Abstract
Background
In Spain the price of all ibuprofen 600 mg tablet generic products is the same due to reimbursement existing rules so for the patient there is not any economic incentive to choose a particular one. Bearing in mind that the quality of generic products should be similar, it could be questioned if differences in patient preferences evaluated as sales could be related to differences on their pharmaceutical properties. The aims of this work were to study the variability on the pharmaceutical characteristics of marketed bioequivalent tablet formulations and its impact on patient preferences.
Methods
Thirty-six batches corresponding to fourteen different generic products were chosen among the best-selling products of the Spanish market in the years 2011 and 2015 and were compared to the reference product. The effect on patient preferences of six variables was studied through a multivariate analysis. The first two variables were marketing characteristics: 1) years in the market and 2) the number of other generic products marketed by the same manufacturer, which could be related to the size and service provided by the manufacturer. The other four variables studied were pharmaceutical tablet properties: 3) mean weight, 4) hardness, 5) disintegration, and 6) dissolution. A multiple linear regression analysis was performed to identify the effect on sales of the six variables studied.
Results
The disintegration time was the most significant (P=0.018) factor affecting the sales of Ibuprofen tablets which may be related to the onset of action.
Conclusion
The faster the tablet disintegration, the higher its sales. Two possible explanations are suggested: 1) the most specialized ibuprofen tablet manufacturer considers fast disintegration as a key parameter and/or 2) habitual consumers of ibuprofen can detect small differences on the onset of action among different marketed formulations. In this work, all marketed ibuprofen tablets comply with the pharmacopoeia specifications.
Introduction
A medicinal product is approved by regulatory authorities such as European Medicines Agency (EMA) or Food and Drug Administration (FDA) when it complies the required in vitro and in vivo assays. Generic medicinal products were defined by EMA as “a product which has the same qualitative and quantitative composition in active substances and the same pharmaceutical form as the reference medicinal product, and whose bioequivalence with the reference medicinal product has been demonstrated by appropriate bioavailability studies”.Citation1 In Spain, the reference product for ibuprofen 600 mg tablet is Neobrufen® 600 mg tablet, commercialized by Abbott. All marketed generic tablets of ibuprofen 600 mg have demonstrated its bioequivalence with this reference product. This bioequivalence has to be proven based on three pharmacokinetic parameters: area under drug concentration vs time curve, maximum drug concentration obtained (Cmax) and time after administration when the Cmax is reached (Tmax). Amongst these parameters, Tmax is the only one directly related to the absorption rate and onset of action. Tmax is the pharmacokinetic parameter with the highest in vivo variability, and a statistical evaluation of Tmax is generally not required for bioequivalence acceptance.Citation1 This general rule is not applicable for ibuprofen formulations because the absorption rate is clinically relevant and important for its onset of action.Citation2 For this reason, Tmax has to be also evaluated in the bioequivalence studies for ibuprofen, although bioequivalence has been reported in formulations with significant Tmax values differences.Citation3 In particular, in Spain the median Tmax should not differ by more than 30 minutes with that of Neobrufen®, as it is expected to observe Tmax in the same or adjacent sampling times. In addition, chiral bioanalytical methods have been recently required to prove bioequivalence of the active S-enantiomer of ibuprofen,Citation4–Citation6 but the first ibuprofen generics were authorized with achiral methods. Based on these bioequivalence requirements, significant differences in the onset of action should not be expected among the different ibuprofen generics in the Spanish market. However, some small changes on formulations of marketed products can be sometimes approved by authorities based on simple in vitro assays (eg, in vitro dissolution tests) and without new bioequivalence studies.Citation2
Due to its low solubility, ibuprofen has been classified as a class II drug in the Biopharmaceutics Classification System (BCS). Moreover, Potthast et alCitation7 proposed ibuprofen as a candidate for BCS biowaivers because it is highly permeable, ie, it is rapidly absorbed as soon as it starts dissolving, which allows the subsequent dissolution of more drug, although its solubility is low at acid pH.Citation8 Theoretically, if dissolution of ibuprofen is the limiting stage for absorption, in vitro comparative dissolution tests should be able to predict its absorption. The problem is that conventional in vitro dissolution tests at pH 1.2, 4.5 and 6.8, at 50 or 75 rpm, have proven not to be good predictors of the in vivo Cmax and/or rate of absorption of ibuprofen.Citation2
In Spain, the standard dose for ibuprofen is 600 mg. Due to the high proportion of the active ingredient, the dilution effect from direct compression excipients is limited, being the drug more affected by process parameters. Due to ibuprofen’s low melting point, the drug can melt during compression resulting in stickiness of the melt ibuprofen to the punches. To avoid this, lubricant agents are frequently added, resulting in an increase of the formulation hydrophobicity. Moreover, the mixture of lubricants with ibuprofen can lead to the formation of eutectic mixtures with even lower melting points.Citation9 Other point to consider is that according to the USP Pharmacopeia the standard dissolution medium used routinely for quality control and batch release of ibuprofen tablets has a pH of 7.2.Citation10 This pH is perfect for the dissolution of an acid drug such as ibuprofen, but its ability to predict in vivo oral behavior of ibuprofen is doubtful. In fact, the FDA Guidance for In Vitro – In Vivo Correlation (IVIVC) studies recommends not to use media with a pH more than 6.8.Citation11
Generics are by definition similar to the reference product, but not the same. Therefore, the small differences that may exist between generics and innovator and also among the different generic products might be detected clinically, eg, the onset of action.Citation12
The objective of this work is to investigate the effect on patient preferences evaluated as sales of different marketing characteristics and physicochemical pharmaceutical variables related to the quality of the tablets. The marketing characteristics are as follows: 1) marketing age and 2) number of other generic products provided by the same manufacturer. The pharmaceutical variables are as follows: 1) mean tablet weight, 2) hardness of the tablet, 3) disintegration time and 4) percentage of ibuprofen dissolved after 5 minutes (Q5).
Material and methods
Marketed products
The following ibuprofen 600 mg film-coated tablet formulations were purchased from Spanish pharmacies: Neobrufen®, Kern Pharma, Cinfa, Teva, Pensa, Normon, Ratiopharm, Mylan, Stada, Alter, Bexal, Sandoz, Aristo, Zentiva, Almus and Apotex. These formulations were codified as A for the reference product and from B to N for the other generic formulations. Details of the batches evaluated and their expiration dates are reported in and corresponding to 2011 and 2015, respectively. The addition of an apostrophe after the letter was used to differentiate formulations from 2015 to 2011. All experimental work was performed before expiration dates of the tablets.
Table 1 Characteristics of the ibuprofen film-coated tablets marketed in 2011
Table 2 Characteristics of the ibuprofen film-coated tablets marketed in 2015
Sales
Sales data expressed as number of packages of 40 tablets sold of the different formulations of 600 mg film-coated tablets marketed in Spain were taken from IMS Health Inc in 2011 and 2015.
Marketing age (years)
The marketing age of each formulation was evaluated and expressed as the number of years passed after the approval of the formulation by the Spanish Agency for Medicines and Health Care Products. These data were obtained from the public database CIMA.Citation13
Number of other generics formulations commercialized by the same company
This number was obtained from the Catálogo de Medicamentos in 2011 and 2015.Citation14,Citation15
Mean weight
Six tablets of each batch were individually weighted.
Hardness
Resistance to crushing of tablets was tested according to European PharmacopoeiaCitation16 using a Pharma Test PTB 311 (Germany). At least ten tablets from each batch were individually evaluated and the mean, minimum and maximum values of the forces in Newtons (N) were reported.
Disintegration
Disintegration of the tablets was tested according to test A of the European PharmacopoeiaCitation16 using a Pharma Test PTZ-1 (Germany). The disintegration was studied in groups of six tablets using purified water at 37°C ± 1°C. Standard discs were placed above the tablets in the tubes, and the time (in minutes) to disintegrate the last tablet was recorded as group’s disintegration time.Citation16
Dissolution
Dissolution studies were performed with apparatus II (paddle) according to the USP specifications for ibuprofen tablets.Citation10 The dissolution characteristics of at least 12 tablets of each batch were studied at pH 7.2 in phosphate buffer at 37°C ± 0.5°C. The dissolution tests were performed in an Erweka DT80 (Heusenstamm, Germany) at a rotating speed of 50 rpm. Samples (5 mL) were withdrawn at different sampling times (0, 5, 10, 15, 20, 30, 45 and 60 minutes) without reposition. Samples were filtered immediately after sampling with a 0.45 µm filter (hydrophilic PVDF Millex-HV, Millipore, Billerica, MA, USA). Ibuprofen was measured by UV spectrophotometry at 266 nm in a Beckman Coulter Du-6 spectrophotometer (Brea, CA, USA). The initial release rate was estimated as the ibuprofen (% of theoretical dose) released during the first 5 minutes (Q5). This parameter was used to compare differences on the release between the formulations.
Statistical data analysis
The statistical data analysis was performed using The Unscrambler® X software (CAMO Software, Oslo, Norway). Seven variables (sales, disintegration time, percentage of dissolution at 5 minutes, tablet weight, hardness, marketing age and number of other ibuprofen generics from the same company in the market) were analyzed by principal component analysis (PCA) and multiple linear regression (MLR) using multiway ANOVA. PCA was employed to study systematic variability and the relationships between variables and scores (ibuprofen products). The correlation loadings of the principal components (PCs) were represented to understand the variance for each variable for a given PC, giving information about the source of the variability inside the dataset.Citation17 MLR was used to assess the impact of the variables on sales and dissolution (Q5).
Results
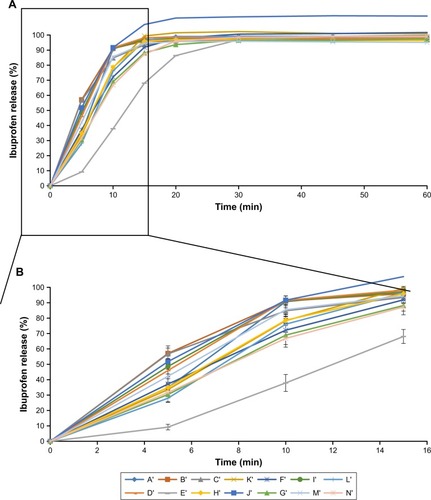
and summarize the sales, marketing age of the products, number of other ibuprofen generics marketed by the same manufacturer and the pharmaceutical properties of the different ibuprofen products in 2011 and 2015. In vitro differences among the marketed formulations are clearly observed in and . All the tablets are white and they have an oblong shape. and show the mean and SD of dissolution results of the products in 2011 and 2015, respectively. According to USP,Citation10 the specification of dissolution for ibuprofen tablets implies that more than 80% of the dose is released at 60 minutes. All tested formulations complied with the USP specifications. Actually, more than 80% of the ibuprofen was already dissolved after 15 minutes in all studied formulations with only one exception. and clearly show that the differences in drug release occur mainly during those first 15 minutes of the test.
Figure 1 Percentage released of ibuprofen (mean and SD) for 60 minutes (A) and the first 15 minutes (B) from 12 marketed formulations in 2011.
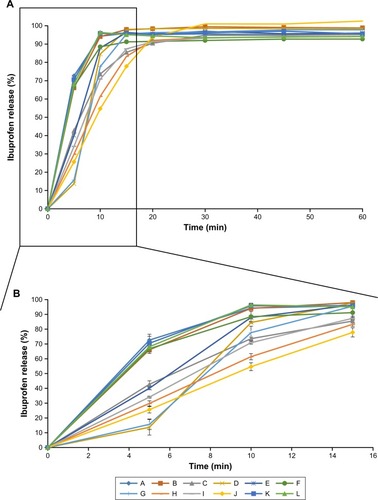
Figure 2 Percentage released of ibuprofen (mean and SD) for 60 minutes (A) and the first 15 minutes (B) from 14 marketed formulations in 2015.
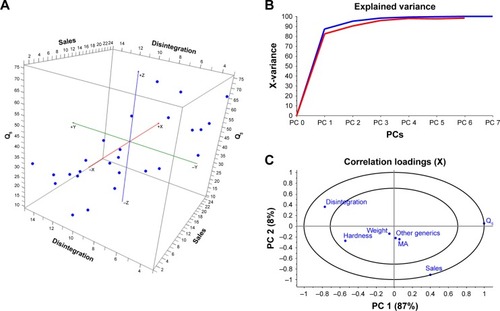
Statistical analysis was performed to understand the relationship among the different variables in 2011 and 2015. The 3D scatter plot showed a clear correlation among sales, disintegration time and Q5 in both years (). This information was also obtained from the results of PCA analysis (). Principal component 1 (PC1) explained 87% of the variability among the different ibuprofen products, while PC2 explained 8%. From the correlation loading graph, it can be inferred that disintegration time and dissolution (Q5) are opposite along the PC1, indicating that lower disintegration time is related to higher dissolution values. Also, a correlation between disintegration and dissolution vs sales was clearly observed. On the other hand, other parameters related to marketing such as marketing age and number of other ibuprofen generics from the same company in the market did not show a strong correlation with number of sales.
Figure 3 Multivariate analysis of marketing data and pharmaceutical properties from ibuprofen film-coated tablets formulations in 2011 and 2015.
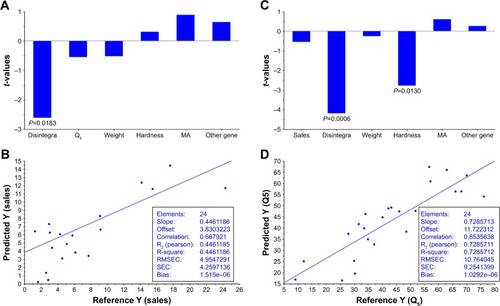
MLR analysis was used to assess the impact of each variable on sales and Q5 (). Interestingly, only the disintegration variable was found to be statistically significant (P=0.018) for sales. The faster the disintegration time, the higher the sales. Other factors did not show statistical significance (P>0.1) with number of sales.
Figure 4 Multiple linear regression (MLR) of marketing data and pharmaceutical properties of products of ibuprofen 600 mg film-coated tablets in 2011 and 2015.
Discussion
In 2010, in Spain 69% of the drugs were financially covered by the government and ibuprofen was the fourth more prescribed drug.Citation18 Ibuprofen 600 mg was the most commonly used self-administered analgesic, even though the 400 mg dose is preferable to avoid side effects.
In a previous work reported by Halme et al,Citation19 approximately half of the consumers were strongly price sensitive when buying one particular over-the-counter analgesic medicine than other. However, the price of the innovator and all generic ibuprofen tablet formulations is the same in the Spanish market. Therefore, ibuprofen sales are not affected by price in Spain. One factor to bear in mind is that pharmacists may sell one brand or another based on promotions and offers from the pharmaceutical companies. Nevertheless, the price of each 40-tablet package is only 1.97 euros. It is reasonable to assume that with such a low price, the marketing promotion and incentives are quite restricted because of the reduced profit left to the pharmaceutical companies. This implies that factors different from marketing promotion might be related to the percentage of market share. Interestingly, the innovator is located at the third position in the sales list in both 2011 and 2015. Considering the ratio between the consumption of generic and the reference 600 mg ibuprofen tablet as an indicator of the quality of the healthcare system, the Spanish market is a mature systemCitation20 and data of ibuprofen sales are in accordance with the increased popularity of generics in Spain as reported by Costa-Font et al.Citation21
Ibuprofen tablets with the highest percentage of sales in Spain were included in this work (IMS, 2011 and 2015); 97% of the overall sales relates to 12 products in 2011 and 91% with 14 products in 2015 indicating a diversification of the generic market. Moreover, these data and the general decrease of percentage of sales of the same companies marketing both in 2011 and 2015 illustrate how tough is the business competition for this product.
In 2011, 22.18 million packages of ibuprofen 40 tablets were sold in Spain, while in 2015 the sales were 20.86 million packages. Related to the changes in the marketing pattern between 2011 and 2015, four products changed its composition, another one decreased its sales and three new products were incorporated in the top list in sales. Therefore, half of the most sold products in 2015 were different from those in 2011. These data illustrates the dynamic current market. It is important to note that some of the studied products were licensed from an initial same product. So, one point to consider is how different are the studied products. In this work, we have taken them into account as different products because, although they have the same initial quantitative composition, small differences in type of excipient, raw material or manufacturing conditions may have an impact on the pharmaceutical characteristics. Moreover, if they are sold by different companies, the marketing strategies could be different. The qualitative composition of the products was obtained through the publicly online available data listed as technical data from CIMA.Citation13 Some of the excipients employed in the manufacturing process can have a key role in the in vivo behavior of the product. For instance, the innovator product is the only one that contains sodium lauryl sulfate in its composition.
Differences in product composition can be related to the differences in pharmaceutical properties. Interestingly, the statistical study shows that disintegration is the most important factor related to the sales. One possible explanation about the relevance of disintegration can be attributed to its relationship with in vivo absorption and onset of action. Important differences in the onset of analgesia have been previously reported for different ibuprofen products.Citation22–Citation24 The analgesic effect of ibuprofen products has been applied recently to study the impact of brand and generic labelingCitation25 and the perception of efficacy of ibuprofen generic medicines.Citation26 Nevertheless, for a BCS class II drug whose absorption is limited by its aqueous solubility, the dissolution variable (Q5) should be also related to the sales even better than disintegration. However, the P-value was far from being significant (P=0.59), which is not surprising as it is known that these dissolution tests conditions of ibuprofen products are not able to predict their in vivo performance.Citation2 When MLR was applied to study the effect on Q5 of the six variables, an inverse significantly correlation was observed between Q5 and disintegration time (P=0.0006) and hardness (P=0.013). The lower the tablet hardness and the lower the disintegration time, the higher the Q5. MLR model for Q5 exhibited better predictive characteristics (R2=0.73) than sales (R2=0.45).
The utility to predict pharmacokinetic and pharmacodynamic in vivo characteristics based on in vitro data such as dissolution and disintegration tests are limited.Citation11 Nevertheless, both disintegration time and Q5 dissolution in vitro parameters are interesting tools for the development of new ibuprofen products. Although since the work of Levy and HollisterCitation27 disintegration test is not generally considered an appropriate tool for IVIVC studies, it is still a fast quality control tool for immediate-release solid dosage forms especially during tableting process control. Moreover, there is scientific justification for using disintegration testing instead of dissolution testing for some immediate release formulations made from highly water soluble drug substances.Citation28–Citation30 Nevertheless, dissolution data on physiological conditions are required for IVIVC studies. The search for a new dissolution test able to predict the in vivo absorption of a low soluble drug such as ibuprofen is a remarkable issue of research. Important new approaches to find biorelevant conditions for the dissolution test of ibuprofen have been published based on more physiology media than the one actually recommended in the USP Pharmacopeia.Citation31
Conclusion
For ibuprofen 600 mg tablets with the same price, disintegration time among other factors affects the percentage of sales or market share. Even though all studied ibuprofen products complied with the USP Pharmacopeia specifications, those products with faster disintegration times were correlated to significantly higher sales.
Acknowledgments
This work was supported by a grant from the Complutense University and Madrid Community Administration to the research group 910,939. The authors would like to thank Industrial Farmaceutica Cantabria and Camo for their support.
Disclosure
This manuscript represents the personal opinion of the authors and does not necessarily represent the views or policy of the Spanish Agency for Medicines and Health Care Products. The authors report no conflicts of interest in this work.
References
- European Medicines AgencyGuideline on the Investigation of BioequivalenceLondonEMA2010 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdfAccessed March 7, 2018
- AlvarezCNúñezITorradoJJGordonJPotthastHGarcía-ArietaAInvestigation on the possibility of biowaivers for ibuprofenJ Pharm Sci201110062343234921491448
- BramlagePGoldisABioequivalence study of three ibuprofen formulations after single dose administration in healthy volunteersBMC Pharmacol20088181818959779
- García-ArietaAAbad-SantosFRodríguez-MartínezMAAn eutomer/distomer ratio near unity does not justify non-enantiospecific assay methods in bioequivalence studiesChirality200517847047516104026
- TorradoJJBlancoMFarréMRosetPGarcía-ArietaARationale and conditions for the requirement of chiral bioanalytical methods in bioequivalence studiesEur J Clin Pharmacol201066659960420195587
- García-ArietaAFerrero-CafieroJMPuntesMImpact of chiral bioanalytical methods on the bioequivalence of ibuprofen products containing ibuprofen lysinate and ibuprofen baseChirality201628542943327094918
- PotthastHDressmanJBJungingerHEBiowaiver monographs for immediate release solid oral dosage forms: ibuprofenJ Pharm Sci200594102121213116136567
- RinakiEDokoumetzidisAValsamiGMacherasPIdentification of biowaivers among Class II drugs: theoretical justification and practical examplesPharm Res20042191567157215497681
- RobertsMFordJLMacleodGSEffect of lubricant type and concentration on the punch tip adherence of model ibuprofen formulationsJ Pharm Pharmacol200456329930515025854
- United States Pharmacopoeia38Rockville2015
- Food and Drug AdministrationGuidance for Industry Extended Release Oral Dosage Forms: Development, Evaluation and Application of In Vitro/In Vivo CorrelationsRockville, MDUS Department of Health and Human Services1997BP2 Available from: https://www.fda.gov/downloads/drugs/guidances/ucm070239.pdfAccessed March 7, 2018
- García-ArietaAGordonJPotthastHOn the biopharmaceutics classification system biowaiver of ibuprofenJ Pharm Sci201510482429243226036644
- Agencia Española Medicamentos. Centro de Información Medicamentos Agencia Española (CIMA) Available from: https://www.aemps.gob.es/cimaAccessed September 7, 2018 Spanish
- Consejo General Colegios de FarmacéuticosCatálogo de Medicamentos [Catalog of Medicines]MadridConsejo General Colegios de Farmacéuticos2011 Spanish
- Consejo General Colegios de FarmacéuticosCatálogo de Medicamentos [Catalog of Medicines]MadridConsejo General Colegios de Farmacéuticos2015 Spanish
- European Pharmacopoeia7th edStrasbourg2011
- FarhaneZBonnierFByrneHJAn in vitro study of the interaction of the chemotherapeutic drug Actinomycin D with lung cancer cell lines using Raman micro-spectroscopyJ Biophotonics2018111e201700112
- Spanish Health System (Ministerio de Sanidad y Consumo)Información Terapéutica del Sistema Nacional de Salud [Therapeutic information of the National Health System]201135124128 Available from: https://www.msssi.gob.es/biblioPublic/publicaciones/Accessed March 7, 2018 Spanish
- HalmeMLindenKKääriäKPatients’ Preferences for Generic and Branded Over-the-Counter Medicines: An Adaptive Conjoint Analysis ApproachPatient20092424325522273245
- ToverudELHartmannKHåkonsenHA Systematic Review of Physicians’ and Pharmacists’ Perspectives on Generic Drug Use: What are the Global Challenges?Appl Health Econ Health Policy201513Suppl 1(Suppl. 1)3545
- Costa-FontJRudisillCTanSLoyaltyBBrand loyalty, patients and limited generic medicines uptakeHealth Policy20141162–322423324573104
- SeymourRAHawkesfordJEWeldonMBrewsterDAn evaluation of different ibuprofen preparations in the control of postoperative pain after third molar surgeryBr J Clin Pharmacol199131183872015175
- DanielsSReaderSBerryPGoulderMOnset of analgesia with sodium ibuprofen, ibuprofen acid incorporating poloxamer and acetaminophen – a single-dose, double-blind, placebo-controlled study in patients with post-operative dental painEur J Clin Pharmacol200965434335319252905
- MehlischDRSykesJIbuprofen blood plasma levels and onset of analgesiaInt J Clin Pract Suppl201317817838
- FaasseKMartinLRGreyAGambleGPetrieKJImpact of brand or generic labeling on medication effectiveness and side effectsHealth Psychol201635218719026462056
- ColganSLFaasseKPereiraJAGreyAPetrieKJChanging perceptions and efficacy of generic medicines: An intervention studyHealth Psychol201635111246125327505191
- LevyGHollisterLEFailure of USP disintegration test to asses physiologic availability of enteric coated tabletsNY State J Med19646430023005
- European Medicines AgencyICH Q6A specifications: Test procedures and acceptance criteria for new drug products: Chemical substancesInternational Conference on Harmonization1999 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002823.pdfAccessed May 28, 2018
- Al-GousousJLangguthPOral Solid Dosage Form Disintegration Testing – The Forgotten TestJ Pharm Sci201510492664267525546430
- UebbingLKlumppLWebsterGKLöbenbergRJustification of disintegration testing beyond current FDA criteria using in vitro and in silico modelsDrug Des Devel Ther20171111631174
- TsumeYLangguthPGarcia-ArietaAAmidonGLIn silico prediction of drug dissolution and absorption with variation in intestinal pH for BCS class II weak acid drugs: ibuprofen and ketoprofenBiopharm Drug Dispos201233736637722815122
