Abstract
Purpose
Clostridioides difficile infection (CDI) is the most prevalent cause of nosocomial infectious diarrhea in Canada and is highly correlated with antibiotic use and contact with health care facilitates. The often-severe symptoms of CDI include diarrhea, dehydration, and abdominal pain. Patients often relapse following symptom resolution, resulting in increased morbidity. Previous research on the impact of CDI centered around the health-care system, clinician perspectives and economic burden, but not on patient experiences. The purpose of this study was to understand the impact of CDI on patients in Canada.
Methods
The Gastrointestinal Society conducted online surveys and gathered data from 167 qualifying participants, who were either patients or their non-treating caregivers. Quantitative parameters were analyzed by descriptive and comparative statistics and contextualized with qualitative insights derived from thematic analysis of open-ended questions.
Results
Our findings, which focused on clinical parameters such as prior exposure to health-care settings, antibiotic use, and patients’ symptoms, mirrored findings from previous research. Interestingly, most surveyed respondents experienced delays in diagnosis and treatment; 29% waited 6–30 days and 10% over 30 days. This delayed diagnosis was further complicated by the report that 62% of respondents did not experience symptom resolution within 7 days of initiating treatment. Importantly, our results suggest a lasting impact after the resolution of CDI and we saw a reduction of self-assessed quality of life from prior to post CDI. Patients’ priorities regarding their experience with CDI focused around concerns about the health-care system, particularly time to diagnosis and treatment, concerns about antibiotic usage and needs from health-care providers.
Conclusion
This is the first Canadian report on patients’ experience with CDI. Our data highlight the symptom-related impact on patients and the long-lasting effect on the quality of life including emotional impact. Reducing time to diagnosis and improving patient education are important priorities to attenuate the impact on patients.
Introduction
Clostridioides difficile infection (CDI) is the leading cause of hospital-acquired infectious diarrhea in adultsCitation1 with infection rates increasing since 1999.Citation2 Symptoms range from mild diarrhea to potentially lethal pseudomembranous colitis.Citation1 Typically, colonic microbiota act as barrier in the gut, providing resistance to CDI. However, disruption of host microbiota, most commonly through antibiotic treatment, results in increased susceptibility to infection.Citation3,Citation4 Furthermore, 20% of adults over the age of 65 are asymptomatically colonized by C. difficile, which increases the chance of CDI and may act as potential vehicles of transmission in a healthcare setting.Citation5,Citation6
Several research groups and national programs have surveyed health-care sites and treating physicians, to evaluate incidence, prevalence, infection control practices and economic burden of CDI in Canada. In 1997, two independent groups surveyed Canadian hospitals and showed a national average of 3.06 cases per 1000 admissions in large hospitals with over 200 bedsCitation7 and 5.9 cases per 1000 patient admissions across 19 Canadian Hospital Epidemiology Committee member sites.Citation8 A follow-up study in 2009 by the Canadian Nosocomial Infection Surveillance Program found similar numbers with 4.6 cases per 1000 admissions.Citation9 While this latter study emphasized patient outcomes using a prospective surveillance method, they did not directly survey patients. In 2011, Wilkinson et alCitation22 surveyed 2880 health-care facilities across Canada, 943 (33%) of which responded to the survey, and identified a broad variation in infection prevention and control practices that were implemented to curb CDI. Overall, acute care sites were more likely to submit liquid stools for testing, compared to mixed care and long-term care sites.Citation22 In 2015, Levy et alCitation21 analyzed direct and indirect medical costs due to CDI in Canada based on nation-wide rates of CDI associated with hospital visits. They estimated the total cost of CDI to the Canadian economy to be over CAD $280 million, 90% of which was directly related to in-hospital cost.Citation21 A recent Ontario population-based matched cohort study based on personal health information identified an increased risk for all-cause mortality and higher cost compared to uninfected control subjects.Citation23
Despite a push toward improved patient experience and a patient-centered health-care system,Citation10 no research group has surveyed Canadian patients living with CDI to understand their perspectives and experiences. Madeo et alCitation11 conducted a pilot study on patient-reported knowledge, awareness and beliefs on nosocomial infections. In their small mixed-method survey of 110 patients, they concluded that patients were aware of the risk of nosocomial infections. However, patients lacked knowledge on routes of infection and prevention. Patients’ main source of knowledge was television and newspaper, with MRSA being named most often as a source of nosocomial infection.Citation11 In a small interview-based study with 15 patients, Madeo and BoyackCitation12 researched the needs and lived experiences of elderly patients with CDI and identified four key themes of experiences – “physical suffering and impact on daily activities of living, lack of control over bowel function, lack of understanding of the illness, and issues around privacy and dignity”. They also found patients lacked an understanding as to how they became infected. As practice points, the group recommended increased patient education and improved staff-patient communication.Citation12 While a few studies investigated patient experiences with nosocomial infections, most focused on MRSA and none of these studies have been conducted in the Canadian context.
To address this knowledge gap, the Gastrointestinal (GI) Society developed a web-based survey to collect lived experiences and perspectives from patients with CDI. Questions were focused on disease severity and management, quality of life, and open-ended input on the most important priority to improve the patient’s experience with CDI.
Materials and Methods
Study Design and Recruitment
The GI (Gastrointestinal) Society developed a mixed-method (quantitative and qualitative) survey with guidance from their medical advisory board as part of their ongoing quality control. Participants were recruited via the English (www.badgut.org) and French (www.mauxdeventre.org) websites and Facebook (www.facebook.com/GISociety/) and Twitter (@GISociety) accounts of the GI Society. Additionally, the GI Society collaborated with five internal medicine physicians across Canada who shared the survey link with their patients. To qualify, participants had to self-identify as either an individual who had experienced CDI or a non-physician caregiver of someone with CDI.
Questionnaire and Data Analysis
The questionnaire was divided into two portions, non-identifiable personal information (six questions total) and questions on C. difficile (18 questions total). Twenty-three questions were multiple-choice and the final two questions were open-ended and focused on the most important priority regarding improved patient experience, as well as an opportunity to share additional comments. The complete questionnaire can be found in Appendix 1.
We included a total of 167 qualifying responses: 119 patient responses and 48 caregiver responses. Caregivers were given the instruction to answer in lieu of the patient. We analyzed each question compiled, as well as stratified by patient and caregiver responder. In this publication, data are presented from the compiled data set unless otherwise specified. Quantitative parameters were analyzed through descriptive and comparative statistics. Quality of life questions yielded non-normal results and were analyzed by Friedman test and Dunn’s multiple comparison post-hoc. These data are presented stratified by patient and caregiver. Open-ended questions were analyzed through inductive thematic analysis. JVS created the initial codebook and DL analyzed all responses using the codebook. Following independent analysis, JVS and DL refined the codebook and added new codes where applicable. JVS then re-analyzed all data using the final codebook, which is provided in Appendix 2. Percentages are calculated by the total number (104) of respondents who answered at least one open-ended question.
Results and Discussion
Demographics
A total of 267 people responded to the survey. Of these, 85 did not complete all demographic and at least one survey question and 15 were non-Canadian residents. After removing these non-eligible participants from the sample set, we analyzed a total of 167 responses, 119 of which came from people infected with C. difficile and 48 from caregivers/loved ones of an infected person. There are no publicly available recent data on CDI incidence in Canada. Our sample size of 167 is representative with a confidence level of 95% and a margin of error of 7.5% based on the estimated incidence of 37,900 in 2012.Citation21 We analyzed responses stratified by patients and caregivers and compiled the data. Most results found no statistical differences between groups and are presented compiled in this report, except when looking at questions based on quality of life (QoL). Of the total cohort, 77% of respondents were female and 22% were male. The majority (77%) were between 30 and 69 years old with a median age range of 50–59 years ().
Table 1 Gender and Age Distribution, Data are Presented as Percent of Total Respondents
Respondents included residents of all Canadian provinces, primarily from Ontario (32%), British Columbia (24%), and Alberta (16%). While the survey was offered in French and English, uptake in Quebec, the second most populated province, was low with only 8% of respondents. We did not receive responses from the Territories. A total of 19% of surveyed respondents identified as health-care professionals with the majority (49%) working in a hospital setting. Furthermore, we observed a bias toward female respondents, an observation made by other researchers when employing online surveys.Citation13
Health-Care System Exposure Preceding Diagnosis
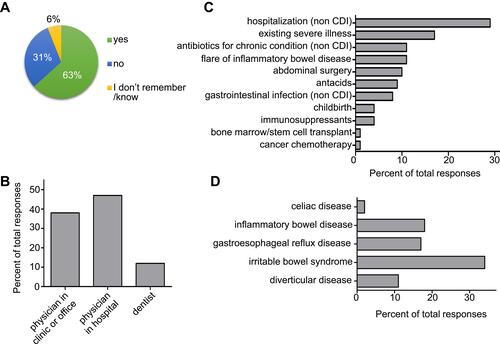
CDI has been most commonly correlated with exposure to antibiotics and prior hospitalization for non-CDI related reasons.Citation14 In line with these correlations, 63% of surveyed respondents indicated taking antibiotics in the 3 months prior to being diagnosed (). Most antibiotics were prescribed by physicians, whereas 12% of respondents were prescribed antibiotics by their dentist (). A total of 73% of respondents indicated to have experienced at least one concurrent situation, for example a medical condition or childbirth. Of these, 29% were hospitalized for non-CDI-related reasons, 17% were suffering from an existing severe illness, 11% suffered from a flare of inflammatory bowel disease, and 10% had received abdominal surgery just prior to their first experience with CDI (). CDI is a GI disease and 60% of survey respondents lived with at least one additional GI condition prior to contracting C. difficile. The majority had irritable bowel syndrome (34%), inflammatory bowel disease (18%), or gastroesophageal reflux disease (17%) ().
Figure 1 Medications and conditions prior to first CDI. (A) Distribution of participants that took antibiotics prior to diagnosis, and (B) health professional who prescribed the antibiotics. (C) Accompanying situations at the time of C. difficile infection and (D) concurrent gastrointestinal conditions.
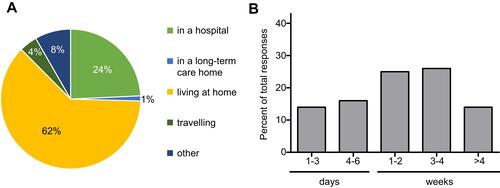
While exposure to health-care settings has been identified as a risk factor for contracting CDI, 62% of surveyed respondents indicated their symptoms began while living at home (). Only 25% indicated that they were hospitalized or living in a long-term care home. Furthermore, 8% indicated “other” and further specified they had been in contact with a health facility prior to developing symptoms. Examples of health facility contact were “shortly after being discharged from the hospital”, “working as RN in ER” and “visiting a patient in hospital”. Of those hospitalized, 69% stated that their hospital stay was prolonged because of their CDI and 33% required further hospitalization for their first infection. Importantly, these percentages might not accurately reflect the real distribution because 24 respondents selected “I was already in the hospital”, while 41% indicated their symptoms began while in the hospital. When asked for the length of their first hospital stay, 30% indicated 1–6 days, 25% indicated 1–2 weeks, 26% indicated 3–4 weeks, and 19% reported “other” and further specified times between 6 weeks and 6 months (). Notably, more people responded to “length of first hospital stay” rather than to “first CDI required hospitalization”. Furthermore, the analysis showed that the majority of those previously hospitalized reported the time of their hospital stay instead of “not applicable”. This reduced internal consistency is a limitation to our report, based on the question design. Overall, these findings confirm previous observations that most respondents had been exposed to either antibiotics, health-care settings, or both prior to their first experience of CDI.
Symptoms, Diagnosis and Treatment
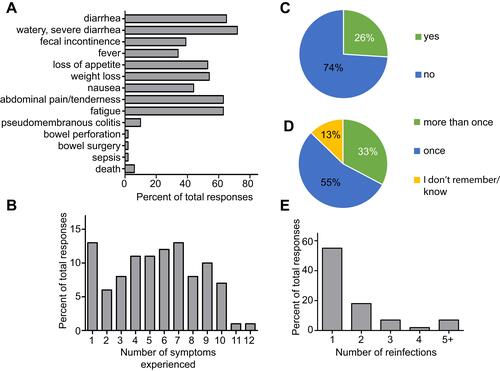
CDI can be a severe infection and the most commonly reported symptoms in our survey mirror those described in the clinical literature: watery, severe diarrhea (72%), diarrhea (65%), fatigue (63%), weight loss (54%) and loss of appetite (53%) (). Most respondents experienced two or more symptoms from a list of 12 with the majority reporting between four and seven (). Past clinical trials estimated the expected recurrence rate for CDI to be between 25% and 30%Citation15,Citation16 which is in line with our observation that 26% of respondents indicated CDI recurrence (). Importantly, 33% of respondents indicated having CDI more than once and 13% could not remember (). This might mean that survey participants used different definitions of recurrence than clinicians, or that not all repeated CDI experiences were diagnosed as recurrence. Of the respondents who had CDI more than once, 27% experienced CDI two to four times while 7% experienced CDI five or more times (). Respondents were given the option to specify the number of recurrences if they selected 5+ times and we found the maximum to be 12 times. These data demonstrate that our participant population is representative compared to the expected symptoms and recurrence rates.
Figure 3 Symptoms and recurrence. Symptoms and complications experienced with CDI, (A) symptoms as percent of total responses and (B) number of symptoms experienced per individual as percent of total responses. (C) Recurrence of C. difficile infection compared to (D) reinfection with C. difficile and (E) number of C. difficile reinfections per respondent.
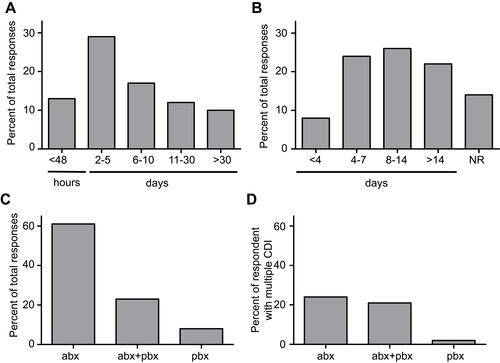
To provide prompt treatment for CDI, time to diagnosis is critical. Nevertheless, only 13% of respondents received their diagnosis within the first 48 hrs after the onset of their symptoms (). In contrast, 29% had to wait between 2 and 5 days and 29% between 6 and 30 days. Surprisingly, 10% of respondents indicated they had to wait over 30 days between the onset of symptoms and diagnosis. The mean time to resolution of diarrhea after initiation of antibiotics in CDI has been reported as 3–4 days,Citation17 but some patients take longer. Current guidelines recommend extending the standard 10d treatment course to 14d if diarrhea has not resolved by day 10.Citation18,Citation19 Interestingly, only 32% of respondents indicated that their symptoms resolved within 7 days. While 26% experienced resolution between 8 and 14 days, 22% were treated for longer than 14 days and 14% did not experience resolution (). As expected, the majority (94%) of respondents were treated with antibiotics for their first CDI (), but 6% indicated they did not receive treatment for their CDI at all. Of these, 27% indicated they concurrently used probiotics while 8% of all respondents used only probiotics to treat their first CDI. In contrast, 96% of respondents treated their subsequent CDI with antibiotics and 47% of these used probiotics in addition to antibiotics (). We did not ask whether people received their probiotics after consultation with a health-care professional.
Figure 4 Diagnosis and treatment. (A) Time between onset of symptoms and diagnosis and (B) time between commencement of treatment and symptom resolution, or no resolution (NR). Antibiotic (abx), probiotic (pbx) or combination (abx+pbx) treatment used to overcome first (C) and any subsequent (D) C. difficile infections.
If antibiotic therapy fails to resolve recurrent CDI, fecal microbial transplantation (FMT) is reported to be a safe and effective alternative.Citation20 One respondent out of the 167 participants indicated receiving FMT for their first CDI episode. More people received FMT for subsequent CDI, albeit much less than expected. A total of five respondents out of 67 who had experienced subsequent CDI indicated treatment by FMT. The open-ended section of the survey revealed that these participants experienced FMT to be the cure to their CDI with one participant commenting they would have liked to try FMT, but it was not available to them. All respondents who received FMTs also indicated they were treated with antibiotics. Overall, these responses demonstrate that most patients were treated with antibiotics as the first line of therapy and more are turning to probiotics during recurrent infections.
CDI Impact on Life
CDI can have a devastating impact on patients and we were interested whether there may be any lasting impact on self-assessed quality of live (QoL). To address this, three questions on the survey accessed participants’ QoL with scores ranging from the “6” (able to carry on normal activity including work; no special care needed, social activities not restricted) to “1” (unable to care for self and requiring institutional or hospital care). Detailed qualifiers for all QoL numbers are listed in . The majority (77%) of responding patients (patient group) who identified as “person who has been infected with the C. difficile bacterium” reported a QoL of 6 before their first CDI experience (). In contrast, respondents who identified as “caregiver/loved one of a person who has been infected with the C. difficile bacterium, but not in a health care provider capacity” (caregiver group) and responded in lieu of the patient, indicated a lower median QoL of 5 before the patient’s first CDI experience, with interquartile ranges of QoL of 3 and QoL of 6 (). The impact that CDI has on patients was evident from the reduced QoL participants indicated for their worst experience of CDI (). The patient group reported a median QoL of 4 with 37% reporting to be unable to care for self (QoL of 1 and 2), 35% unable to work and/or needing some assistance with normal activities (QoL of 3 and 4) and 28% with minor or no restrictions to their normal activities (QoL of 5 and 6). Again, the caregiver group indicated a lower QoL compared to the patient group with a median QoL of 3 (). Interestingly, while 41% of this group reported a QoL of 1, compared to only 23% in the patient group, 30% of caregivers indicated a QoL of 6 at the worst stage of CDI compared to only 10% of the patient group. Taken together, these observations suggest that someone who experienced a reduced quality of life prior to CDI, experienced on average a more severe impact on their quality of life during active disease. However, a subgroup might be less affected by the symptoms of CDI.
Table 2 Survey Qualifiers to Query Quality of Life Scores
Figure 5 Impact of C. difficile infection on quality of life, data separated by (A) patient and (B) caregiver response. Data presented as violin plot of individual survey responses. Thickness of plot corresponds to number of responses whereas all responses are plotted.
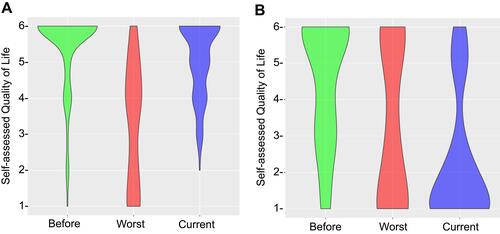
Finally, we were interested in the long-term impact of CDI on people’s lives. To answer this question, we asked participants for their current quality of life. We acknowledge that this question does not conclusively address our question as participants might have answered the survey at different stages after recovering. We found a dramatically reduced median QoL of 1 in the caregiver group but cannot conclude that this QoL is related to CDI or another disease. We also observed a slightly reduced median QoL of 5.5 and broader interquartile ranges in the patient group. Those patients who received FMT to treat their CDI reported current QoL at the same level as before their first CDI experience and perceived FMT to have cured their CDI. Current clinical trials on FMT include assessing health-related quality of life measures and will likely be able to provide further insights into this observation. For the purpose of our analysis, we assumed that people would not have filled out our survey while experiencing CDI symptoms and therefore “currently” means post-CDI. This is a limitation to our analysis and follow-up research should address this point. While 77% indicated a QoL of 6 pre-CDI, 50% indicated this post-CDI. As analyzed by paired analyses, these respondents now indicate a QoL of 5 (24%), of 4 (18%), and of 3 (8%). From the open-ended question, we learned that many respondents suffer from long-term consequences after their CDI was cured. We did not have enough participants to further stratify our analysis based on time between symptom onset and diagnosis or treatment. Overall, the data demonstrate a significant impact of CDI on patient’s self-assessed QoL and the lasting impact CDI has on a subgroup of patients, highlighting the importance of early diagnosis and a rapid cure.
Most Important Priority to Improve Patient Experience
In the final two survey questions, participants were asked to highlight their most important priority in improving the experience with CDI and to share further comments. We found their answers to overlap sufficiently, compiled both questions and analyzed the answers using inductive thematic analysis. While reading the answers, four major themes emerged, each of which we sub-categorized into two to six individual codes (Appendix 2). We then compared our codebook with the key insights described in the 15-patient interview study by Madeo and BoyackCitation12 and identified each of their themes in our cohort. The key emerging themes in our survey were concerns about the healthcare system (63%), concerns about antibiotics (17%), needs from healthcare providers (45%) and impact on life (49%) (). Percentages represent number of people who responded to the open-ended questions and not all survey respondents. Most of those who answered the question on important priorities commented on several themes in their response. Of the comments about the healthcare system, we found the most important priority to be time to diagnosis (26%) and speed and selection of treatment (10%), which relates to the importance of timely diagnosis and treatment discussed above. Importantly, 5% highlighted that they received a wrong initial diagnosis while 4% were not referred to a specialist fast enough. A total of 13% observed insufficient prevention protocols such as “I witnessed cleaning personnel not cleaning hospitals properly, while I have been in Emergency at all hours”. Furthermore, 6% experienced poor attitudes by doctors and hospital staff to patients, for example. “I had a nurse berate me in emerge because I ‘contaminated’ the bathroom, screaming that in front of all the patients”. This insight is reflected in Madeo and Boyack’sCitation12 theme as “issues around privacy and dignity”. Of the respondents indicating concerns about antibiotics, five mentioned their CDI was related to a prescription for clindamycin by a dentist.
Table 3 Emerging Themes and Distribution of Responses. Data Presented as Percentages of Respondents Who Answered to Open-Ended Questions (104 Total Responses) and Percentage of Themes
Needs from health-care providers were focused around patient education (12%) which aligns with Madeo and Boyack’sCitation12 theme “lack of understanding of the illness” and “lack of knowledge on how the patients got infected”. Furthermore, respondents highlighted the importance of getting cured (9%) which was related to ongoing bowel concerns and recurrence (12%). Many respondents indicated their preference for using pro/prebiotics (9%) and some considered seeing alternative providers (2%) most important to improving their experience.
Half of the respondents commented on the impact of CDI on their lives. Specifically, 12% commented on the severity of symptoms. Our code merged Madeo and Boyack’sCitation12 “physical suffering” and “lack of control over bowel function”. Furthermore, 11% indicated changes in daily habits, such as “ensure you always wash hands and clean areas you touch” and “Before contracting c diff., I was a very healthy and active individual”, which is mirrored in “impact on daily activities of living” by Madeo and Boyack.Citation12 Finally, 22% highlighted the emotional impact of CDI on either patients or family and caregivers. One significant answer summarized this as “My mother was isolated in the hospital once she contracted the disease and became so depressed that she decided to stop all medications and dialysis and die”. Overall, the responses support and mirror our insights from the quantitative portion of the survey and highlight a need for further education of health-care professionals to compassionately deal with CDI in a fast and effective manner.
Conclusion
This report is the first survey of Canadian residents with lived experience of CDI and among the first internationally. We recognize that a limitation of our study is potential selection bias, as survey recruitment targeted followers of the GI Society. Patients who experience recurrence or additional gastrointestinal conditions might be more likely to follow the GI Society which might have skewed our results toward those more severe cases. The survey link was also shared by internal medicine doctors, which we believe reduces possible selection bias. Follow-up studies to our exploratory survey should aim to get a random sample with a larger sample size from across Canada to minimize selection bias and improve generalizability.
Our results confirm expected clinical parameters and report important insights regarding the lasting impact of CDI on patients’ lives. As previously reported, contraction of CDI was correlated with prior exposure to antibiotics and/or health facilitates and two-thirds of our survey respondents experienced at least one other GI condition. This population might be more likely to visit health facilities than the general public, thereby increasing their risk for infection with C. difficile.
Time to diagnosis is critical for fast and effective treatment; however, only a minority of respondents received their diagnosis within 7 days after symptom onset. A similar-sized group experienced complete resolution of symptoms within 7 days after starting treatment. Our sample size was too small to stratify treatment success based on time to diagnosis.
We identified a long-lasting impact of CDI and we found overlapping and different themes as described by Madeo and Boyack.Citation12 Clearly, the acute impact of CDI on patient’s lives is related to the severity of symptoms which bring upon physical suffering and precluded patients from participating in their daily activities. As CDI is a GI condition with symptoms such as severe diarrhea, patients are concerned with their privacy and dignity. Several patients experienced poor attitudes of hospital staff related to their bowel issues and highlighted a need for improved patient-staff communication.
Finally, we identified a lack of education. Patients indicated the need for understanding the illness and its potential impact on their future lives. Greater awareness among hospital staff and adherence to cleaning protocols could potentially reduce future infections with C. difficile. Several patients observed poor hygiene and cleaning in their respective health-care settings. Additionally, as many patients experienced slow diagnosis and referral to a specialist, which translated to a delay in treatment commencement, we see a need for education of physicians to correctly identify the symptoms. We saw several comments on misdiagnosis of CDI for anxiety during ER visits which could be addressed through communication and education strategies within hospitals across Canada.
Our data clearly indicate a need for further standardizing prevention and control practices across Canada. Reducing infection rates and curbing the spread of CDI would benefit the patient’s lives and ultimately reduce the cost of CDI to the Canadian economy.
Ethics Approval and Informed Consent
The original survey was hosted through the GI Society as quality control for their ongoing programming. Survey respondents were provided with a cover letter to the survey (Appendix 1) that stated reasons for conducting the survey, eligibility criteria, and information on how the results will be used. Consent to participate was provided by selecting one of the two eligibility criteria. All participants were 19 years of age and older. The GI Society was not required to obtain ethics approval for this quality control study and did not explicitly ask participants to provide informed consent. Prior to sharing the anonymous data for scientific analysis, JVS and TS obtained ethics approval for secondary use of data by the UBC Clinical Research Ethics Board (UBC CREB Number H17-00747) to ensure it was ethical to analyze and publish these data.
Data Sharing Statement
The data used to support the findings of this study were supplied by the Gastrointestinal Society, a Canadian registered charity under license and so cannot be made freely available. Requests for access to these data should be made to Gail Attara, President & Chief Executive Officer, Gastrointestinal Society, [email protected].
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. Survey design and implementation, GPA; methodology, formal analysis, data curation, JVS; qualitative analysis, JVS and DL, figure preparation, JVS and DL, writing – manuscript preparation, JVS; writing – review and editing, JVS, GPA, DL, TSS; supervision, TSS; funding acquisition, GPA, JVS.
Funding
The Gastrointestinal Society received funding from Merck Canada Inc. to conduct this independent survey. Jens Vent-Schmidt received stipend funding through a CIHR Vanier Canada Graduate Scholarship.
Disclosure
Gail Attara is an employee of the Gastrointestinal Society. Gail Attara reports grants from Merck, during the conduct of the study. Jens Vent-Schmidt reports personal fees from the Government of Canada, during the conduct of the study. Theodore S Steiner reports grants, personal fees from Merck, during the conduct of the study; grants from Merck, personal fees from Pendopharm, Verity, clinical trial support from Rebiotix, Seres, and Sanofi Pasteur, personal fees from Avir, outside the submitted work. The opinions expressed in this manuscript are those of the authors, based on the results, and do not necessarily reflect the views of Merck Canada Inc., which has not been involved in survey development data collection and data analysis. The authors report no other conflicts of interest in this work.
References
- Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. Can Med Assoc J. 2004;171(1):51–58. doi:10.1503/cmaj.1031189
- Zoutman DE, Ford BD. A comparison of infection control program resources, activities, and antibiotic resistant organism rates in Canadian acute care hospitals in 1999 and 2005: pre- and post-severe acute respiratory syndrome. Can J Infect Control off J Community Hosp Infect Control Assoc = Rev Can Prev Des Infect. 2009;24(2):109–115.
- Wilson KH. The microecology of Clostridium difficile. Clin Infect Dis. 1993;16 Suppl 4:S214–8. doi:10.1093/clinids/16.Supplement_4.S214
- Rees WD, Steiner TS. Adaptive immune response to Clostridium difficile infection: a perspective for prevention and therapy. Eur J Immunol. 2018;48(3):398–406. doi:10.1002/eji.201747295
- Furuya-Kanamori L, Marquess J, Yakob L, et al. Asymptomatic Clostridium difficile colonization: epidemiology and clinical implications. BMC Infect Dis. 2015;15(1):1–11. doi:10.1186/s12879-015-1258-4
- Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758. doi:10.7326/0003-4819-145-10-200611210-00008
- Alfa MJ, Du T, Beda G. Survey of incidence of Clostridium difficile infection in Canadian hospitals and diagnostic approaches. J Clin Microbiol. 1998;36(7):2076–2080.
- Hyland M, Ofner-Agostini M, Miller M, Paton S, Gourdeau M, Ishak M. N-CDAD in Canada: results of the Canadian Nosocomial Infection Surveillance Program 1997 N-CDAD Prevalence Surveillance Project. Can J Infect Dis. 2001;12(2):81–88.
- Gravel D, Miller M, Simor A, et al. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48(5):568–576. doi:10.1086/596703
- Cochrane BS, Hagins M, King JA, Picciano G, McCafferty MM, Nelson B. Back to the future: patient experience and the link to quality, safety, and financial performance. Healthc Manag Forum. 2015;28(6_suppl):S47–S58. doi:10.1016/j.ajodo.2015.07.024
- Madeo M, Shields L, Owen E. A pilot study to investigate patients reported knowledge, awareness, and beliefs on health care-associated infection. Am J Infect Control. 2008;36(1):63–69. doi:10.1016/j.ajic.2007.01.008
- Madeo M, Boyack M. Using the lived experiences of patients with Clostridium difficile infection to improve care. Nurs Times. 2010;106(36):10–13.
- Becker HM, Grigat D, Ghosh S, et al. Living with inflammatory bowel disease: a Crohn's and Colitis Canada survey. Can J Gastroenterol Hepatol. 2015;29(2):77–84. doi:10.1155/2015/815820
- Delate T, Albrecht G, Won K, Jackson A. Ambulatory-treated Clostridium difficile infection: a comparison of community-acquired vs. nosocomial infection. Epidemiol Infect. 2015;143(6):1225–1235. doi:10.1017/S0950268814001800
- Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile Infection. N Engl J Med. 2017;376(4):305–317. doi:10.1056/NEJMoa1602615
- Tieu JD, Williams RJ, Skrepnek GH, Gentry CA. Clinical outcomes of fidaxomicin vs oral vancomycin in recurrent Clostridium difficile infection. J Clin Pharm Ther. 2018;(June):1–9. doi:10.1111/jcpt.12771
- Wilcox MH, Howe R. Diarrhoea caused by clostridium difficile: response time for treatment with metronidazole and vancomycin. J Antimicrob Chemother. 1995;36(4):673–679. doi:10.1093/jac/36.4.673
- McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2017;66(7):e1–e48. doi:10.1093/cid/cix1085
- Loo VG, Davis I, Embil J, et al. Association of medical microbiology and infectious disease Canada treatment practice guidelines for Clostridium difficile infection. Off J Assoc Med Microbiol Infect Dis Canada. 2018;3(2):71–92. doi:10.3138/jammi.2018.02.13
- Daniels LM, Kufel WD. Clinical review of Clostridium difficile infection: an update on treatment and prevention. Expert Opin Pharmacother. 2018;00(00):1–11. doi:10.1080/14656566.2018.1524872
- Levy AR, Szabo SM, Lozano-Ortega G, et al. Incidence and costs of Clostridium difficile infections in Canada. Open Forum Infect Dis. 2015;2(3):ofv076. doi:10.1093/ofid/ofv076
- Wilkinson K, Gravel D, Taylor G, et al. Infection prevention and control practices related to Clostridium difficile infection in Canadian acute and long-term care institutions. Am J Infect Control. 2011;39(3):177–182. doi:10.1016/j.ajic.2011.01.007
- Nanwa N, Sander B, Krahn M, et al. A population-based matched cohort study examining the mortality and costs of patients with community-onset Clostridium difficile infection identified using emergency department visits and hospital admissions. PLoS ONE. 2017;12(3):e0172410. doi:10.1371/journal.pone.0172410