Abstract
Purpose
The role of specialized pharmacy services remains unexplored in clinical practice for hepatitis C patients in Pakistan. This study aimed to evaluate the impact of clinical pharmacy interventions on treatment outcomes, health-related quality of life (HRQoL), and medication adherence among hepatitis C patients.
Methods
A randomized control trial was conducted at two tertiary-care teaching hospitals in Pakistan. Hepatitis C patients who attended the outpatient clinics between October 2015 and September 2018 were randomized to two groups [usual care (UC) and pharmaceutical care (PC)] in a 1:1 ratio, applying simple envelope method. The PC group received pharmaceutical care led by a clinical pharmacist. The care that patients received included education and counseling on medication compliance, labeling of medication packs, and monitoring of adverse drug events, led by a qualified clinical pharmacist during the 15- to 20-minute monthly sessions, while the UC group received standard care at hospital, which did not involve clinical pharmacist input. Outcome measures, such as sustained virological response, HRQoL, and adherence rate (pharmacy data) were assessed at enrolment and distinct time intervals: 4 weeks, 8 weeks, and end of treatment.
Results
A total of 931 patients were included in the study (UC 466 and PC 465), with mean age 42.35±1.9 years. Sustained virological response at 12 weeks was achieved in 86.0% patients in the PC group, significantly (p<0.001) higher than the UC (69.3%) group. Fewer patients (9.9%) in the PC group reported mobility problems, significantly fewer (p<0.001) than the UC group (11.8%). Self-care, usual activity, pain, and depression were relieved significantly in the PC group compared to the UC group. The EuroQol visual analogue scale (baseline 56.1 of UC group versus 55.2 for PC group) was raised to 71.8 and 71.9 in the UC and PC groups, respectively. Medication adherence was significantly improved (p<0.001) in the PC group (88.6%) when compared to the UC group (77.9%, 95% CI 88.9%–91.9%).
Conclusion
Pharmacist-led clinical pharmacy interventions as part of multidisciplinary care had a significant impact on improving cure rates, HRQoL, and medication adherence for hepatitis C patients. This study suggests that clinical pharmacists should be incorporated into the multidisciplinary health-care team for care of hepatitis C patients.
Introduction
Hepatitis C virus (HCV) infection is a health-care problem worldwide.Citation1 The estimated global prevalence of HCV has been reported to be approximately 2.2%–3.0% (130–170 million people) in the literature.Citation1–Citation3 Recent advances in HCV pharmacotherapies and the development of a World Health Organisation HCV-elimination strategy has resulted in a significant increase in “test and treat” programs.Citation4–Citation6 Novel direct acting antiviral (DAA)–based therapies are effective in 95% of HCV patients.Citation7 However, long-term therapy with DAAs and pill burden often results in nonadherence that leads to suboptimal treatment outcomes in HCV patients.Citation8–Citation10
Although the major determinants of therapeutic response to pharmacotherapy include HCV genotype and viral load, additional factors, such as medication adherence and optimal duration of treatment, contribute to therapeutic outcomes.Citation11 Various studies have highlighted the significance of educating patients to improve adherence and treatment outcomes, including education on treatment schedules and side effects, especially prior to initiation of antiviral therapy.Citation12–Citation14
The key element of effective HCV clinical management is access to a multidisciplinary team (MDT).Citation15 As a member of a multidisciplinary team, the pharmacist is in an ideal position to serve patients with chronic HCV, not only through patient counseling regarding the disease state, therapeutic regimen, and associated adverse events but also by educating patients on the vital role of treatment adherence in the achievement of optimal therapeutic outcomes.Citation16 Pharmacist interventions and patient education on medication use have played a central role in improving adherence and management of other chronic diseases.Citation17,Citation18 The European Society for the Study of the Liver in 2018 also emphasized theCitation19,Citation20 vital role of pharmacists in educating patients on potential drug–drug interactions (DDIs) and improving adherence to the treatment regimen that will eventually result in significant improvement in health-related quality of life (HRQoL) for all patients receiving HCV therapies.Citation21
A recent comprehensive pharmaceutical care programme in Spain demonstrated improved adherence and treatment outcomes in HCV patients.Citation22 A similar pilot study revealed that safe and effective management of HCV infection can be achieved through the involvement of clinical pharmacists in patient care.Citation23 Published studies have unveiled significant improvement in treatment outcomes as a consequence of clinical pharmacist–led and person-centered care.Citation24–Citation26 Limited literature exists on the role of pharmacists in optimization of treatment regimens and management of adverse effects in HCV infection,Citation27 particularly in the context of low- and middle-income countries (LMICs). LMICs harbour 80% of the global HCV burden. Currently, Pakistan has approximately 10 million HCV patients, and health-care system is not fully equipped with clinical pharmacists specialized in chronic HCV.Citation28 Alongside this considerable burden, there is limited literature on the context of benefits of pharmacist interventions within the infrastructure of health care in LMICs among HCV patients being treated with new DAAs. This study aimed to evaluate the impact of pharmacist-led clinical interventions on treatment outcomes, HRQoL, and medication adherence in HCV patients in Pakistan.
Methods
Study Design
A randomized control study was undertaken. The allocation ratio was 1:1.
Participants and Eligibility
Confirmed HCV-positive patients aged ≥18 years who presented to the gastroenterology department or HCV clinic during the study period were eligible for inclusion in the study. Those had been initiated on DAA treatment [sofosbuvir (Sof), daclatasvir (Dac), or a combination thereof with or without ribavirin (Rv)] and had given informed consent were enrolled in the study. All patients who were pregnant or coinfected with HBV, HDV, or autoimmune hepatitis were excluded.
Patients were recruited from the outpatient clinic of gastroenterology departments at two tertiary-care hospitals in Islamabad and Lahore with a combined bed capacity of 2,346 (1,150 and 1,196 respectively). These hospitals have dedicated pharmacies to dispense HCV medicines to referred patients. The hospitals included in the study accept referrals from primary- and secondary-care hospitals, as well as basic health-care units situated in remote areas of Pakistan. This study was conducted between October 2015 and September 2018. Patients were divided into two groups [usual care (UC) and pharmaceutical care (PC)]. Written consent was obtained from each recruited patient, and all patients had the purpose and conduct of the study explained to them. Any queries raised by patients were addressed to satisfy each recruited subject.
Usual Care (Control)
This group was given usual care by the hospital staff, which included the input of physicians, a nurse, and/or pharmacy technician. They were treated in accordance with the routine clinical practices of hospital, which include diagnosis, dispensing/issuance of medication by hospital-pharmacy staff, and routine follow-up of patients at the health-care facility. They did not receive interventions from the pharmacist. These patients were provided guidance as per their requirements, and were not bound to attend any pharmacists' counseling session. Hospital staff were also informed to refer their patients to a pharmacist for any additional medicine–related information or guidance.
Pharmaceutical Care (Interventional)
In addition to usual care, all the patients in the PC group were provided with individualized patient care provided by a clinical pharmacist. The additional care provided by the pharmacist included direct patient monitoring, education on lifestyle modifications, and counseling on the appropriate use of HCV medication. All patients who consented to the pharmacist’s counseling session were enrolled at baseline and followed up until 12 weeks after the end of treatment. Each patient was instructed to report to the pharmacist every month during his/her visit for routine checkup by the physician. Clinical pharmacy services continued until treatment completion. Moreover, patients were asked to inquire any information pertaining to the medication by telephone after the end of treatment.
Patients’ Pharmaceutical Care and Interventions
Individualized patient care comprised the following:
Pharmacist counseling (15- to 20-minute sessions) on the proper use of medication and the provision of an educational pack inclusive of medication diary, a laboratory profile book, and a medication-adherence chart.
Labeling of medication packs to assist pill sorting. Labels included instructions on dose and frequency/timing of medication doses.
Patient education using both oral and written approaches. This protocol included an educational leaflet pertaining to medicine administration, medicine storage, and lifestyle modification.
Detection or assessment of potential DDIs by a pharmacist prior to start of treatment and recommendations for their management.
Pharmacist counseling on the safe use of medication (self-medication or over-the-counter [OTC] medicines), monitoring, and prompt detection of adverse drug events (ADEs).
A trained pharmacist provided the interventions described at each health-care facility, along with the existing pharmacy staff during the study period. The educational materials were delivered in Urdu (local language). The pharmacist was responsible for monitoring adherence to the HCV treatment. Patients were interviewed at their first visit and follow-up visit each month in a dedicated room adjacent to the gastroenterologist's office. Information pertaining to medicine usage, missed doses/adherence, hematology and biochemistry data, incidence of any ADE, and evaluation of clinical outcomes was retrieved from patients. Medication usage and missed doses were monitored by retrieving the empty blister packs of medicines from the patients in the pharmacy area, followed by a pill count.Citation27 Additionally, dose charts (filled) were retrieved from the patients. Any missing information was requested verbally from the patient. A separate room was availed for patients’ counseling sessions and interviews and to avoid any contamination with the UC group. The clinical pharmacist had no access to or was not involved in the care of the patients in the UC arm. To maintain the ethical role of health-care provision, at the end of the study all patients who attended the gastroenterology clinic after screening were provided with an educational booklet containing information on preventive care for family members.
Outcome Measures
The primary outcome measure was sustained virological response at week 12 posttreatment (SVR12),Citation29,Citation30 while secondary outcome measures were assessment of number of ADEs, HRQoL, and medication adherence.
Sustained Virological Response
Clinical outcomes were assessed based on HCV viral load measured at baseline, end of treatment, and 12 weeks after the end of treatment (SVR12). A viral load of <12 IU/mL at 12 weeks after the end of treatment was considered undetectable and regarded as “cure”.Citation31 HCV RNA levels were measured using an Abbott m2000 real-time HCV assay. Samples were sent to allied laboratories of these hospitals for reporting of quantitative viral loads through PCR. Data were extracted from the medical records of patients for this parameter.
Adverse Drug Events, DDIs, and Concomitant Medicines
The incidence of ADEs was assessed by trained pharmacist based on the Common Terminology Criteria for Adverse Events version 4.Citation32 Data pertaining to ADEs were collected at three time intervals: 4 weeks, 8 weeks, and 12 weeks from the start of treatment in both arms. Assessment of clinical potential DDIs, as well as recommendations for DDI management, were made in accordance with the Drug Interaction Checker, University of Liverpool (http://www.hep-druginteractions.org). The number and type of concomitant medications used was assessed based on the previously published literatureCitation19 and 73rd edition of the British National Formulary.
Health-Related Quality of Life
HRQoL was assessed at three time points: before, during, and after treatment (at day 1, 8th week, and 12th week (end of treatment). A validated tool (EuroQol 5D-3L) was administered by the researcher during the patient’s visit to the pharmacy. The EQ-5D-3L consists of five dimensions: mobility, self-care, usual activity, pain/discomfort, and anxiety/depression. These dimensions were dichotomized into two levels for analysis, ie, patient has no/mild problems (level 1) and patient has moderate/severe problems (level 2). The EQ VAS records the patient’s self-rated health on a scale numbered from 0 (worst perceived health) to 100 (best perceived health).Citation33
Medication Adherence
Medication adherence was measured using data of pharmacy refills (obtained from the pharmacy database). Pharmacy refills were recorded at patients' hospital visits each month. Data relating to adherence was collected at three time intervals: 4 weeks, 8 weeks, and 12 weeks from the start of treatment. The total number of pharmacy refills attended by each patient divided by the number of advised refills by 100 was the algorithm used to calculate Citation34,Citation35 Finally, adherence was measured as the proportion of patients who had taken at least 80% of their prescribed doses. The metric was used as an approximation of the 80/80/80 rule. This rule refers to the greater possibility of successful treatment outcome that is associated with taking 80% Sof and 80% Rv for 80% of the prescribed treatment duration.Citation35
Sample Size
Based on the prevalence of HCV infection and sample size that has been reported in the literature,Citation24,Citation36 we estimated a minimum sample size of 384 from each center (95% CI with confidence interval 5%). The target sample size from the two health-care facilities was 922, taking into account a potential 20% dropout rate. SVR12 was the major outcome measure to assess the success of treatment and power analyses.
Randomization
Enrolled patients were randomized at a ratio of 1:1 using a simple envelope method with modification.Citation37 Briefly, the physician at the time of first visit/diagnosis picked one of the envelopes marked UC or PC alternately, which was the assigned to patients.Citation36,Citation37 A separate room was availed for patients’ counseling sessions and interviews and to avoid any contamination with the UC group. The pharmacist had no access to or was not involved in the care of patients in the UC arm. The principal investigator (TR) was responsible for checking the pharmacists to assure the delivery of all components of the interventions as intended.
Data Sources and Collection
Patient demographics and treatment variables were collected on a predesigned data sheet moderated by the research team, which consisted of a senior medical consultant, a specialist pharmacist, and a statistician. Data extraction was carried out by the principal investigator and validated by the gastroenterologist. Variables were age, sex, HCV genotype, HCV viral load, liver-health status, presence of comorbidities, concomitant medication, baseline hematology and biochemistry data, and treatment regimens. During follow-up visits (at weeks 4, 8, and 12), each variable wwas re-recorded to assess any changes.
The information was obtained from patients' profiles and electronic records available via the hospital database and the logistics management and information system. All data were anonymized by the data collector prior to analysis by the research team. Data sets were stored on a password-protected computer at the Department of Pharmacy, Quaid-i-Azam University, Islamabad, Pakistan. The necessary ethical approvals were obtained from the Ethical Review Board of PIMS Hospital Islamabad (F.1-1/2015/ERB/SZABMU), SIMS (IRB/2017/333/SIMS), and the Bioethics Committee of Quaid-i-Azam University, Islamabad (DFBS/2015-248). All questionnaires or tools were adopted after the necessary permissions from the corresponding organizations or the authors.
Statistical Analysis
Statistical analyses were performed using SPSS version 24. Frequency distribution and descriptive statistics were calculated for demographics. Results are reported in terms of differences between groups with 95% CIs where appropriate. Pearson's χ2 was applied to compare demographic and end-point variables. Mann–Whitney U tests were conducted to compare groups. McNemar's χ2 was used to analyze whether changes in percentage HRQoL between the PC group and UC group were4 significant.Citation38 Missing data were analyzed by missing-value analysis with SPSS and any missing values replaced by mean values. p≤0.05 was taken as statistically significant.
Results
Overall, 1,050 patients were enrolled. Of those, 931 were eligible for randomization postscreening (n=757 from hospital A and n=174 from hospital B), while 119 were excluded. All patients consented to take part. Patients were assigned to one of the two groups (UC, n=466; PC, n=465). The meanage of patients was 42.35±1.9 years. There were 418 (44.9%) males and 513 (55.1%) female s. Of the total cohort, 671 (72.1%) were urban residents. A total of 109 (11.7%) were cirrhotic, and genotype 3a the most prevalent genotype (96.6%). There was no significant difference between the UC and PC groups (p=0.88) for baseline viral load; and ).
Figure 1 Flow diagram showing patient recruitment and follow-up.
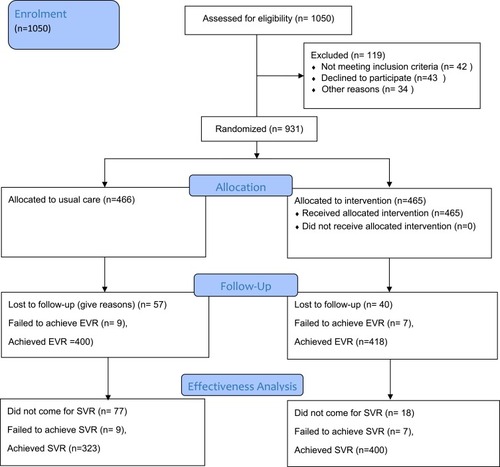
Table 1 Baseline Demographic and Clinical Characteristics of Study Population
[CONSORT diagram () to appear here]
The treatment regimen Sof/Rv was the most prescribed for 608 (65.3%) patients, followed by Sof/Dac/Rv for 201 (21.5%) and Sof/Dac for 13.1%. Baseline characteristics of patients and treatment regimens are summarized in .
Clinical Outcomes
A total of 400 (86.0%) patients in the PC group achieved SVR12, significantly (p<0.001) more than the UC group — 323 (69.3%). A total of 192 (20.6%) did not attend their 12-week posttreatment follow-up appointment (134 [28.8%] UC group vs 58 (12.5%) PC group, p<0.001). Overall, 287 (30.8%) patients presented with a moderate baseline viral load. At the end of treatment, 818 (87.9%) had achieved a response showing viral load below the detectable level, while 16 (1.7%) had failed to achieve a response (p=0.16). Viral clearance was achieved in 723 (77.7%) patients at 12 weeks after the end of treatment, ie, SVR12, while 16 (1.7%) failed to achieve SVR12. contains the clinical outcomes of both groups included in the study.
Table 2 Comparison of Outcome Parameters (Adherence and Clinical Outcomes) Among Groups
Adverse Drug Events
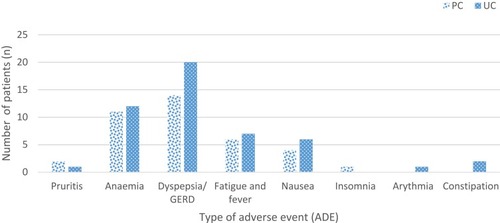
Fewer patients (38 [8.2%]) experienced an ADE in the PC group than the UC group (49 [10.5%]). Dyspepsia/gastroesophageal reflux was the most frequent ADE (n=20 [3.8%] in UC and n=14 [2.9%] in PC), followed by anemia (n=12 [2.5%] in UC and n=11 [2.4%] in PC) and fatigue (1.5% and 1.3% in UC and PC groups, respectively; )
Concomitant Medication and Drug-Drug Interactions with DAAs
There were 52 (11.2%) patients in UC group and 47 (10.1%) in the PC group using OTC or regular medications along with the DAAs. When assessed by the pharmacist, the possibility of DDIs between DAAs and self-medicated OTC products was low: n=8 (1.7%) in the UC group and n=5 (1.1%) in the PC group. In the UC group, proton-pump inhibitorss were the most frequent concomitant medications (n=14), followed by multivitamins (n=10) and NSAIDs (n=6), while in the PC group multivitamins (n=12) were the most frequent concomitant medicines, followed by proton-pump inhibitors (n=11) and NSAIDs (n=8). details the potential DDIs identified in the study.
Table 3 Drug–drug Interactions Between HCV DAAs and Concomitant Drugs
Health-Related Quality of Life
Before treatment in the UC group, 51.5%, 24.2%, and 15.5% patients reported problems in these dimensions, respectively. These problems were alleviated significantly (p<0.001) to 11.8%, 4.9%, and 5.8%, respectively, at the end of treatment. In the PC group, baseline dimensions (52.9%, 27.3%, and 15.9%) were relieved significantly (p<0.001) to 10.1%, 4.0%, and 4.1% respectively (p<0.001, ).
Table 4 Summary of EQ5D-3L Data for Health Related Quality of Life Domains
Overall, 47.0% of patients reported moderate levels of pain or discomfort at baseline that were relieved, and only 0.3% had a complaint of pain at the end of treatment. No significant difference (p>0.05) was observed with regard to reduction in pain between the PC group (baseline 221 [47.5%], final two [0.4%])] and the UC group (baseline 217 [46.6%], final three [0.6%]). However, McNemar's χ2 showed significant (p=0) change in HRQoL dimensions between the PC group and the UC group. Moreover, anxiety was significantly reduced (p=0.00) in the PC group (baseline 329 [70.8%] vs final 109 [23.5%]) in comparison with the UC group (baseline 343 [73.4%] vs final 104 [22.3%]).
EuroQol Visual Analogue Scale
Overall, mean EQ VAS was 55.7±6.9. Baseline EQ VAS for the UC (56.1±7.0) and PC (55.2±6.8) groups rose to 71.8±8.4 and 71.9±8.7, respectively, showing a significant change in both groups (p=0, )
Medication Adherence
Based on pharmacy refills, 775 (83.2%) patients had a >80% adherence rate to the prescribed treatment, with 12.4% of patients receiving <60% of their expected refills vs 3.7% (n=17) of patients in the PC group. Adherence was significantly better (>80% [p<0.001]) in the PC group (412 [88.6%]) than the UC group (363 [77.9%], 95% CI 88.9%–91.9; ).
Discussion
This study evaluated the impact of introducing a clinical pharmacy service to HCV patients in optimizing clinical outcomes, with an aim to improve medication adherence and HRQoL. Viral clearance results revealed that SVR12 was achieved in 86.0% of PC-group patients and 69.3% in the UC group. Unfortunately, as the DNA rate for SVR12 was significantly higher in the UC group than the PC group (28.8% vs 12.5%), it is difficult to conclude the true SVR12 rates of both groups, as a fair number of the UC group vs the PC group (28.8% vs 12.5%) did not appear for SVR12 follow-up. Despite the high published SVR12 rates seen in the literature with novel DAAs,Citation39 several factors may have contributed to the slightly decreased SVR12 rate seen in this study: the financial burden to the patient from having to pay for laboratory tests and travel costs being possible factors. An earlier study demonstrated the burden of out-of-pocket costs accrued by patients receiving HCV treatment.Citation40
Interestingly, more patients (10.5%) in the UC group experienced ADEs than the PC group (8.2%). Dyspepsia/gastroesophageal reflux was the most frequent ADE (3.8% in UC and 2.9% in PC). This low incidence of ADEs may be attributed to the increased safety of DAAs in comparison to previous regimens.Citation41 There were 12.1% of follow-up cases for whom interventions were required, including 49 and 38 patients who experienced ADEs in UC and PC, respectively. Of those, 26 had significant comorbidities. Studies have reported that the provision of specialized pharmacy clinical services, including medication-administration counseling and management of ADEs, improves patient outcomes, and in this studyCitation42,Citation43 36 (3.9%) patients were specifically counseled by the pharmacist to take their concomitant medicines at different times to the HCV medicines. The GRUviC trial also demonstrated that a comprehensive pharmaceutical care programme involving direct patient counseling before initiation of treatment improves patient education and safety consequently.Citation22
Our results show that HRQoL of HC patients was significantly improved in the PC group. According to EQ5D scores, the three most frequent dimensions (of moderate severity) were mobility (49.0%), pain/discomfort (46.7%), and anxiety (70.8%). Similarly, HRQoL complaints (fatigue, depression, and neurocognitive deficits) were reported by Foster et al among HCV patients.Citation44 These results emphasize the vital role a pharmacist has as a member of the multidisciplinary team, in improving the QoL and disease management of patients with chronic HCV infections. The provision of educational and supportive material in the form of counseling, adequate labeling of medication, medication diaries, and other compliance tools serves to facilitate adherence to routine follow-up tests and encourages patients to take their medication as per prescribed doses.
Our study demonstrates that pharmacist-led individualized patient care of HCV patients is an effective approach that improves patient adherence when following DAA regimens. The adherence rate was significantly improved >80% (p<0.001) in the PC group 412 (88.6%) over the UC group — 363 (77.9%). Studies have shown that nonadherence to combination therapy is common in routine patients,Citation8 and clinical trial-adherence data suggest that SVR decreases when patients have <60% dose-interval adherence.Citation45 Our results are also consistent with other interventional studies where pharmacist-led interventions significantly improved patient adherence to medication regimes.Citation25,Citation36
This study has explored the role of the pharmacist as a member of the health-care team in the management of HCV. Patient data were collected from two major tertiary hospitals with adequate sample size for the planned statistical analyses. Validated and recognised data sources were used for all information on prescription parameters and clinical outcomes. The study is not without its limitations. Firstly, a significant number of patients did not attend their SVR12 follow-up appointment, hence missing the final assessment by the pharmacist. Secondly, HRQoL complaints may have been underreported by patients who received treatment during their follow-up appointments. Thirdly, this study was specific to the population served by the tertiary-care centers, and may not be representative of the entire patient population affected by HCV in Pakistan. Finally, at the time of the study, DAAs were newly approved for use in hospital B, so the recruited subjects from this health-care facility were fewer in number. The inclusion of HBV and HDV patients would have been of benefit for improved adherence to HCV medications, but the management of these infections requires different approaches from HCV. Some contamination could have occurred during patients’ clinic visits and sharing the same waiting area. Local validation of the translation could not be done, due to resource constraints.
This study highlights the valuable role of the pharmacist as part of a multidisciplinary team in the provision of individualized care for HCV patients. It emphasises the potential benefits of adopting a collaborative-care approach when managing HCV-infected patients at tertiary-care hospitals in Pakistan. Based on our findings, it is recommended that future research be directed toward exploring the role of a specialist pharmacist in the management of HCV-infected patients in secondary and primary health-care settings. The development of policies to outline the role of specialist pharmacists is something that should be considered in Pakistan. The implementation of such roles, which include specialist pharmacy services and patient-centered care, in the context of LMICs is an emerging concept.Citation46,Citation47 The management of chronic or communicable diseases, such as HCV, are potential areas where pharmacists can play a coordinated role in achieving optimum therapeutic outcomes.Citation48 Future qualitative research from LMICs would result in beneficial additions to the existing literature in developing clinical pharmacist roles in LMICs.
Conclusion
This study shows that the provision of a specialist clinical pharmacy service focused for patient-centered care is an effective approach that would result in a positive impact on cure rates, HRQoL, and medication adherence in the HCV-infected population. Close monitoring of patients contributed to the achievement of positive outcomes in terms of goals of the therapy. This study suggests that clinical pharmacists be incorporated into multidisciplinary health-care team for care of HCV patients.
Ethics Approval and Informed Consent
Ethical approvals was obtained from the Ethical Review Board of PIMS Hospital, Islamabad (F.1-1/2015/ERB/SZABMU), SIMS (IRB/2017/333/SIMS), and the Bioethics Committee of Quaid-i-Azam University, Islamabad (DFBS/2015-248). Written consent was obtained from all patients before the start of interview.
Data Sharing
The appendix, statistical sheets, and data set will be available from the corresponding author at [email protected].
Author Contributions
All authors contributed to data analysis and drafting and revising the article. MA, FR, and SA contributed substantially in acquisition of data. VP and TR made the initial concept and design. Interpretation of data was conducted by SA, SU, and SH. The initial draft of the manuscript was written by SA and TR. Finally, all authors approved the manuscript prior to submission and agreed to be accountable for all aspects of the work.
Acknowledgments
We thank the Administration of Services, Institute of Medical Sciences, Lahore (SIMS), Pakistan and the Pakistan Institute of Medical Sciences, Islamabad (PIMS), Pakistan for their kind support. We are grateful to Professor Dr Muhammad Imran (consultant) and Jamshaid Iqbal (pharmacy technician) at SIMS, who supported during the data collection. The authors are thankful to Ms. Eleri Lougher, Principal Pharmacist Patient Services, Princess of Wales Hospital, UK for watchful insight into the final manuscript, proofreading, and suggestions for English-language improvement.
Disclosure
The authors declare that they have no financial or conflict of interest.
Additional information
Funding
References
- Hanafiah KM, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. doi:10.1002/hep.2614123172780
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29:74–81. doi:10.1111/liv.2009.29.issue-s1
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Inf. 2011;17(2):107–115. doi:10.1111/j.1469-0691.2010.03432.x
- Organization WH. Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection. World Health Organization; 2014.
- Pockros PJ, Reddy KR, Mantry PS, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology. 2016;150(7):1590–1598. doi:10.1053/j.gastro.2016.02.07826976799
- Wedemeyer H, Duberg A-S, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat. 2014;21:60–89. doi:10.1111/jvh.1224924713006
- Walker DR, Pedrosa MC, Manthena SR, Patel N, Marx SE. Early view of the effectiveness of new direct-acting antiviral (DAA) regimens in patients with hepatitis C virus (HCV). Adv Ther. 2015;32(11):1117–1127. doi:10.1007/s12325-015-0258-526538232
- Marcellin P, Chousterman M, Fontanges T, et al. Adherence to treatment and quality of life during hepatitis C therapy: a prospective, real‐life, observational study. Liver Int. 2011;31(4):516–524. doi:10.1111/liv.2011.31.issue-421382162
- Younossi ZM, Stepanova M, Henry L, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH-C). J Hepatol. 2014;60(4):741–747. doi:10.1016/j.jhep.2013.12.00624333184
- Rodis JL, Kibbe P. Evaluation of medication adherence and quality of life in patients with hepatitis C virus receiving combination therapy. Gastroenterol Nurs. 2010;33(5):368–373. doi:10.1097/SGA.0b013e3181f443cb20890160
- Manns MP. Adherence to combination therapy: influence on sustained virologic response and economic impact. Gastroenterol Clin. 2004;33(1):11–24. doi:10.1016/j.gtc.2003.12.003
- Craxi A, Pawlotsky J, Wedemeyer H; European Association for the Study of the Liver, et al. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264.21371579
- Surjadi M, Torruellas C, Ayala C, Yee HF, Khalili M. Formal patient education improves patient knowledge of hepatitis C in vulnerable populations. Dig Dis Sci. 2011;56(1):213–219. doi:10.1007/s10620-010-1455-320972850
- Kolor B. Patient education and treatment strategies implemented at a pharmacist‐managed hepatitis C virus clinic. Pharmacother. 2005;25(9):1230–1241. doi:10.1592/phco.2005.25.9.1230
- Nazareth S, Piercey C, Tibbet P, Cheng W. Innovative practice in the management of chronic hepatitis C: introducing the nurse practitioner model. Aust J Adv Nurs. 2008;25(4):107.
- Smith JP. Treatment options for patients with hepatitis C: role of pharmacists in optimizing treatment response and managing adverse events. Pharmacother. 2008;28(9):1151–1161. doi:10.1592/phco.28.9.1151
- Ravi S, Toosi MN, Karimzadeh I, Ahadi-Barzoki M, Khalili H. Adherence to chronic hepatitis C treatment regimen: first report from a referral center in Iran. Hepat Mon. 2013;13:6. doi:10.5812/hepatmon
- Re III V L, Amorosa VK, Localio AR, et al. Adherence to hepatitis C virus therapy and early virologic outcomes. Clin Inf Dis. 2009;48(2):186–193. doi:10.1086/595685
- Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.01929628281
- Pawlotsky J-M, Aghemo A, Back D, et al. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63(1):199–236.25911336
- Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1):S45–S57. doi:10.1016/j.jhep.2014.07.02725086286
- Chamorro‐de‐Vega E, Rodriguez‐Gonzalez CG, Gimenez‐Manzorro A, et al. Improving pharmacotherapy outcomes in patients with hepatitis C virus infection treated with direct‐acting antivirals: the GRUviC project. Int J Clin Pract. 2017;71(8):e12988. doi:10.1111/ijcp.12988
- Mariño EL, Álvarez-Rubio L, Miró S, et al. Pharmacist intervention in treatment of patients with genotype 1 chronic hepatitis C. J Managed Care Pharm. 2009;15(2):147–150. doi:10.18553/jmcp.2009.15.2.147
- Blobel B. Comparing approaches for advanced e-health security infrastructures. Int J Med Inform. 2007;76(5–6):454–459. doi:10.1016/j.ijmedinf.2006.09.01217074532
- Pande S, Hiller JE, Nkansah N, Bero L. The effect of pharmacist‐provided non‐dispensing services on patient outcomes, health service utilisation and costs in low‐and middle‐income countries. Cochrane Database Syst Rev. 2013;2.
- Foster GR, Irving WL, Cheung MC, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi:10.1016/j.jhep.2016.01.02926829205
- Yang S, Britt RB, Hashem MG, Brown JN. Outcomes of pharmacy-led hepatitis C direct-acting antiviral utilization management at a veterans affairs medical center. J Managed Care Specialty Pharm. 2017;23(3):364–369. doi:10.18553/jmcp.2017.23.3.364
- Waheed Y, Shafi T, Safi SZ, Qadri I. Hepatitis C virus in Pakistan: a systematic review of prevalence, genotypes and risk factors. World j Gastroenterol. 2009;15(45):5647. doi:10.3748/wjg.15.564719960560
- Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi:10.1056/NEJMoa140235524720702
- Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144(7):1450–1455. doi:10.1053/j.gastro.2013.02.03923470616
- Craxì A, Dusheiko G, Flisiak R. EASL clinical practice guidelines: management of hepatitis C. J Hepatol. 2011;55(2):245–264.21371579
- Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the common terminology criteria for adverse events version 4.0. J Am Acad Dermatol. 2012;67(5):1025–1039. doi:10.1016/j.jaad.2012.02.01022502948
- Kularatna S, Whitty JA, Johnson NW, Jayasinghe R, Scuffham PA. EQ-5D-3L derived population norms for health related quality of life in Sri Lanka. PLoS One. 2014;9(11):e108434. doi:10.1371/journal.pone.010843425365171
- Grossberg R, Gross R. Use of pharmacy refill data as a measure of antiretroviral adherence. Curr HIV/AIDS Rep. 2007;4(4):187–191. doi:10.1007/s11904-007-0027-418366950
- Alam I, Stainbrook T, Cecil B, Kistler K. Enhanced adherence to HCV therapy with higher dose ribavirin formulation: final analyses from the ADHERE registry. Aliment Pharmacol Ther. 2010;32(4):535–542. doi:10.1111/apt.2010.32.issue-420500732
- Butt M, Ali AM, Bakry MM, Mustafa N. Impact of a pharmacist led diabetes mellitus intervention on HbA1c, medication adherence and quality of life: a randomised controlled study. Saudi Pharm j. 2016;24(1):40–48. doi:10.1016/j.jsps.2015.02.02326903767
- Kagawa T, Morizane T, Saito H, et al. A randomized, controlled trial of weekly administration of lymphoblastoid interferon in patients with chronic hepatitis C. J Hepatol. 1993;17(1):91–96. doi:10.1016/S0168-8278(05)80527-68445225
- Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practiceand problems. Stat Med. 2002;21(19):2917–2930. doi:10.1002/(ISSN)1097-025812325108
- Asselah T, Boyer N, Saadoun D, Martinot‐Peignoux M, Marcellin P. Direct‐acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN‐free treatment and future perspectives. Liver Int. 2016;36:47–57. doi:10.1111/liv.1302726725897
- Federico CA, Hsu PC, Krajden M, et al. Patient time costs and out‐of‐pocket costs in hepatitis C. Liver Int. 2012;32(5):815–825. doi:10.1111/liv.2012.32.issue-522221745
- Smith MA, Love BL, Mohammad RA. The Changing Landscape of Adverse Drug Events Associated with Chronic Hepatitis C Virus Therapy. Taylor & Francis; 2015.
- Mohammad RA, Bulloch MN, Chan J, et al. Provision of clinical pharmacist services for individuals with chronic hepatitis C viral infection: joint opinion of the GI/liver/nutrition and infectious diseases practice and research networks of the American College of Clinical Pharmacy. Pharmacother. 2014;34(12):1341–1354. doi:10.1002/phar.2014.34.issue-12
- Wenzler E, Dickson W, Vibhakar S, Adeyemi OM, Danziger LH. Hepatitis C management and the infectious diseases pharmacist. Clin Inf Dis. 2015;61(7):1201–1202. doi:10.1093/cid/civ545
- Foster GR. Quality of life considerations for patients with chronic hepatitis C. J Viral Hepat. 2009;16(9):605–611. doi:10.1111/jvh.2009.16.issue-919674284
- Weiss JJ, Alcorn MC, Rabkin JG, Dieterich DT. The critical role of medication adherence in the success of boceprevir and telaprevir in clinical practice. J Hepatol. 2012;56(2):503–504. doi:10.1016/j.jhep.2011.05.01421718669
- Kerpel-Fronius S, Rosenkranz B, Allen E, et al. Education and training for medicines development, regulation, and clinical research in emerging countries. Front Pharmacol. 2015;6:80. doi:10.3389/fphar.2015.0008025926798
- Lemay V, Hamet P, Hizel C, Lemarié É, Tremblay Y. Personalized medicine: interdisciplinary perspective, world tidal wave, and potential growth for the emerging countries In: Mukesh Verma, editor. Progress and Challenges in Precision Medicine. Elsevier; 2017:301–314.
- Larrey D, Ripault M-P, Pageaux G-P. Patient adherence issues in the treatment of hepatitis C. Patient Prefer Adherence. 2014;8:763. doi:10.2147/PPA24920888
- Rabin R, Oemar M, Oppe M. EQ-5D-3L User Guide, Version 4.0. Rotterdam, Netherlands: EuroQoL Group; 2011.