Abstract
Background
Daily tenofovir disoproxil fumarate (TDF)/emtricitabine as HIV pre-exposure prophylaxis (PrEP) causes subclinical decreases in bone mineral density (BMD). We surveyed PrEP users to assess feasibility for a clinical trial of vitamin D supplementation to mitigate TDF-induced BMD loss.
Methods
We recruited participants using or starting PrEP in Toronto and Vancouver. The primary objective was to assess the acceptability of daily or weekly vitamin D supplementation. We also assessed the acceptability of calcium supplementation, existing use of non-pharmacological bone health interventions, prevalence of osteoporosis risk factors, and bone health knowledge (Osteoporosis Knowledge Test, OKT).
Results
Of 161 participants, 72.1% were current PrEP users, 18.0% were starting PrEP, and 9.9% did not indicate their PrEP status. All identified as males, 88.8% as gays, and 67.1% as Whites. Median (IQR) age was 32.0 (29.0, 40.0) years, and 62.1% reported family income $$60,000/year. Among those not already using the interventions, willingness to supplement with daily vitamin D, weekly vitamin D, or daily calcium was very high at 90.9%, 96.4%, and 93.0 %, respectively. Only 31.0% reported adequate dietary calcium intake, while 42.9% reported $1 osteoporosis risk factor (most commonly, alcohol and smoking). Overall bone health knowledge was low, as median (IQR) OKT score was 16/32. In post hoc comparisons, current PrEP users may have been more likely than new PrEP users to engage in bone loading exercise (Bone-specific Physical Activity Questionnaire score=12.5 vs 3.6, P=0.001) and have greater bone health knowledge (OKT=17 vs 14, P=0.08), but they had similar levels of current vitamin D supplementation (37.4% vs 21.4%, P=0.11), calcium supplementation (11.2% vs 13.8%, P=0.70), and adequate dietary calcium intake (32.7% vs 25.0%, P=0.43).
Discussion
The high acceptability of vitamin D and calcium supplementation in this cohort suggests that enrollment into a clinical trial of such interventions to mitigate PrEP-induced BMD loss is feasible.
Background
The use of daily oral tenofovir disoproxil fumarate with emtricitabine (TDF/FTC) as HIV pre-exposure prophylaxis (PrEP) has been shown to reduce the risk of HIV acquisition when users’ adherence is high.Citation1 This co-formulated combination tablet has been approved for the prevention of sexually transmitted HIV since 2012 in the US and 2016 in Canada. However, TDF/FTC causes subclinical adverse effects, including reversible decreases in renal function and bone mineral density (BMD). The pre-exposure prophylaxis initiative [Iniciativa Profilaxis Pre-Exposición] trial involving 498 gay, bisexual, and other men who have sex with men (gbMSM) randomized to TDF/FTC-based PrEP or placebo found that the treatment group experienced modest but statistically significant decreases in BMD of 0.91% (95% CI, 0.38–1.44) at the spine and 0.61% (95% CI, 0.27–0.96) at the hip compared with the placebo group at 24 weeks.Citation2 Similarly, reductions in BMD have also been observed in PrEP trials conducted among young adults in BotswanaCitation3 and gbMSM in San Francisco.Citation4 To date, no study has reported increased fractures in PrEP users, although follow-up has been relatively short.
Because of the adverse effects caused by TDF/FTC, a new prodrug of tenofovir called tenofovir alafenamide (TAF) has been introduced. TAF transfers tenofovir into leukocytes more efficiently than TDF, allowing the drug to concentrate less in plasma and yield less renal and bone toxicity.Citation5 Clinical trials of TAF/FTC as HIV treatment have shown that it has similar antiviral efficacy and causes smaller decreases in BMD and less bone turnover compared with TDF/FTC.Citation6–Citation8 An ongoing international clinical trial is testing the efficacy of TAF/FTC for use as PrEP.Citation9 If successful, there may be pressure on payers to cover this agent due to its favorable toxicity profile, even though the cost of the drug will be high, and its manufacturing protected by patent laws for many years. In contrast, generic versions of TDF/FTC already exist in many markets, and are much more affordable.Citation10–Citation13 If adverse effects caused by TDF can be mitigated by low-cost interventions, then combining such interventions with TDF/FTC could provide a more efficient way of deploying PrEP at scale.
Vitamin D and calcium supplements have been shown to mitigate TDF-induced BMD loss in HIV patients taking TDF-containing anti-retroviral therapy,Citation14 and there is interest in studying the impact of these interventions on the BMD of PrEP users in randomized controlled trials (RCT) as well.Citation15 To assess the feasibility of such a trial, we conducted a cross-sectional survey of current and prospective TDF/FTC-based PrEP users.
Methods
Study design
This was a cross-sectional survey of people using or considering the use of oral TDF/FTC-based PrEP. The primary objective was to assess the acceptability of daily or weekly vitamin D supplementation in patients using daily TDF/FTC-based PrEP. Secondary objectives were to assess the acceptability of dietary calcium interventions, the existing use of non-pharmacological interventions, the prevalence of osteoporosis risk factors, and the awareness of bone health in the study population. An exploratory objective was to determine what participant characteristics were associated with willingness to accept vitamin D interventions.
Participants
We recruited participants during routine clinic visits to an academic infectious disease clinic and an academic family practice in Toronto, Ontario, as well as a community clinic serving gbMSM in Vancouver, British Columbia between May and November 2017. The eligibility criteria included being able to read and write in English, and currently taking (current PrEP users) or planning to use (new PrEP users) oral TDF/FTC-based PrEP. All participants were offered $10 CAD as compensation for completing the survey.
Survey instrument
We administered the survey either in paper form or online through the Google Cloud Platform. The 78-item survey comprised six domains.
Clinical characteristics
We obtained basic demographicsCitation16 and information about participants’ PrEP regimens, and quantified PrEP adherence using the AIDS Clinical Trials Group adherence questionnaire and a previously published scoring system, which rescales responses from 0 to 100.Citation17
Knowledge
We asked participants a “yes/no” question regarding whether or not PrEP causes a decrease in BMD. We also used the Osteoporosis Knowledge Test (OKT)Citation18 to gauge participants’ knowledge of bone health. This questionnaire asks participants about their knowledge of osteoporosis, bone-loading exercises, and dietary sources of calcium.
Acceptability of bone health interventions
We provided a brief preamble stating, “Although TDF/FTC (tenofovir disoproxil fumarate + emtricitabine, or Truvada®) as PrEP is generally very safe, it is known to cause a small decrease in bone density”. This decrease in bone density is something that you cannot feel. If a person’s bone density gets very low, it is called ‘low bone mass’ or even ‘osteoporosis’, and the person can get a fracture (broken bone) more easily. However, no PrEP studies have ever shown an increased risk of fracture. It is not clear how to prevent PrEP-related bone loss, so our research team is considering future studies to try to find out”. We then asked participants, “Would you be willing to take a daily vitamin D pill as a way to try to prevent PrEP-related bone loss?” as a yes/no question. We further asked four identically structured questions about weekly vitamin D pills, daily calcium pills, and daily or weekly dietary surveys with advice about calcium intake.
Existing uptake of bone health interventions
We asked participants about their current use of vitamin D and/or calcium supplements, including dosages.
Prevalence of osteoporosis risk and mitigating factors
We determined the presence of osteoporosis risk factors in survey respondents as defined by the Fracture Risk Assessment Tool (FRAX).Citation19 FRAX assesses one’s risk of fracture in the next 10 years based on age, sex, body mass index, and family and medical risk factors. In assessing mitigation factors, we asked participants about their dietary calcium intake using the Calcium Assessment Tool (CAT)Citation20 and performance of bone-building exercises using the Bone-specific Physical Activity Questionnaire (BPAQ).Citation21 The CAT calculates and categorizes one’s daily calcium intake by asking respondents to quantify the number of servings of calcium-rich foods that they eat from an inventory. The BPAQ questionnaire quantifies how much one participates in bone-loading exercises by asking respondents to report the frequency and age at which they perform(ed) certain sports. We also asked participants whether or not they used tanning beds, which have been shown to increase serum vitamin D levels.Citation22–Citation26
Self-Efficacy
We administered the Osteoporosis Self-Efficacy (OSE) scale,Citation27 which asks participants to indicate how confident they feel in accomplishing tasks that are conducive to improving bone health, such as bone-loading activities and integrating more calcium-rich foods into one’s diet.
Analysis
Participant characteristics were summarized using descriptive statistics. To achieve our main objectives, we calculated the proportion of participants who responded “yes” to each question about willingness to use bone health interventions. The high proportion of respondents willing to use vitamin D and calcium supplementation precluded our ability to conduct exploratory logistic regression analyses to identify predictors of these outcomes. We used previously published scoring methods for standardized scales, including the OKT, CAT, BPAQ, and OSE scale.Citation18–Citation21,Citation27
In post hoc analysis, we used the chi-squared test to determine whether or not there were differences between current and new PrEP users in current vitamin D and calcium supplementation. We also used the Wilcoxon signed-rank test to compare osteoporosis knowledge and participation in bone-loading activities between the two groups. All analyses were conducted using SAS version 9.0 (SAS Institute Inc., Cary, NC, USA).
Sample size considerations
The target sample size was based on the minimum number of participants required to determine the proportion of PrEP users willing to adopt a vitamin D supplementation intervention, with reasonable precision. Using the most conservative estimate of 50%, and using a standard equation for the estimating sample size for a proportion, we determined that a minimum of 96 respondents were required to generate a 95% CI of ±10% around our estimate. Our target sample size was, therefore, 100 participants.
Ethics approval and informed consent
We obtained ethical approval from the Research Ethics Boards of St Michael’s Hospital and Providence Health Care/University of British Columbia before initiating any study activities. Consent from participants was implied by their completion of the survey.
Results
Demographic and clinical characteristics
Of 168 eligible patients who were approached regarding the study, we recruited 163 participants. Of these, one was excluded due to missing primary outcomes and another for loss of data, leaving a final sample of 161 individuals. shows the demographic characteristics of participants. Most (133) participants were recruited from Toronto, while 28 were from Vancouver. Most (72.1%) participants were already taking PrEP and 18.0% were considering taking PrEP; PrEP status was unknown for 9.9%. All participants were identified as males, 64.6% as Whites, and 89.4% as gays. The median (IQR) age was 32 (29, 40) years and a large proportion (62.1%) reported an annual family income of $$60,000 CAD. Basic demographic characteristics were not significantly different for current vs new PrEP users (data not shown).
Table 1 Basic demographic and clinical characteristics of survey participantsTable Footnotea
Acceptability of supplementation interventions
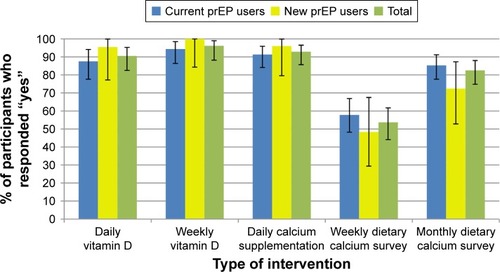
There was very high willingness to use all supplementation interventions, with 90.5%, 96.2%, and 92.9% of respon-dents overall indicating willingness to use daily vitamin D, weekly vitamin D, and daily calcium supplementations, respectively (). Willingness was similar between current and new PrEP users. With regard to dietary surveys to monitor calcium intake, monthly surveys were preferred over weekly ones overall (82.5% vs 53.7%).
Bone health characteristics
The majority of respondents (69.0%, 95% CI, 60.8%–76.4%) reported a CAT score of 1,000 mg/day or less, putting them in the inadequate (40.0%, 95% CI, 32.0%–48.5%) and deficient (29.0%, 95% CI, 21.7%–37.1%) categories (). Only 32.7% (95% CI, 25.4%–40.7%) were currently taking vitamin D supplements and 11.3% (95% CI, 6.8%–17.2%) were taking calcium supplements.
Table 2 Bone health characteristics, presence of non-pharmacological, and bone health awareness of survey participantsTable Footnotea
A total of 42.9% (95% CI, 31.5%–50.9%) of respondents reported ≥1 osteoporosis risk factor. The most common risk factors were consuming ≥3 units of alcohol per day (24.1%), a reported fragility fracture in adult life (13.8%), and smoking (10.9%). The mean (SD) 10-year probability of fracture using the FRAX tool was low at 2.3% (1.6%) ().
Knowledge about bone health in general was modest with a median (IQR) OKT score of 16/32,Citation13,Citation19 although 75.4% (95% CI, 67.3%–82.3%) of respondents were aware that PrEP causes a decrease in BMD (). The mean (SD) diet and exercise self-efficacy scores were 74.0 (21.9) and 75.1/100 (23.7), respectively, suggesting moderate-high self-efficacy.
In post hoc analysis, we found that current PrEP users were more likely than new PrEP ones to engage in bone loading exercise (P=0.001), but had similar bone health knowledge (P=0.08), vitamin D supplementation (P=0.11), calcium supplementation (0.70), and dietary calcium intake (P=0.43) ().
Table 3 Comparison of osteoporosis mitigating factors and bone health knowledge between current and new PrEP usersTable Footnotea
Discussion
In this cross-sectional survey of PrEP users in Toronto and Vancouver, we found a high degree of willingness to take vita-min D (90.5%–96.2%) and calcium (92.9%) supplements, but more modest willingness to undergo regular surveys of calcium intake (53.7%–82.5%). Although participants had only moderate knowledge of bone health, as measured by the OKT, most knew that PrEP causes a decrease in BMD. Many had at least one osteoporosis risk factor, most commonly smoking or alcohol use, although the absolute risk of fracture in this relatively young population was low overall.
The finding that more than 90% of participants were willing to use each vitamin D intervention is striking. This finding may reflect the high awareness of the relationship between PrEP use and BMD loss in our sample, and the high income of participants, since people of high socioeconomic status are likely to be more health conscious.Citation28 It may also relate to the timing of our study (conducted only a year and a half after TDF/FTC was approved for use as PrEP in Canada, and before PrEP was publicly reimbursed in either Ontario or British Columbia), in that our sample may consist of “early adopters” of PrEP who are disproportionately health-conscious compared with other at-risk gbMSM. That 11.3% of participants were already using calcium and 32.7% vitamin D supplements is further reflective of this possibility.
It is noteworthy that vitamin D supplementation is usually well accepted by the general population due to its affordability and low toxicity. In fact, current guidelines from Health Canada recommend that all Canadians consume vitamin D-fortified foods, and that all those .50 years of age take vitamin D supplements.Citation29 The high acceptability of vitamin D supplementation found in our study is similar to the finding from a previous study that 95%–100% of osteopenic individuals enrolled in a randomized trial of docosahexaenoic acid, vitamin D, and calcium supplementation rated the pills as acceptable.Citation30 In addition, we found that weekly vitamin D supplements were viewed as more favorable than daily supplements. This finding is consistent with that of a previous RCT, which found that weekly supplements were better accepted than daily supplements in older populations at risk of osteoporosis.Citation31
Our study has several implications for the potential design of a future interventional trial aimed at mitigating the effects of TDF/FTC-based PrEP on bone health. First, our acceptability findings suggest that it would be highly feasible to rapidly enroll participants into clinical trials of vitamin D and calcium supplementation. Second, the very high percentage of participants willing to take such supplements suggests that a trial design in which everyone receives an active agent, such as a dose–response study, may be more appropriate than a placebo-controlled design. Third, that less than one-third of our participants reported adequate calcium intake emphasizes that calcium intake should be carefully monitored in such a trial.
Although much of our sample reported ≥1 osteoporosis risk factor, the median number of osteoporosis risk factors was only one, and most commonly included smoking and alcohol use, which are common in the general population.
Strengths of our study include our use of validated survey tools to measure PrEP adherence, osteoporosis knowledge, calcium intake, physical activity, and fracture risk, as well as our enrollment of participants in a variety of care settings. Our study also has limitations that warrant consideration. For instance, responses to our survey may have been subject to recall bias and social desirability bias, potentially leading to overestimations of willingness to take supplements, PrEP adherence, and OSE. In addition, most of our respondents were identified as gay white males of higher socioeconomic status, so our findings may not be generalizable to other PrEP-using populations. Finally, some physicians at practice sites incorporate education about the impact of PrEP on BMD into routine patient counseling, which may have inflated interest in bone health interventions.
In summary, the acceptability of vitamin D and/or calcium supplementation appears to be high in PrEP-using gbMSM. Clinical trials are warranted to evaluate the efficacy of such interventions.
Author contributions
All authors have seen and approved the final submitted version of the article. All six contributed significantly to the work, as follows: DHST conceived the study idea, designed the study, and supervised all aspects of its execution; SW oversaw enrollment of participants into the study, planned and performed the statistical analysis, and wrote the original draft of the manuscript; JLK contributed to conception and design; MWH, GA, and JRMO contributed substantially to data acquisition; and all authors contributed to data analysis, drafting and revising the article, approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to acknowledge all study participants; Alexandre Schnubb and Allan Lal for coordinating the study; and the staff at the Positive Care Clinic, 410 Sherbourne Family Health Team, and Health Initiative for Men for assisting with data collection.
This research was supported by a grant from the Canadian Institutes of Health Research (Grant No. HIM 145368). DHST’s intitution has received research support for investigator-initiated research studies from Gilead and ViiV Healthcare. DHST is supported by a New Investigator Award from the Canadian Institutes of Health Research/Ontario HIV Treatment Network.
Disclosure
SW, JLK, and JRMO have no conflicts to disclose. MWH reports receiving honoraria for advisory board representation and speaking engagements regarding HIV and the Hepatitis C virus from BMS, Gilead, Merck, andViiV Healthcare, paid to his institution. GA has been a member of the scientific advisory board for Gilead and Viiv, received research grants from Gilead, and received honoraria from Gilead and Merck. In the past 3 years, DHST has received honoraria from Merck for conducting educational lectures of his own design. DHST is a Site Principal Investigator for clinical trials sponsored by GlaxoSmithKline. All authors report no others conflicts of interest in this work.
References
- RiddellJAmicoKRMayerKHHIV Preexposure prophylaxis: a reviewJAMA2018319121261126829584848
- MulliganKGliddenDVAndersonPLEffects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trialClin Infect Dis201561457258025908682
- KasondeMNiskaRWRoseCBone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofoviremtricitabine or placebo in BotswanaPLoS One201493e9011124625530
- LiuAYVittinghoffESellmeyerDEBone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San FranciscoPLoS One201168e2368821897852
- SaxPEWohlDYinMTTenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trialsLancet201538599872606261525890673
- MillsAArribasJRAndrade-VillanuevaJSwitching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority studyLancet Infect Dis2016161435226538525
- ArribasJRThompsonMSaxPEBrief report: randomized, double-blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat, and emtricitabine (E/C/F) for initial HIV-1 treatment: week 144 resultsJ Acquir Immune Defic Syndr201775221121828282300
- SaxPEZolopaABrarITenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 studyJ Acquir Immune Defic Syndr2014671525824872136
- Safety and Efficacy of Emtricitabine and Tenofovir Alafenamide (F/TAF) Fixed-Dose Combination Once Daily for Pre-Exposure Pro-phylaxis in Men and Transgender Women Who Have Sex With Men and Are At Risk of HIV-1 Infection (DISCOVER) [Internet]US Library of Medicine2016 [cited 2018-08-16]. Available from: https://www.nlm.nih.gov/bsd/uniform_requirements.html#electronic
- Health CanadaPMS-emtricitabine-tenofovir2017 Available from: https://pdf.hres.ca/dpd_pm/00040337.PDFAccessed October 11, 2018
- Health CanadaAPO-tenofovir2018 Available from: http://www.health.gov.nl.ca/health/_scripts/class_search_nl.asp?din=02451980&GReturn=GenericName&subtitle1=Generic%20or%20Brand%20Name%20:%20%20%20&subtitle2=&tempformulary=Accessed October 11, 2018
- Health CanadaMylan-emtricitabine/tenofovir disoproxil2017 Available from: http://www.gilead.ca/application/files/1715/3185/3013/Truvada_English_PM_e133222-GS-017.pdfAccessed October 11, 2018
- Health CanadaTEVA-emtricitabine/tenofovir2017 Available from: https://pdf.hres.ca/dpd_pm/00040336.PDFAccessed October 11, 2018
- OvertonETChanESBrownTTVitamin D and calcium attenuate bone loss with antiretroviral therapy initiation: a randomized trialAnn Intern Med20151621281582426075752
- HavensPLStephensenCBvan LoanMDDecline in bone mass with tenofovir disoproxil fumarate/emtricitabine is associated with hormonal changes in the absence of renal impairment when used by HIV-uninfected adolescent boys and young men for HIV preexposure prophylaxisClin Infect Dis201764331732528013265
- HealthTTri-Hospital and Toronto Public Health: Health Equity Data Collection Research Project ReportToronto Public Health, St Michael’s Hospital, Centre for Addiction and Mental HealthMount Sinai Hospital2013
- ReynoldsNRSunJNagarajaHNGiffordALWuAWChesneyMAOptimizing measurement of self-reported adherence with the ACTG Adherence Questionnaire: a cross-protocol analysisJ Acquir Immune Defic Syndr200746440240918077832
- GendlerPECoviakCPMartinJTRevision of the osteoporosis knowledge test: reliability and validityWest J Nurs Res201537121623164324923463
- ParkerSCiaccioMCookEValidation of a modified FRAX® tool for improving outpatient efficiency – part of the “Catch Before a Fall” initiativeArch Osteoporos201510125
- HungAHamidiMRiazantsevaEValidation of a calcium assessment tool in postmenopausal Canadian womenMaturitas201169216817221450422
- WeeksBKBeckBRThe BPAQ: a bone-specific physical activity assessment instrumentOsteoporos Int200819111567157718414964
- de GruijlFRPavelSThe effects of a mid-winter 8-week course of sub-sunburn sunbed exposures on tanning, vitamin D status and coldsPhotochem Photobiol Sci201211121848185423104230
- LagunovaZPorojnicuACAksnesLEffect of vitamin D supple-mentation and ultraviolet B exposure on serum 25-hydroxyvitamin D concentrations in healthy volunteers: a randomized, crossover clinical trialBr J Dermatol2013169243444023551243
- TangprichaVTurnerASpinaCDecastroSChenTCHolickMFTanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral densityAm J Clin Nutr20048061645164915585781
- ThiedenEJørgensenHLJørgensenNRPhilipsenPAWulfHCSunbed radiation provokes cutaneous vitamin D synthesis in humans – a randomized controlled trialPhotochem Photobiol20088461487149218513233
- WeberBBachmannCCBraunRAbrahamAGSerraALHofbauerGFL25-Hydroxyvitamin-D3 serum modulation after use of sunbeds compliant with European Union standards: a randomized open observational controlled trialJ Am Acad Dermatol2017771485428416344
- HoranMLKimKKGendlerPFromanRDPatelMDDevelopment and evaluation of the Osteoporosis Self-Efficacy ScaleRes Nurs Health19982153954039761137
- WardleJSteptoeASocioeconomic differences in attitudes and beliefs about healthy lifestylesJ Epidemiol Community Health200357644044312775791
- Health CanadaEating well with Canada’s food guide2011 Available from: http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.phpAccessed October 11, 2018
- VanlintSJRiedKEfficacy and tolerability of calcium, vitamin D and a plant-based omega-3 oil for osteopenia: a pilot RCTMaturitas2012711444822078660
- BruyèreODeroisyRDardenneNA phase IV, two-armed, randomized, cross-over study comparing compliance with once-a-month administration of vitamin D3 to compliance with daily administration of a fixed-dose combination of vitamin D3 and calcium during two 6-month periodsOsteoporos Int201526122863286826100413