 million people of all ages suffer from depression.Citation1–Citation3 It is expected that by 2030, depression will be the leading cause of disease burden globally.Citation4
Depressive disorders account for 6% of total disease burden in terms of disability-adjusted life years. In Europe, the total annual cost of depression has been estimated at Euro 118 billion, including direct and indirect costs, which corresponds to a cost of Euro 253 per inhabitant and a 1% of European gross domestic product.Citation5 The percentage of the total economy of Spain is similar to that generally observed in Europe.Citation6
Depression is often a chronic and/or recurrent disorder with consequences over the entire life span.Citation7 At least half of those who recover from a first episode of depression will experience additional episodes, and approximately 80% of those with a history of two episodes will have another recurrence.Citation8 Depression may become a serious health condition and is the leading cause of suicide. Approximately 800,000 people commit suicide each year due to depression.Citation9
Despite that there are effective pharmacological treatments for depression, nonadherence to appropriately prescribed medications compromises the effectiveness of available treatments and interferes with recovery.Citation10,Citation11 Although the rates of early adherence to antidepressant medication have been estimated ranging from 72% to 78%,Citation12 approximately 50% of patients prematurely discontinue antidepressant therapy.Citation13–Citation16 Despite the fact that 49%–84% of the patients with depression perceive the need for antidepressant treatment,Citation17 one-third of patients stop medication within 6 weeks, and up to 55%, at 10–12 weeks.Citation18 Moreover, the degree of nonadherence may vary according to the severity of the disease and the evolutionary moment. Adherence is greater in patients with more severe symptoms.Citation19 In the maintenance phase of treatment, clinical improvement and previously acceptable adverse effects, such as sexual dysfunction, may reduce adherence. Precisely, reduced perceived effectiveness and increased perceived adverse effects are related to higher nonadherence rates.Citation17
Nonadherence to antidepressants has an impact on health care utilization and charges. Nonadherence is associated with relapse and recurrence, emergency department visits, and higher hospitalization rates.Citation20 Therefore, there is a need for effective and cost-effective interventions to improve adherence.
There are many approaches to address adherence issues in patients with depression, such as collaborative care,Citation21–Citation33 counseling,Citation34,Citation35 cognitive-behavioral approaches,Citation36–Citation43 psycho-education,Citation37,Citation44–Citation48 support,Citation34,Citation35,Citation49–Citation54 coaching,Citation55,Citation56 and shared decision-making skills training.Citation57,Citation58 However, these approaches are only effective to improve short-term adherence but insufficient to appreciate long-term effect.
Multifaceted interventions directed to the patient and physician are comparatively more effective in improving medication adherence than interventions with a single component (such as information or education).Citation59–Citation61 Intervention programs should attend to patients’ specific health beliefs and attitudes concerning their condition and antidepressant treatment,Citation62 and patients’ perception of control over health (psychological reactance and health locus of control).Citation10,Citation11 It appears useful and important that mental health professionals improve communication and negotiation skills to overcome communication barriers with patients: doctors’ interventions which can cause nonadherence and hostile attitudes in the patients toward changes.Citation63,Citation64 Moreover, the complexity of adherence phenomenon requires multifaceted interventions that can be reinforced by the use of information and communication technology.Citation65–Citation68 In this sense, health applications (apps), including medication reminder apps, are becoming more and more popular and are promising tools to improve medication adherence and decrease the costs of traditional interventions on adherence.Citation69–Citation72
There is a need for cost-effective alternative strategies involving patients, relatives, and physicians that improve short- and long-term adherence to depression treatment. With this purpose, the Adherence Improvement in Patients with Depression study (Mejora de la Adherencia en Pacientes con Depresión, MAPDep) is designed. The purpose of MAP-Dep is to assess the effectiveness and cost-effectiveness of a multicomponent strategy to enhance adherence toward medications in patients with depression.
Methods
Trial design
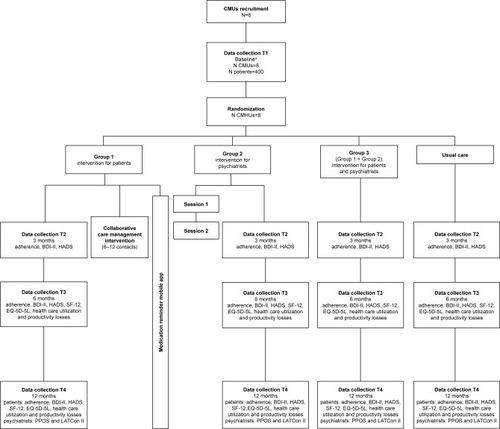
MAPDep study is an open, multicenter, four-arm cluster randomized controlled trial comparing three interventions and usual care (control arm). In group 1, only patients and relatives receive intervention; in group 2, only psychiatrists receive intervention; and in group 3, patients and psychiatrists receive intervention. In the control group, patients/relatives and psychiatrists receive the usual care provided by the Canary Islands Health Service ().
Figure 1 Flowchart of MAPDep study procedures.
Abbreviations: BDI-II, Beck Depression Inventory-II; BMQ, Beliefs about Medicines Questionnaire; CMHU, Community Mental Health Unit; CPS, Control Preferences Scale; DAI-10, Drug Attitude Inventory – 10 Items; EQ-5D-5L, EuroQol-5D-5L; HADS, Hospital Anxiety and Depression Scale; HPRS, Hong Psychological Reactance Scale; LATCon II, Leeds Attitude Towards Concordance II Scale; MHLC-C, Multidimensional Health Locus of Control, Form C; PPOS, Patient-Practitioner Orientation Scale; SF-12, Short Form-12.
Subjects
Mental health professionals
The unit of recruitment for psychiatrists is the Community Mental Health Unit (CMHU). Psychiatrists working in the selected CMHUs who volunteer to participate in the study and have the intention to stay in their CMHU during the follow-up period will be included after signing informed consent.
Patients
Inclusion criteria are as follows: (1) patients diagnosed with depressive disorder (major depressive disorder and/or dysthymia) according to the criteria of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, taking antidepressant medication for an existing diagnosis of depression and (2) aged 18–65 years; (3) mobile phone users; (4) patients who have visited their CMHU at least once in the last 6 months; and (5) consent to participate in the study. Exclusion criteria are as follows: (1) patients with bipolar disorder and/or any psychotic disorder; (2) insufficient Spanish language skills; (3) pregnancy; and (4) participation in another experimental study.
Setting and recruitment
The setting is urban or rural CMHUs located on Tenerife (Canary Islands, Spain). From the total pool of CMHUs, eight will be randomly selected using computer-generated randomization numbers. We will have an initial contact with psychiatrists working on each selected CMHU by e-mail or telephone to give a brief explanation of the objectives of the study and to request their collaboration. A more detailed explanation of the study (objective, time frame and tasks, expected resources utilization, and funding) will be subsequently provided in a 60- to 80-minute face-to-face or virtual meeting. To minimize information bias and selection bias, all included psychiatrists will complete both an informed consent form and the baseline questionnaire, and then they will consecutively invite patients meeting selection criteria to participate before CMHUs randomization. Accepting patients will sign an informed consent form and will complete the baseline questionnaires in their first visit.
Random assignment
We will perform allocation by clusters–with the CMHUs as the randomization unit. After professionals and patients are selected, an investigator blinded to CMHUs identity will randomly assign participants to interventions or control group.
Blinding
Participating psychiatrists and patients from each selected CMHU will be blind to intervention assignment (groups 1–4) until the last patient is recruited. Psychiatrists and patients cannot be blinded after assignment to interventions or control group. The investigator responsible for data analysis will be blinded to the intervention assignment.
Interventions
The study will evaluate the effectiveness and cost-effectiveness of interventions for psychiatrists and/or patients and relatives (). These interventions are compared with a control group receiving usual care.
Intervention for patients
Groups 1 and 3 receive an intervention combining the following components: 1) a collaborative care management intervention and 2) the use of an already available high-quality medication reminder app.
Collaborative care management intervention
This intervention has been designed in accordance with the Chronic Care Model,Citation73 including depression education, medication management, and behavioral activation.
Patients will receive a set of 6–12 contacts with researchers along a period of no more than 3 months. Patient and relative, if accompanied, will receive the initial face-to-face contact that will last 30–40 minutes. Subsequent telephone sessions with patient will last 15–20 minutes.
Depression education
Its goal is to provide adequate information about depression and antidepressant treatment.
Medication management
Its goal is to support appropriate antidepressant use and reinforce information from the psychiatrists by: 1) assessing patients’ attitudes toward pharmacological treatment of depression, medication-taking behaviors, and symptoms and emotional outcomes; 2) providing education concerning the appropriate use of antidepressants; 3) negotiating shared decisions about the use of antidepressants; and 4) promoting patients’ perception of control over health.
Behavioral activation
This is a brief structured intervention that aims to help people interrupt patterns of avoidance that maintain depression and increase engagement in adaptive activities. It is focused on developing a plan to reestablish daily activities and increase the number of both pleasant activities and positive interactions with their environment.
Supervision
Mental health specialists–psychiatrists and psychological therapists – supervise the researches. Supervisors help and support researchers to review the patient’s progress and the team’s plan for the patient.
Medication reminder mobile app
In the first session, participants will be informed about the use of a medication reminder mobile app. Participants will download the app and enter their prescription data (medication, time of administration, and dose). Participants will be required to use the medication reminder app along the 12-month follow-up.
Intervention for psychiatrists
Participating psychiatrists of groups 2 and 3 receive an educational group program of 4-hour duration consisting in two interactive sessions, 1 month apart. Contents of the first session have been designed to develop skills to promote shared decision making and patient-centered care and to improve communication and negotiation abilities. To deliver this intervention, a set of short video films with role-playing exercises representing different types of complex sham patients will be used.
Contents of the second session have been designed to promote motivational interviewing methods and shared decision making in the context of the patient-centered care model. A mental health professional with expertise in patient-centered care methods and communication skills will lead these sessions.
Usual care
Patients will receive care from their psychiatrists according to usual activities provided by the Canary Islands Health Service, including antidepressant therapy and referral for other treatments. Psychiatrists will not have access to the educational group program.
Outcome measures
Primary outcome
The primary end point is rate of adherence to the antidepressant drug at 6 months assessed using the Sidorkiewicz adherence instrument in Spanish.Citation74,Citation75 This is a five-item instrument with two or three possible answers to assess different medication-taking behaviors for each individual drug taken by patients. The results generate adherence levels ranging from 1 (high drug adherence) to 6 (drug discontinuation). Adherence will also be measured at baseline, at 3 months, and at 12 months ().
Table 1 Outcome measurements
Secondary outcomes
Beck Depression Inventory-II (BDI-II)
This is a 21-item self-report multiple-choice inventory used for measuring the severity of depression. Each item has a four-point (0–3) scale, and the score of scale ranges from 0 to 63.Citation76 Severity score ranges are as follows: 0–13 (minimal depression), 14–19 (mild depression), 20–28 (moderate depression), and 29–63 (severe depression). The BDI-II is a reliable and well-validated measure for screening depression symptoms in adults.Citation76–Citation78 Depression will be measured at baseline, at 3 months, at 6 months, and at 12 months.
Hospital Anxiety and Depression Scale (HADS)
This is a 14-item self-reporting screening scale that contains two seven-item Likert scales, one for anxiety and one for depression. Each item has a four-point (0–3) Likert scale, and the scores of both scales range from 0 to 21. Higher scores indicate greater anxiety and/or depression. HADS is scored by summing the ratings for the 14 items to yield a total score, and by summing the ratings for the seven items of each subscale to yield separate scores for anxiety and depression. HADS has been shown to be a valid and reliable measure.Citation79,Citation80 HADS score will be measured at baseline, at 3 months, at 6 months, and at 12 months.
Short Form-12 (SF-12) health survey
This is a 12-item index designed to examine eight health domains to assess quality of life (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health). Two summary subscales may be derived from the SF-12, including a mental health summary and a physical health summary. Higher scores represent better health status.Citation81 SF-12 is a reliable and well-validated instrument.Citation82 SF-12 will be administered at baseline, at 6 months, and at 12 months.
EuroQol-5D-5L (EQ-5D-5L)
This is a measure of health-related quality of life (HRQoL) comprising five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension is graded on five levels (no problems, slight problems, moderate problems, severe problems, and extreme problems).Citation83 HRQoL will be measured at baseline, at 6 months, and at 12 months.
Patient-Practitioner Orientation Scale (PPOS)
This is an 18-item reliable and validated self-administered tool that assesses patient-centeredness in both health care professionals and patients. Each item is rated on a six-point Likert scale (1–6).Citation84,Citation85 PPOS will be used to assess health care professionals’ attitudes. The cutoffs used to classify professionals according to their patient-centeredness score are: low (≤4.57), medium (>4.57 and <5), or high (≥5).Citation85 PPOS questionnaire will be administered at baseline and at 12 months.
Leeds Attitude Towards Concordance II Scale (LATCon II)
This is a 20-item scale for measuring health care professionals’ attitudes toward concordance in medicine taking. Each item is rated on a six-point Likert scale (0–3). Scores on the LATCon II range from 0 to 60, with higher scores representing more positive attitude toward concordance.Citation86,Citation87 LATCon II will be administered at baseline and at 12 months.
Health care utilization and productivity losses
Costs will be assessed from the health care services perspective, and the costs derived from the development and use of all components for each intervention assessed (sessions, app, etc.) will be included. Information about prescribed medication and doses, contacts with mental health and primary care providers, hospital admissions and duration of stay, and productivity losses will be obtained from a self-administered questionnaire and the electronic clinical record. Collected information will cover the 6-month period prior to the study. Health care utilization and indirect costs will be measured at baseline, at 6 months, and at 12 months.
Additional measures
Sociodemographic and clinical data will be collected at baseline from the patients and the psychiatrists. Professionals will be asked about their age, sex, and professional profile, while patients will be asked about their type of depression, number of prior episodes and the duration of the current episode, sex, age, education level, occupation, marital status, and family living status (alone or accompanied). The prescription date, the quantity of prescribed medication, the dispensation date, and the quantity of dispensed medication will be downloaded from the electronic clinical record.
In addition, the following measures will be collected at baseline from the patients.
Drug Attitude Inventory – 10 Items (DAI-10)
DAI-10, a 10-item self-report scale, assesses psychiatric patients’ attitudes toward their psychopharmacological medications. Response options are true/false, and each response is scored as +1 if correct or −1 if incorrect. The score of the scale ranges from −10 to −10, with positive scores indicating positive attitudes and negative scores indicating negative attitudes toward medication.Citation88,Citation89
Multidimensional Health Locus of Control, Form C (MHLC-C)
MHLC-C is an 18-item self-report scale composed of one scale on internal locus of control (six items), and three scales on external locus of control: Chance (six items), Doctors (three items), and Others (three items); these scales assess patients’ belief in their ability to control health. Patients are asked to rate, on a six-point Likert scale, the degree to which they agree or disagree with each statement. Higher scores on each subscale indicate a stronger belief in that kind of control.Citation90,Citation91
Hong Psychological Reactance Scale (HPRS)
HPRS is a 14-item self-report questionnaire to assess individual differences in reactance proneness, that is, individuals’ trait propensity to experience psychological reactance. Participants indicate the extent to which they endorsed each statement on a five-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree).Citation92,Citation93
Control Preferences Scale (CPS)
CPS consists of five cards that illustrate different roles in decision making using a statement and a cartoon. Patients have to choose between the cards, observing them one at a time, to establish an order of preference that ranges from a completely active role to a more passive style (from 0 to 5; the higher the score, the more passive the style). There are six response categories: active–active, active–collaborative, collaborative–active, collaborative– passive, passive–collaborative, and passive–passive. The responses can be further collapsed into the following three categories of role responses: active (active–active or active–collaborative), collaborative (collaborative–active or collaborative–passive), and passive (passive–collaborative or passive–passive).Citation94,Citation95
Beliefs about Medicines Questionnaire (BMQ)
BMQ is an 18-item questionnaire that contains a specific and a general scale. The BMQ-Specific scale is subdivided into two subscales (Concern and Necessity) that assess representations of medication prescribed for personal use. The BMQ-General scale is subdivided into two subscales (Overuse and Harm) that assess beliefs about medicines in general. Patients are asked to rate, on a five-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree), the degree of agreement with each statement.Citation96,Citation97
Statistical methods
To analyze changes in outcomes over time for the intervention and control groups, generalized linear mixed models with repeated measures will be used. Intervention groups will be treated as a “factor within”. For multiple comparisons, Bonferroni adjustment will be used. In order to incorporate cluster effects across three levels (patients, psychiatrist, and CMHU), a multilevel model approach will be implemented.
A structural equation model will be applied to assess the relationships among sociodemographic and clinical characteristics of patients, interventions, and results in the emotional variables and adherence.
Both per-protocol and intention-to-treat analyses will be performed. Missing data will be classified into the following categories: mistakenly randomized; did not receive allocated intervention; withdrew consent; did not adhere to the protocol; dropped out, crossed-over; or lost to follow-up.Citation98
Economic evaluation
Cost-effectiveness analysis of group 3 (multicomponent intervention) vs the control group (usual care) will be undertaken. The cost-effectiveness measure will be the incremental cost per quality-adjusted life year (QALY) gained. QALYs will be measured by the EQ-5D-5L questionnaire, which will be collected for each individual patient. The analyses will take the perspective of the National Health Service and personal social services; therefore, direct and indirect costs will be included. The direct costs per patient will be calculated based on the use of health care resource utilization, and the indirect costs will be estimated focusing on productivity losses due to the disorder applying the human capital approach. Results will be summarized as the incremental cost-effectiveness ratio (ICER). ICER is the ratio of the differences in costs to the differences in effects observed.
Nonparametric bootstrap methods for calculating confidence regions for the ICERs will be used. The bootstrap replications will also be performed to construct a cost-effectiveness acceptability curve, which will reveal the probability that an intervention is cost-effective compared with the alternative for different values of willingness to pay for certain future QALY gains. We will also subject the results to one-, two-, and multi-way deterministic sensitivity analysis. All analyses will be in line with accepted economic evaluation methods.Citation99
The willingness-to-pay threshold is defined at Euro 25,000/QALY on the basis of the values reported in the latest Spanish literature.Citation100
Sample size
For detecting a difference of at least 20 points in the rate of adherence of patients to the drug assessed by use of the Sidorkiewicz adherence instrument at 6 months, assuming an alpha of 0.05 (two-tailed) with 80% power and an intra-cluster correlation coefficient of 0.01 (IQR 0–0.04) based on literature data, the estimated number of patients required per arm is 91 (total in the study =364 patients). Assuming losses of 10% at 6 months, a total sample of 400 patients is estimated.
Ethics
The Scientific and Ethics Committees of both the University Hospital of the Nuestra Señora de Candelaria and the University Hospital of Canary Islands have approved the study protocol.
The trial will be conducted in accordance with the latest version of the Declaration of Helsinki and the Organic Law 15/1999, of 13 December, on the Protection of Personal Data (LOPD). We will obtain informed consent from all participants of the study.
Trial status
This trial is not yet recruiting.
Discussion
The MAPDep study is a cluster randomized controlled trial involving actors who play a role in decision making in depression management in specialized mental health care (patients, relatives, and psychiatrists). The effectiveness and cost-effectiveness of three interventions promoting collaborative care, patient-centered care, and shared decision making with the aim of improving adherence to antidepressants among patients with depression against usual care will be assessed.
MAPDep offer tools for depressive patients and mental health professionals to establish an appropriate engagement with medication. These interventions could therefore help to decrease the risks of disease relapse or recurrence and soften the impact of depression on health care utilization and costs.
Limitations
This study is not free from limitations. Firstly, MAPDep interventions can be blinded neither to patients nor to psychiatrists. However, mental health professionals will be blind to intervention assignment until the last patient is recruited to warrant patients’ and professionals’ cooperation.
Secondly, MAPDep intervention for psychiatrists has been developed to stimulate its uniform implementation through training psychiatrists to develop skills to promote shared decision making and patient-centered care and to improve communication and negotiation abilities. However, psychiatrists may differ in their motivation to include these resources in daily practice and the way in which motivation influences their intervention engagement. On the other hand, this variability can be observed in daily patient care and thereby enhance the external validity. Another limitation is that, despite the information about the use of a medication reminder mobile app, misuse and nonuse of the app may occur. To prevent nonuse, one session will be conducted in a face-to-face manner. Finally, there are concerns about the validity of self-report measures due to their vulnerability to memory biases and social desirability that tend to overestimate adherence behavior compared with other assessment methods.Citation101 However, self-reporting is the most simple and inexpensive method of measuring adherence which can feasibly be used in clinical settings.Citation102
Despite all these limitations, few previous studies have assessed not only the effectiveness but also the cost-effectiveness of multicomponent interventions for all actors involved in decision making in depression management in specialized mental health care through a randomized controlled design.
Conclusion
Depression affects million people around globe, and numerous studies have consistently shown lower adherence toward medications is associated with poor recovery among patients with depression and higher treatment costs per patient. Therefore, it is important to enhance medication adherence to improve patient health and soften the impact of depression on health care utilization and costs. The MAPDep study will show whether a multicomponent strategy to promote collaborative care, patient-centered care, and shared decision making in the management of patients with depression is effective and cost-effective.
Author contributions
All authors contributed to the design of the study. In addition, all authors contributed toward drafting and critically revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgments
This work is supported by the Cabildo de Tenerife within the framework of the Agustín de Betancourt Program and the Institute of Health Carlos III (PI18/00767). The funding institution had no role in designing the study, in drafting, reviewing, and approving the manuscript, or in the decision to submit it for publication.
Disclosure
The authors have no conflict of interest in the subject matter or materials discussed in the manuscript.
References
- AlonsoJLépineJPBrughaSESEMeD/MHEDEA 2000 Scientific CommitteeOverview of key data from the European study of the epidemiology of mental disorders (ESEMeD)J Clin Psychiatry200768Suppl 239
- KesslerRCAguilar-GaxiolaSAlonsoJThe global burden of mental disorders: an update from the WHO World mental health (WMH) surveysEpidemiol Psichiatr Soc2009181233319378696
- KesslerRCBerglundPDemlerOJinRMerikangasKRWaltersEELifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National comorbidity survey replicationArch Gen Psychiatry200562659360215939837
- World Health OrganizationThe Global Burden of Disease: 2004 UpdateGeneva SwitzerlandWorld Health Organization2008
- SobockiPJönssonBAngstJRehnbergCCost of depression in EuropeJ Ment Health Policy Econ200692879817007486
- Parés-BadellOBarbagliaGJerinicPGustavssonASalvador-CarullaLAlonsoJCost of disorders of the brain in SpainPLoS One201498e10457125121608
- HardeveldFSpijkerJDe GraafRNolenWABeekmanATPrevalence and predictors of recurrence of major depressive disorder in the adult populationActa Psychiatr Scand2010122318419120003092
- KupferDJFrankEWamhoffJMood disorders: update on prevention of recurrenceMundtCGoldsteinMJHahlwegKFiedlerPInterpersonal Factors in the Origin and Course of Affective DisordersLondon (UK)Gaskell/Royal College of psychiatrists1996289302
- World Health OrganizationDepression and Other Common, Mental Disorders: Global Health EstimatesGeneva, SwitzerlandWorld Health Organization2017
- De Las CuevasCDe LeonJPeñateWBetancortMFactors influencing adherence to psychopharmacological medications in psychiatric patients: a structural equation modeling approachPatient Prefer Adherence20171168169028405160
- De Las CuevasCPeñateWCabreraCPerceived health control: a promising step forward in our understanding of treatment adherence in psychiatric careJ Clin Psychiatry20167710e1233e123927529827
- SimonGEJohnsonEStewartCDoes patient adherence to antidepressant medication actually vary between physicians?J Clin Psychiatry2018793710
- BambauerKZSoumeraiSBAdamsASZhangFRoss-DegnanDProvider and patient characteristics associated with antidepressant nonadherence: the impact of provider specialtyJ Clin Psychiatry200768686787317592910
- LeeMSLeeHYKangSGVariables influencing antidepressant medication adherence for treating outpatients with depressive disordersJ Affect Disord20101231–321622119914719
- SawadaNUchidaHSuzukiTPersistence and compliance to antidepressant treatment in patients with depression: a chart reviewBMC Psychiatry20099111019133132
- YehMYSungSCYorkerBCSunCCKuoYLPredictors of adherence to an antidepressant medication regimen among patients diagnosed with depression in TaiwanIssues Ment Health Nurs200829770171718592422
- PrinsMAVerhaakPFBensingJMvan der MeerKHealth beliefs and perceived need for mental health care of anxiety and depression – the patients’ perspective exploredClin Psychol Rev20082861038105818420323
- MaddoxJCLeviMThompsonCThe compliance with antidepressants in general practiceJ Psychopharmacol199481485222298480
- van GeffenECHeerdinkERHugtenburgJGSieroFWEgbertsACvan HultenRPatients’ perceptions and illness severity at start of antidepressant treatment in general practiceInt J Pharm Pract201018421722520636673
- HoSCChongHYChaiyakunaprukNTangiisuranBJacobSAClinical and economic impact of non-adherence to antidepressants in major depressive disorder: a systematic reviewJ Affect Disord201619311026748881
- AdlerDABungayKMWilsonIBThe impact of a pharmacist intervention on 6-month outcomes in depressed primary care patientsGen Hosp Psychiatry200426319920915121348
- BungayKMAdlerDARogersWHDescription of a clinical Pharmacist Intervention administered to primary care patients with depressionGen Hosp Psychiatry200426321021815121349
- KatonWCollaborative management to achieve treatment guidelinesJAMA19952731310267897786
- LinEHVon KorffMCiechanowskiPTreatment adjustment and medication adherence for complex patients with diabetes, heart disease, and depression: a randomized controlled trialAnn Fam Med201210161422230825
- RichardsDABowerPChew-GrahamCClinical effectiveness and cost-effectiveness of collaborative care for depression in UK primary Care (CADET): a cluster randomised controlled trialHealth Technol Assess201620141192
- CapocciaKBoudreauDBloughDRandomized trial of pharmacist interventions to improve depression care and outcomes in primary careAm J Heal Pharm200461364372
- DietrichAJOxmanTEWilliamsJWRe-engineering systems for the treatment of depression in primary care: cluster randomised controlled trialBMJ2004329746660260515345600
- FinleyPRRensHRPontJTImpact of a collaborative care model on depression in a primary care setting: a randomized controlled trialPharmacotherapy20032391175118514524649
- FortneyJCPyneJMEdlundMJA randomized trial of telemedicine-based collaborative care for depressionJ Gen Intern Med20072281086109317492326
- GensichenJvon KorffMPeitzMAnnals of internal medicine article case management for depression by health care assistants in smallAnn Intern Med200915136937819755362
- KatonWRobinsonPVon KorffMA multifaceted intervention to improve treatment of depression in primary careArch Gen Psychiatry199653109249328857869
- KatonWRutterCLudmanEJA randomized trial of relapse prevention of depression in primary careArch Gen Psychiatry200158324124711231831
- KatonWvon KorffMLinEStepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trialArch Gen Psychiatry199956121109111510591288
- Al-SaffarNDeshmukhAACarterPAdibSMEffect of information leaflets and counselling on antidepressant adherence: open randomised controlled trial in a psychiatric hospital in KuwaitInt J Pharm Pract2005132123132
- Al-SaffarNAbdulkareemAAbdulhakeemASalahAQHebaMDepressed patients’ preferences for education about medications by pharmacists in KuwaitPatient Educ Couns20087219410118337052
- ArnowBABlaseyCManberRDropouts versus completers among chronically depressed outpatientsJ Affect Disord2007971–319720216857266
- AzocarFBranstromRBUse of depression education materials to improve treatment compliance of primary care patientsJ Behav Health Serv Res200633334735316752111
- HigginsNLivingstonGKatonaCConcordance therapy: an intervention to help older people take antidepressantsJ Affect Disord200481328729115337334
- LinEHvon KorffMLudmanEJEnhancing adherence to prevent depression relapse in primary careGen Hosp Psychiatry200325530331012972220
- PerlisRHNierenbergAAAlpertJEEffects of adding cognitive therapy to fluoxetine dose increase on risk of relapse and residual depressive symptoms in continuation treatment of major depressive disorderJ Clin Psychopharmacol200222547448012352270
- WilesNThomasLAbelAClinical effectiveness and cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: the cobalt randomised controlled trialHealth Technol Assess201418311167
- WilesNThomasLAbelACognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the cobalt randomised controlled trialLancet2013381986437538423219570
- WilesNJHollinghurstSMasonVA randomized controlled trial of cognitive behavioural therapy as an adjunct to pharmacotherapy in primary care based patients with treatment resistant depression: a pilot studyBehav Cogn Psychother200836012133
- MyersEDCalvertEJThe effect of Forewarning on the occurrence of side-effects and discontinuance of medication in patients on dothiepinJ Int Med Res1976442372401026548
- MyersEDCalvertEJInformationCEInformation, compliance and side-effects: a study of patients on antidepressant medicationBr J Clin Pharmacol198417121256691885
- PerahiaDGQuailDGandhiPWalkerDJPevelerRCA randomized, controlled trial of duloxetine alone vs. duloxetine plus a telephone intervention in the treatment of depressionJ Affect Disord20081081–2334117905442
- Rubio-ValeraMMarch PujolMFernándezAEvaluation of a pharmacist intervention on patients initiating pharmacological treatment for depression: a randomized controlled superiority trialEur Neuropsychopharmacol20132391057106623219937
- Rubio-ValeraMPeñarrubia-MaríaMTFernández-VergelRImpacto de Una intervención farmacéutica en La prevención de recaídas en depresión en atención primariaAtención Primaria201648530831526415743
- AkerbladACBengtssonFEkseliusLvon KnorringLEffects of an educational compliance enhancement programme and therapeutic drug monitoring on treatment adherence in depressed patients managed by general practitionersInt Clin Psychopharmacol200318634735414571155
- DattoCJThompsonRHorowitzDDisbotMOslinDWThe pilot study of a telephone disease management program for depressionGen Hosp Psychiatry200325316917712748029
- HunkelerEMMeresmanJFHargreavesWAEfficacy of nurse telehealth care and peer support in augmenting treatment of depression in primary careArch Fam Med20009870070810927707
- KutcherSLeblancJMaclarenCHadravaVA randomized trial of a specific adherence enhancement program in sertraline-treated adults with major depressive disorder in a primary care settingProg Neuropsychopharmacol Biol Psychiatry200226359159611999913
- MundtJCClarkeGNBurroughsDBrennemanDOGriestJHEffectiveness of antidepressant pharmacotherapy: the impact of medication compliance and patient educationDepress Anxiety200113111011233454
- IsaacsAPradeepJShanbagDSelvanSSrinivasanKEnhanced care by community health workers in improving treatment adherence to antidepressant medication in rural women with major depressionIndian J Med Res20034612236245
- BrookOHvan HoutHStalmanWA pharmacy-based coaching program to improve adherence to antidepressant treatment among primary care patientsPsychiatr Serv200556448748915812103
- BrookOvan HoutHNieuwenhuyseHHeerdinkEImpact of coaching by community pharmacists on drug attitude of depressive primary care patients and acceptability to patients; a randomized controlled trialEur Neuropsychopharmacol20031311912480116
- LeblancAHerrinJWilliamsMDShared decision making for antidepressants in primary care: a cluster randomized trialJAMA Intern Med2015175111761177026414670
- AljumahKHassaliMAImpact of pharmacist intervention on adherence and measurable patient outcomes among depressed patients: a randomised controlled studyBMC Psychiatry20151511925609320
- PampallonaSBolliniPTibaldiGKupelnickBMunizzaCPatient adherence in the treatment of depressionBr J Psychiatry20021800210410911823317
- VergouwenACBakkerAKatonWJVerheijTJKoerselmanFImproving adherence to antidepressants: a systematic review of interventionsJ Clin Psychiatry200364121415142014728101
- WilliamsJWGerrityMHolsingerTDobschaSGaynesBDietrichASystematic review of multifaceted interventions to improve depression careGen Hosp Psychiatry20072929111617336659
- De Las CuevasCPeñateWSanzEJRisk factors for non-adherence to antidepressant treatment in patients with mood disordersEur J Clin Pharmacol2014701899824013851
- De Las CuevasCPeñateWSanzEJThe relationship of psychological reactance, health locus of control and sense of self-efficacy with adherence to treatment in psychiatric outpatients with depressionBMC Psychiatry201414132425412702
- BertGRebuilding the relationship between doctor and patientRecent Prog Med20069710548555
- HammondsTRickertKGoldsteinCAdherence to antidepressant medications: a randomized controlled trial of medication reminding in college studentsJ Am Coll Health201563320420825338175
- KrishnaSBorenSABalasEAHealthcare via cell phones: a systematic reviewTelemed J E Health200915323124019382860
- World Health OrganizationAdherence to Long-Term Therapies: Evidence for ActionGeneva, SwitzerlandWorld Health Organization2003
- WeingartenSRHenningJMBadamgaravEInterventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reportsBMJ2002325737092512399340
- MiraJJNavarroIBotellaFA Spanish pillbox APP for elderly patients taking multiple medications: randomized controlled trialJ Med Internet Res2014164e9924705022
- McgillicuddyJWGregoskiMJWeilandAKMobile health medication adherence and blood pressure control in renal transplant recipients: a proof-of-concept randomized controlled trialJ Med Internet Res2013159112
- HarriesTWalking in the wild? Using an always-on smartphone application to increase physical activityIFIP Conference on Human-Computer InteractionBerlin20131936
- DayerLHeldenbrandSAndersonPGubbinsPOMartinBCSmart-phone medication adherence apps: potential benefits to patients and providersJ Am Pharm Assoc2013532172181
- WagnerEHAustinBTDavisCHindmarshMSchaeferJBonomiAImproving chronic illness care: translating evidence into actionHealth Aff20012066478
- De Las CuevasCPeñateWManuel García de CeciliaJde LeonJPredictive validity of the Sidorkiewicz instrument in Spanish: assessing individual drug adherence in psychiatric patientsInt J Clin Health Psychol201818213314230487918
- SidorkiewiczSTranV-TCousynCPerrodeauERavaudPDevelopment and validation of an instrument to assess treatment adherence for each individual drug taken by a patientBMJ Open201665e010510
- BeckASteerRBrownGBeck Depression Inventory–Second Edition ManualSan Antonio, TXThe Psychological Corporation1996
- SanzJAlPVázquezCAdaptación española del Inventario para La Depresión de Beck-II (BDI-II): 2. Propiedades psicométricas en población GeneralClin y Salud2003143249280
- PenleyJAWiebeJSNwosuAPsychometric properties of the Spanish Beck depression Inventory-II in a medical samplePsychol Assess200315456957714692850
- ZigmondASSnaithRPThe hospital anxiety and depression scaleActa Psychiatr Scand19836763613706880820
- HerreroMJBlanchJPeriJMde PabloJPintorLBulbenaAA validation study of the hospital anxiety and depression scale (HADS) in a Spanish populationGeneral Hospital Psychiatry200325427728312850660
- WareJKosinskiMKellerSDA 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validityMed Care19963432202338628042
- GandekBWareJEAaronsonNKCross-validation of item selection and scoring for the SF-12 health survey in nine countriesJ Clin Epidemiol19985111117111789817135
- HerdmanMGudexCLloydADevelopment and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L)Qual Life Res201120101727173621479777
- ShawWSWoiszwilloMJKrupatEFurther validation of the Patient-Practitioner orientation scale (PPOs) from recorded visits for back painPatient Educ Couns201289228829122954491
- KrupatEYeagerCMPutnamSPatient role orientations, doctor-patient fit, and visit satisfactionPsychol Health2000155707719
- KnappPRaynorDKThistlethwaiteJEJonesMBA questionnaire to measure health practitioners’ attitudes to partnership in medicine taking: LATCon IIHeal Expect2009122175186
- De Las CuevasCRivero-SantanaAPerestelo-PerezLMental health professionals’ attitudes to partnership in medicine taking: a validation study of the Leeds attitude to concordance scale IIPharmacoepidemiol Drug Saf201221212312921956875
- Robles GarcíaRSalazar AlvaradoVPáez AgrazFRamírez BarretoFAssessment of drug attitudes in patients with schizophrenia: psychometric properties of the DAI Spanish versionActas Esp Psiquiatr2004323138142 Spanish15168263
- HoganTPAwadAGEastwoodRA self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validityPsychol Med19831311771836133297
- De Las CuevasCPeñateWBetancortMCabreraCWhat do psychiatric patients believe regarding where control over their illness lies? validation of the multidimensional health locus of control scale in psychiatric outpatient careJ Nerv Ment Dis20152032818625594790
- WallstonKASteinMJSmithCAForm C of the MHLC scales: a condition-specific measure of locus of controlJ Pers Assess19946335345537844739
- HongS-MFaeddaSRefinement of the Hong psychological Reactance scaleEduc Psychol Meas1996561173182
- De Las CuevasCPeñateWBetancortMde RiveraLPsychological reactance in psychiatric patients: examining the dimensionality and correlates of the Hong psychological Reactance scale in a large clinical samplePers Individ Dif2014708591
- DegnerLSloanJVenkateshPThe control preference scaleCan J Nurs Res19972932143
- De Las CuevasCPeñateWValidity of the control preferences scale in patients with emotional disordersPatient Prefer Adherence2016102351235627895470
- HorneRWeinmanJHankinsMThe beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medicationPsychol Health1999141124
- De Las CuevasCRivero-SantanaAPerestelo-PerezLGonzalez-LorenzoMPerez-RamosJSanzEJAdaptation and validation study of the beliefs about medicines questionnaire in psychiatric outpatients in a community mental health settingHum Psychopharmacol Clin Exp2011262140146
- AklEABrielMYouJJLost to follow-up information in trials (LOST-IT): a protocol on the potential impactTrials20091014019519891
- BriggsAClaxtonKSculpherMDecision Modelling for Health Economic EvaluationNew YorkOxford University Press2006
- Vallejo-TorresLGarcía-LorenzoBSerrano-AguilarPEstimating a cost-effectiveness threshold for the Spanish NHSHealth Econ201827474676129282798
- StirrattMJDunbar-JacobJCraneHMSelf-report measures of medication adherence behavior: recommendations on optimal useTransl Behav Med20155447048226622919
- GarfieldSCliffordSEliassonLBarberNWillsonASuitability of measures of self-reported medication adherence for routine clinical use: a systematic reviewBMC Med Res Methodol201111114922050830
