Abstract
Purpose: This study aimed to evaluate Japanese patient preferences regarding features of intermediate or advanced (Progressed) hepatocellular carcinoma (HCC) treatments: transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), and oral anti-cancer therapy.
Methods: Patients with HCC, recruited from clinical sites and a patient panel in Japan, completed a cross-sectional web-based survey. Preferences were quantified using best–worst scaling, where patients identified the best and worst among 13 treatment features. Direct elicitation was used to identify preference for TACE, HAIC, or oral therapy, including the likelihood of trying each. Additional items asked for the willingness to try an oral medication that delays progression by six months but has an 8% or 21% risk of severe hand-foot skin reaction (HFSR).
Results: The sample (N=119; 29 early stage; 90 Progressed) most preferred “oral medication”, “artery branches plugged”, and “prevents formation of new blood vessels”, and least preferred “risk of liver damage” and “risk of catheter-related complications”. Overall, 51%, 40%, and 8% preferred oral therapy, TACE, and HAIC, respectively (p<0.05), and the mean likelihood of trying each were 59%, 52%, and 35%, respectively (p<0.001). Patients with sorafenib or TACE experience most preferred what they had received; however, both groups were equally willing to try the other treatment. Patients preferring oral therapy favored “oral medication” over “artery branches plugged”, “surgery is repeated as required when the cancer grows again”, and “risk of liver damage”, compared to those preferring TACE (p<0.05). Sixty-eight percent would probably try therapy with an 8% risk of severe HFSR, compared to 50% with a 21% risk.
Conclusion: Treatment type, mode of action, and risks may drive HCC patient preferences. Such features likely should be incorporated into physician–patient interactions regarding treatment decision-making.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of cancer originating in the liver. It is the fifth most common cancer in men and the seventh in women, and it represents the third most frequent cause of cancer death, accounting for approximately 500,000 deaths each year worldwide.Citation1,Citation2 HCC rates are particularly high in eastern/south-eastern Asia and in Africa, intermediate in Southern Europe, and low in most high-income countries. Persistent infections by hepatitis B virus (HBV) or hepatitis C virus (HCV) are the main recognized risk factors for HCC. In high-income countries, heavy alcohol drinking, tobacco smoking, overweight, diabetes, familial/genetic factors, and selected dietary aspects, have a relevant role.Citation1,Citation3 Recent statistics have shown that the incidence and mortality in HCC in Japan have been decreasing in recent years; however, the importance of developing more effective treatments remains important.Citation4,Citation5
Prognostic modeling of HCC patients considers tumor stage, degree of liver function impairment, patient’s general condition, and treatment efficacy.Citation6 Furthermore, current treatment paradigms for HCC rely on the Barcelona Clinic Liver Cancer algorithm, which classifies HCC into five stages based on extent of disease, Child-Pugh score, and ECOG performance status, enabling prognostication and informing allocation of first-line treatment.Citation7 Transarterial chemoembolization (TACE), which combines embolization with chemotherapy using tiny beads that emit chemotherapy, or giving chemotherapy through a catheter directly into the artery, is the first-line treatment for unresectable intermediate HCC. TACE may be repeated as clinically necessary over time. For TACE-refractory patients, oral preparations, including sorafenib, or hepatic arterial infusion chemotherapy (HAIC) with a reservoir system, are recommended, the choice of which depends in part upon whether or not there is minor and/or main portal branch invasion. Sorafenib also may be considered when cancer has spread beyond the liver.Citation8
In addition to the extent of cirrhosis and extrahepatic spread, patient preferences should be considered in treatment decisions in HCC.Citation9 There is a paucity of data on how patients value the features of different HCC treatment options in Japan. Understanding patient preferences may possess implications not only for treatment decision-making, but also for increasing patient satisfaction and adherence with follow-up care.Citation10,Citation11 The primary objective of this study was to understand the preferences of Japanese patients with HCC for key features associated with treatments for intermediate or advanced HCC, specifically TACE, sorafenib, and HAIC. The secondary objective was to evaluate trade-offs that HCC patients are willing to make regarding treatment features specific to sorafenib.
Methods
A non-interventional cross-sectional online survey was implemented among patients with HCC in Japan. Although the study focused on treatments provided in the intermediate/advanced setting, the study also included early-stage patients so that their perspectives could be captured. Patients were recruited through seven cancer centers in Japan and via an online panel to complete the survey. To be eligible for participation in the study, individuals must have been diagnosed with HCC, be at least 20 years old, reside in Japan, and be able to read and understand Japanese. Patients recruited via the online panel included early-stage patients as well as patients who had progressed into the intermediate or advanced stage (“Progressed”), whereas the cancer centers recruited only Progressed patients. All study participants endorsed an informed consent form. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethical Review Committees of all the participating institutions: Chiba University, Ehime Prefectural Central Hospital, Kikkoman General Hospital, Numazu City Hospital, Osaka Red Cross Hospital, Kimitsu Chuo Hospital, and Mitsui Memorial Hospital, as well as the central IRB, Magil IRB (Rockville, MD). Patient recruitment was conducted from May to September 2016 (CT.gov: NCT02616692).
Survey content
The survey assessed preferences using best–worst scaling (BWS) case 1, a stated preference method developed to scientifically measure relative preferences for a set of items.Citation12,Citation13 The BWS exercise involved prioritizing 13 features representing key differentiating characteristics of TACE, HAIC, and sorafenib, the only oral anti-cancer agent available with evidence of extension of overall survival at the time that this survey was conducted. Based on the literature, we included type of treatment (oral medication, surgery, procedure to implant catheter), mode of action (prevents formation of new blood vessels, liver arteries plugged), selected outcomes/follow-up interventions (clinical trial evidence of increased survival in advanced cancer, surgery is repeated when cancer grows again, follow-up visits to refill chemotherapy, stopping treatment due to side effects), and risks of adverse events (hand-foot skin reaction (HFSR); fever, abdominal pain, and nausea; catheter-related complications; liver damage).Citation14–Citation21
Each BWS item presented a subset of four selected features, and respondents identified which was most favorable and which was least favorable. An example BWS item is shown in . In preparation for the BWS exercise, respondents familiarized themselves with the different features by rating how much they liked or disliked them on a Likert scale.
In addition, a direct preference elicitation item was used comparing repeated TACE, HAIC, and oral anti-cancer therapy (). The direct preference elicitation item involved showing standardized descriptions of each of the three treatments, and respondents identified which they preferred most. Respondents also reported the percentage likelihood that they would try each treatment, assuming their symptoms worsened. Specifically, for each of the three treatments, the survey asked: “If your symptoms worsened and [treatment] is available free of charge, would you want to try [treatment]? Please answer from 0% (do not want to try at all) to 100% (definitely would try it).”
Figure 2 Direct preference elicitation item.

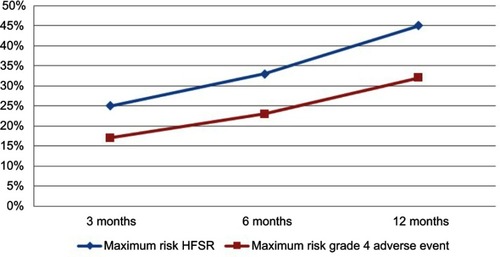
The survey also included trade-off items asking patients their willingness to try an oral anti-cancer medication that can stop the deterioration of cancer for six months, but it has a risk of severe HFSR, including pain and swelling on the palms and soles of the feet. Willingness to try was gauged for a treatment with an 8% risk, and for one with a 21% risk, of severe HFSR.Citation14,Citation22,Citation23 A six-month delay in disease progression was incorporated in the description as this was the overall mean time to progression observed for sorafenib in phase III clinical trial.Citation24 Responses ranged from “definitely would try” to “definitely would not try”. Open-ended items also prompted patients to identify the maximum risk of severe HFSR and of a life-threatening side effect that they were willing to accept for an oral anti-cancer therapy that would stop the deterioration of cancer by 3, 6, and 12 months, respectively.
The survey concluded with questions to obtain demographic and clinical information. The survey was translated from English to Japanese, including forward translation and reconciliation by two independent, native Japanese speakers. The survey underwent two rounds of cognitive debriefing interviews with a total of 22 patients with HCC in Japan to confirm that the items were interpreted accurately and consistently across respondents.
Analysis
The primary analyses were descriptive, reporting means and proportions, as applicable. The BWS scores for each of the 13 features were calculated based on the rates that each feature was identified as best and as worst across the set of BWS items. Specifically, to compute BWS scores, the number of times a feature selected as worst was subtracted from the number of times it was selected as best, and then divided by the total number of times that the feature appeared. The scores range from −1.0 to 1.0, where −1.0 reflects the worst (feature selected as worst in every question), and 1.0 reflects the best (feature selected as best in every question). For the direct preference elicitation item inquiring which treatment was most preferred, the proportions choosing each were reported. For the willingness to try treatment items, each response was expressed as a percentage ranging from 0% (do not want to try at all) to 100% (definitely would try it).
Exploratory subgroup analyses were performed using chi-square or analysis of variance tests, as applicable. BWS scores were compared among subgroups based on patients’ most preferred treatment. Among patients with TACE experience, BWS scores were compared between patients who had undergone three or fewer TACE procedures and more than three TACE procedures (three TACE procedures were the mean and median number experienced by patients). BWS scores also were evaluated among early-stage HCC patients, defined as online panel-recruited patients who had not had TACE, HAIC, or an oral anti-cancer therapy and whose cancer had not spread beyond the liver, and Progressed patients (online panel patients who were not early-stage patients as well as all clinic-recruited patients). The analyses were performed using SPSS, version 22.0.
Results
Demographic background
The sample comprised 68 (57.1%) patients recruited via the online panel, and 51 (42.9%) patients recruited via the seven participating clinical sites (). Of the total of 119 patients, 29 were early-stage patients, and 90 were progressed (intermediate or advanced) patients. reports the demographic and clinical characteristics of the sample. The mean age was 64.6±11 years, and 97 (81.5%) were male. The mean time since HCC diagnosis was 4.6±3.9 years. Sixty (50.4%) patients reported having a relapse, with a mean number of relapses of 4.8±4.2, and 19 (16%) reported that their cancer had spread beyond the liver. Approximately one-half (52.1%) of patients had TACE experience, with a mean number of 3.26±2.6 procedures [range=1–10], and one quarter (23.5%) had experience with sorafenib; 18.5% were currently receiving it. Twenty-four (20%) patients had HAIC experience, the majority of whom reported having it without a pump.
Table 1 Demographic and clinical characteristics
BWS and direct preference elicitation
reports the mean BWS scores for the treatment features. Of the 13 features included in the exercise, “Oral medication taken twice a day (two tablets each time)” was perceived as most favorable (mean: 0.61; 95% CI: 0.54, 0.68), followed by “Arteries in the liver are plugged to make it difficult for blood and nutrients to reach the cancer” (0.46; 0.40, 0.53), and “Prevents formation of new blood vessels that the cancer needs to grow” (0.41; 0.35, 0.47). The least favorable features were “Risk of liver damage that may prevent liver cancer treatment in the future” (−0.65; −0.72, −0.58), “Risk of complications due to implanting catheter” (−0.44; −0.50, −0.38), and “Risk of stopping treatment due to side effects” (−0.34; −0.42, −0.26). Early stage patients and Progressed patients had BWS scores consistent with the overall sample.
In the direct elicitation question, approximately one-half of the patients (51.3%; 95% CI: 42–61%) most preferred oral anti-cancer therapy, 40.3% (95% CI: 31–50%) most preferred TACE, and 8.4% (95%CI: 3.0–14%) most preferred HAIC. Progressed patients most preferred TACE (48.9%; 38–60%) or sorafenib (41.1%; 30–51%) vs HAIC (10%; 3.2–17%).
The comparison of BWS scores among groups stratified by which treatment the patients most preferred showed that, compared to patients most preferring TACE, patients who most preferred oral anti-cancer therapy favored “Oral medication taken twice a day (2 tablets each time)” (0.69 vs 0.51; p<0.05), and were more averse to “Arteries in the liver are plugged to make it difficult for blood and nutrients to reach the cancer” (0.34 vs 0.66; p<0.05), “Surgery is repeated as required when the cancer grows again” (−0.30 vs 0.03; p<0.05), and “Risk of liver damage that may prevent liver cancer treatment in the future” (−0.74 vs −0.52; p<0.05).
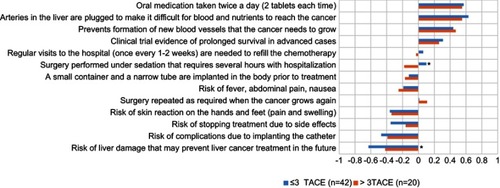
Among TACE-experienced patients, BWS scores were compared between patients who had undergone three or fewer TACE procedures (n=42) and more than three TACE procedures (n=20) (). Those who had undergone more than three TACE procedures perceived “Surgery performed under sedation that requires several hours with hospitalization” to be worse than those with fewer or equal to three procedures (−0.23 vs 0.14; p=0.003). In contrast, those with fewer or equal to three TACE procedures considered “Risk of liver damage that may prevent liver cancer treatment in the future” to be worse than those with greater than three procedures (−0.67 vs −0.39; p=0.026).
Figure 4 Best–worst scaling scores by the number of TACE procedures (≤3 vs >3).
Abbreviation: TACE, transarterial chemoembolization.
Most preferred treatment and percentage likelihood of trying treatment were compared among non-mutually exclusive subgroups based on treatment experience: TACE-experienced, HAIC-experienced, and oral anti-cancer therapy-experienced. shows the proportions of patients most preferring each treatment by previous treatment experience. Patients with TACE or oral anti-cancer therapy experience most preferred what they had received. Those with HAIC experience, however, equally preferred TACE or oral anti-cancer therapy. In a further analysis, it was found that, among the 15 patients with both oral anti-cancer therapy and TACE experience, 6 (40%) most preferred oral anti-cancer therapy, 5 (33%) most preferred TACE, and 4 (27%) most preferred HAIC.
Figure 5 Most preferred treatment by treatment experience.
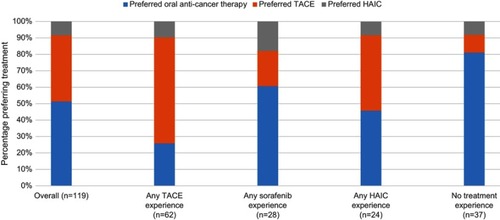
The mean likelihood estimates of trying oral anti-cancer therapy, TACE, and HAIC, were 59.1±33%, 52.2±33%, and 34.5±27%, respectively (p<0.001). Patients with TACE or oral anti-cancer therapy experience reported the highest mean likelihood of trying what they experienced; however, they were equally willing to try the other treatment. Those with HAIC experience were equally willing to try TACE or oral anti-cancer therapy, with a slightly lower mean likelihood of trying HAIC. Among Progressed patients, mean willingness to try treatment also was higher for oral anti-cancer therapy (58.0%) and TACE (57.8%) vs HAIC (34.7%) (p<0.001).
A subgroup analysis was performed examining the patients recruited only via the clinical sites (these patients were clearly identified by their physicians as having either intermediate or advanced stage HCC). The findings showed that the BWS scores, direct preference elicitation responses, and willingness to try scores among the patients recruited only via clinical sites were comparable with those of the overall Progressed group.
Oral anti-cancer therapy trade-off items
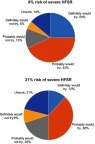
The majority of patients (68%) reported that they “definitely would try” or “probably would try” an oral anti-cancer medication that delayed disease progression by six months if it had a 8% risk of severe HFSR, compared to 50% of the patients if the medication had a 21% risk of severe HFSR (). In response to the open-ended items, the mean maximum acceptable risk estimates of severe HFSR that patients were willing to accept for 3 -, 6 -, and 12 month delays in time to progression were of 26.1±22.5%, 33.5±24.8%, and 45.5±30.6%, respectively (p<0.001). The patients reported mean maximum acceptable risk estimates of a life-threatening side effect (grade 4) that they were willing to accept for 3-, 6-, and 12-month delays in time to progression to be 17.7±21.5%, 23.1±23.2%, and 32.7±29.3%, respectively (p<0.001) ().
Discussion
This non-interventional study examined preferences for 13 features associated with sorafenib, TACE, and HAIC, and found that preference for treatment approach may be affected by the balance among the type of treatment, side effects, and burden. HCC patients are willing to take an oral anti-cancer medication with high risks of severe HFSR or a life-threatening event in exchange for a three-month delay in time to disease progression. Incorporating discussion of the key features of treatments into the patient–physician interactions may help to enhance shared decision-making about treatment and increase HCC patient satisfaction with care. Physicians could discuss the features examined in this study, including treatment type, mechanism of action, risks, and effectiveness, so that patients are aware of their choices before actually bearing the burden of the treatment.
This study found that preferences for different features varied by which treatment patients most preferred. Consistent with previous research findings that the perceptions of worse features generally provide better predictors of patient preferences,Citation25 the features driving the choice of oral anti-cancer therapy in this study were primarily negative features associated with repeated TACE. Another patient preference study in early-stage HCC found that 50% of the participants who chose radiofrequency ablation reported that the fear of complications from surgery was the main reason for their selection; only 9% reported that five-year overall survival was the main reason for preferring radiofrequency ablation.Citation26 Another preference study of arthritis medications found that patients’ preferences were most relevant when patients disliked a proposed treatment.Citation27
Patients with sorafenib or TACE experience most preferred what they had received; however, both treatment groups were equally willing to try the other treatment. With respect to TACE, patients may perceive undergoing TACE once as acceptable; however, if it should lose its effectiveness and repeated TACE procedures are needed, patients may not wish to continue. It would be useful to explore this in a future study. Patients who had experienced HAIC most preferred alternative treatment options; however, based on the responses to the question on willingness to try each treatment, when HAIC is the only option, they would still try it. This may in part be because HAIC patients typically have advanced disease when there are few, if any, treatment options, and as such are willing to try HAIC, particularly if they have heard that it is the optimal treatment for them.
The finding that patients’ experience with treatment was associated with preferences may be attributable to recommendations from their primary physicians. As found in a study of patients with asymptomatic HCC in Japan, patient preference for a specific treatment often stemmed from their consultations with a clinician.Citation28 A study of women with adjuvant breast cancer found that the majority of the patients preferred to make their treatment decisions collaboratively with a clinician vs on their own.Citation29 The finding that those without treatment experience, or the early stage patients, most preferred oral anti-cancer therapy may be attributable to being most familiar with oral medications as opposed to surgery.
Unlike those most preferring TACE and oral anti-cancer medication, patients with HAIC experience did not most prefer HAIC, possibly because these patients actually underwent HAIC and found it to be a difficult and burdensome procedure. This study found that patients who had undergone greater than three TACE procedures perceived “Surgery performed under sedation that requires several hours with hospitalization” to be worse than those with three or fewer procedures. It may be that, after several TACE procedures, patients become more averse to undergoing one again. As Cao and colleagues found in a patient-reported outcomes study of patients undergoing TACE, some patients eventually refuse repeated TACE because they cannot tolerate the repeated painful symptoms caused by TACE.Citation17
Collaboration and open communication between the patient and physician are especially important when outcomes are uncertain or when the optimal treatment in a particular population is not scientifically evident. In a study of patients with small HCC in compensated cirrhosis, no significant differences were observed between the different types of treatment, and it was concluded that choice of treatment should in part be based on patient preferences, after they have been properly informed on the survival, morbidity, and mortality related to each treatment option.Citation30 The alignment between intermediate to advanced HCC patients and their treating physicians, including hepatologists, gastroenterologists, and/or clinical oncologists, may lead to greater patient satisfaction. Feedback from interviews with patients with HCC in Taiwan found that there was a difference between HCC patients’ treatment preferences and their physicians’ recommendations, and that patients with advanced stage still prefer active treatment.Citation31
This study found that HCC patients are willing to accept an oral medication with relatively high risks of severe HFSR and of a life-threatening side effect for a delay in disease progression as small as three months. The maximum acceptable risk estimates that patients reported for these events were higher than the observed rates for these in sorafenib clinical studies.Citation14,Citation22,Citation23,Citation32
Limitations
A potential study limitation is that the patients recruited from the online panel, representing approximately one-half of the sample, self-reported their diagnosis and treatment experience. Given that these data could not be verified, there may be recall bias and inaccuracies with respect to these data. Another limitation of this study was that the description of HAIC in the direct elicitation item included discussion of a reservoir. Although this is consistent with the common perception among physicians of HAIC as that using a reservoir, patients who indicated that they had undergone HAIC may instead have undergone transcatheter arterial infusion.
With the evolving techniques in TACE therapy, including low profile microcatheters and drug-eluting beads, it is possible that the tolerability, acceptance, and hence, preference for TACE treatment could have differed among patients who experienced different kinds of TACE therapy. However, because it is possible that some patients may not have been fully aware of which TACE treatment they had, the type of TACE therapy was not asked in this study, any differences in preferences that may have been affected by the type of TACE cannot be inferred from these results. Another limitation is that the patients enrolled in this study were relatively younger than published estimates in Japan.Citation33 It is possible that this was due to the recruitment from an online patient panel, as well as interest in accessing the online survey among only those site-recruited patients who have familiarity with this type of data collection. It would be useful to confirm the findings, particularly those resulting from the subgroup analyses, in future larger studies.
Conclusion
Oral medication is perceived as most favorable, and risk of liver damage is perceived as worse, from the perspective of patients with HCC. Although patients highly prefer an oral medication, the decision on the optimum treatment still rests on the clinician’s judgment on what is best for the patient. The type of treatment, mode of action, and perceptions of treatment-related risks may drive HCC patient preferences. Patients’ previous experience with treatment likely will influence their preferences regarding future treatments, where they likely will favor the option with which they are most familiar. However, patients generally are equally willing to try sorafenib and TACE. Finally, HCC patients are willing to take an oral anti-cancer medication with substantial risks of severe HFSR or a life-threatening event in exchange for relatively small delays in time to disease progression. This study highlights the importance of understanding the patient’s perspective when choosing the best therapy for the disease. Asking HCC patients about their preferences may help inform overall disease management and enhance shared clinical decision-making.
Ethics approval and informed consent
The study protocol was approved by the Ethical Review Committees of all the participating institutions: Chiba University, Ehime Prefectural Central Hospital, Kikkoman General Hospital, Numazu City Hospital, Osaka Red Cross Hospital, Kimitsu Chuo Hospital, and Mitsui Memorial Hospital, as well as the central IRB, Magil IRB (Rockville, MD). Patient recruitment was conducted from May to September 2016 (CT.gov: NCT02616692).
Author contributions
T. Chiba and O. Yokosuka contributed to the questionnaire clinical review, study design, data collection, data analysis and interpretation, and manuscript preparation. A. Hiraoka, S. Mikami, M. Shinozaki, Y. Osaki, M. Obu and T. Ohki contributed to the data collection, data analysis, and data interpretation. N. Mita, D. Ledesma, N. Yoshihara, K. Beusterien, K. Amos, and J. Bridges contributed to the study design, data analysis and interpretation, and manuscript preparation. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to acknowledge Masaaki Jitsu and Yuki Nishiyama for logistical support and Erika Tanaka for study concept support. All are employees of Bayer Yakuhin, Ltd. Conduct of this study and preparation of this paper were funded by Bayer Yakuhin, Ltd. Bayer approved the submission of the manuscript.
Data availability
The study data are available upon request from the corresponding author.
Disclosure
T. Chiba reports commercial research funding from Bayer Yakuhin, Ltd. He also reports grants from Bayer Inc., during the conduct of the study and outside the submitted work. N. Mita, D. Ledesma, and N. Yoshihara are employees of Bayer Yakuhin, Ltd. K. Beusterien and K. Amos worked for ORS Health, which provided consulting services to Bayer Yakuhin, Ltd. during this study. J. Bridges has consulted for Bayer and has received travel support from Bayer. He also received personal fees from ORS Health, during the conduct of the study. T. Ohki reports personal fees from Bayer Yakuhin, personal fees from Eisai, outside the submitted work. O. Yokosuka reports personal fees from BMS, personal fees from Bayer, grants from Eisai, Takeda, Chugai, MSD, DS Pharma, Daiichi-Sankyo, Astellas, Asahi-kasei, Tanabe, and Nipponkayaku, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28(5):753–770. doi:10.1016/j.bpg.2014.08.00725260306
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: globocan 2000. Int J Cancer. 2001;94:153–156.11668491
- Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25(2):143–154. doi:10.1055/s-2005-87119415918143
- Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015;4(1):39–50. doi:10.1159/00036772726020028
- Umemura T, Ichijo T, Yoshizawa K, et al. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44(Suppl 19):102. doi:10.1007/s00535-008-2251-019148802
- Bruix J, Llovet J. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2001;35(3):519–524. doi:10.1053/jhep.2002.32089
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.04126795574
- Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3:458‐68. doi:10.1159/000343875
- American Society of Clinical Oncology (ASCO). In: liver cancer: treatment options. Cancer.Net editorial board; 2016 http://www.cancer.net/cancer-types/liver-cancer/treatment-options. Accessed 220, 2017.
- Eek D, Krohe M, Mazar I, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence. 2016;10:1609–1621. doi:10.2147/PPA.S10662927601886
- Zdenkowski N, Butow P, Hutchings E, Douglas C, Coll JR, Boyle FM. A decision aid for women considering neoadjuvant systemic therapy for operable invasive breast cancer: development and protocol of a phase II evaluation study (ANZ1301 DOMINO). JMIR Res Protoc. 2016;5(2):e88. doi:10.2196/resprot.564127207563
- Flynn TN. Valuing citizen and patient preferences in health: recent developments in three types of best-worst scaling. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):259–267. doi:10.1586/erp.10.2920545591
- Gallego G, Bridges J, Flynn T, Blauvelt BM, Niessen LW. Using best-worst scaling in horizon scanning for hepatocellular carcinoma technologies. Int J Technol Assess Health Care. 2012;28(3):339–346. doi:10.1017/S026646231200027X22980714
- Ogasawara S, Chiba T, Ooka Y, et al. Sorafenib treatment in Child-Pugh A and B patients with advanced hepatocellular carcinoma: safety, efficacy and prognostic factors. Invest New Drugs. 2015;33(3):729–739. doi:10.1007/s10637-015-0237-325861764
- Ogasawara S, Chiba T, Ooka Y, et al. Liver function assessment according to the Albumin–bilirubin (ALBI) grade in sorafenib-treated patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33(6):1257–1262. doi:10.1007/s10637-015-0292-926462681
- Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10(5):425–434. doi:10.3348/kjr.2009.10.5.42519721826
- Cao W, Li J, Hu C, et al. TACE symptom clusters and symptom interference of HCC patients undergoing TACE: a cross-sectional study in China. Support Care Cancer. 2013;21(2):475–483. doi:10.1007/s00520-012-1541-523010958
- Clark T. Complications of hepatic chemoembolization. Semin Intervent Radiol. 2006;23(2):119–125. doi:10.1055/s-2006-94144221326755
- Ikeda M, Arai Y, Park SJ, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol. 2013;24:490–500. doi:10.1016/j.jvir.2013.01.00323466316
- Ganeshan A, Upponi S, Lye-Quen H, Warakaulle D, Uberoi R. Hepatic arterial infusion of chemotherapy: the role of diagnostic and interventional radiology. Ann Oncol. 2008;19:847–851. doi:10.1093/annonc/mdm52818029972
- Oh MJ, Lee HJ, Lee SH. Efficacy and safety of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma as first-line therapy 2013. Clin Mol Hepatol. 2013;19:288–299. doi:10.3350/cmh.2013.19.3.28824133667
- Arizumi T, Ueshima K, Iwanishi M, et al. Real-life clinical practice with sorafenib in advanced hepatocellular carcinoma: a single-center experience second analysis. Dig Dis. 2015;33(6):728–734. doi:10.1159/00043907926488730
- Bruix J, Raoul JL, Sherman M. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalysis of a phase III trial. J Hepatol. 2012;57:821–829. doi:10.1016/j.jhep.2012.06.01422727733
- Llovet JM, Ricci S, Mazzaferro V, et al. for the SHARP investigators study group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa070885718650514
- Louviere JJ, Flynn TN, Marley AAJ. Best-Worst Scaling: Theory, Methods and Applications. University Printing House: Cambridge University Press; 2015.
- Molinari M, De Coutere S, Krahn M, Helton S, Urbach DR. Patients‘ preferences and trade-offs for the treatment of early stage hepatocellular carcinoma. J Surg Res. 2014;189(1):57–67. doi:10.1016/j.jss.2014.02.01524650457
- Hifinger M, Hiligsmann M, Ramiro S, et al. Patients‘ preferences and economic considerations play an important role in treatment decisions: a discrete choice experiment among rheumatologists. Rheumatology (Oxford). 2017;56(1):68–76. doi:10.1093/rheumatology/kew32828028156
- Sato K, Sato T, Furuse J, et al. A conundrum for randomized controlled trials: experience from a small hepatocellular carcinoma trial. Jpn J Clin Oncol. 2010;40(10):949–953. doi:10.1093/jjco/hyq07420495193
- Harder H, Ballinger R, Langridge C, Ring A, Fallowfield LJ. Adjuvant chemotherapy in elderly women with breast cancer: patients‘ perspectives on information giving and decision making. Psychooncology. 2013;22(12):2729–2735. doi:10.1002/pon.333823813806
- Farinati F, Gianni S, Marin G, Fagiuoli S, Rinaldi M, Naccarato R. Does the choice of treatment influence survival of patients with small hepatocellular carcinoma in compensated cirrhosis? Eur J Gastroenterol Hepatol. 2001;13(10):1217–1224.11711779
- Chen CM, Hsu CY, Bai CH Building a patient oriented treatment decision system for liver cancer. 2012 3rd Global Congress on Intelligent Systems; 2012; Wuhan, China.
- Nexavar® (Sorafenib) [Package Insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; 2010.
- Minami T, Tateishi R, Shiina S, et al. Comparison of improved prognosis between hepatitis B- and hepatitis C-related hepatocellular carcinoma. Hepatol Res. 2015;45:E99–E107. doi:10.1111/hepr.1246825559860