Abstract
Purpose
Adolescents and young adults (AYAs) with severe hemophilia use prophylaxis that requires a high level of adherence. The present study aimed to explore the underlying reason for adherence and non-adherence to prophylaxis in hemophilia from the perspective of AYAs.
Patients and Methods
A qualitative study in Dutch AYAs with hemophilia (14–25 years) using prophylaxis was executed. Focus group interviews and individual interviews were recorded, transcribed, coded and analyzed using an iterative process. Member checking in three respondents was used to validate the potential model.
Results
A total of 21 interviews were performed. Parental support decreased when AYAs gained more treatment responsibilities, which resulted in a higher risk for non-adherence. AYAs were weighing their potential bleeding risk per activity based on the wish to do what they prefer while also wanting to simultaneously feel safe. When bleeding with low impact on their daily life occurred, or when bleeding remained absent, AYAs felt safe and the perceived need for prophylaxis decreased.
Conclusion
The level of treatment responsibility per AYA and estimated risks per activity were the two main underlying reasons for (non-)adherence.
Clinical implications
We suggest using a conversation technique to discuss adherence, especially during bleeding assessment visits.
Graphical abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Adolescents and Young Adults (AYAs) struggle with “normal” changes in biological, physical and emotional wellbeing.Citation1 Adolescents with a chronic illness are exposed to additional challenges such as accepting responsibility for their disease and its treatment. The physical and emotional changes of AYAs can result in difficulties in imagining the future and the rejection of parents or medical professionals.Citation1 Potential peer pressure and AYAs’ desire to be normal can easily lead to non-adherence to their chronic treatments.Citation2 Notably, self-management skills must be learned during adolescence. These skills are defined as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a chronic condition”.Citation3
Hemophilia is a rare X-linked congenital bleeding disorder affecting ±1/10.000 males. People with hemophilia A lack clotting factor VIII, those with hemophilia B lack clotting factor IX. Bleeding tendency in hemophilia A and B is similar. People with severe hemophilia (clotting factor VIII/IX <1%) need prophylactic factor replacement therapy.Citation4 Prophylactic treatment consists of intravenous injections twice weekly to every other day (i.e. 2–3.5 injections/week) to maintain minimal clotting factor activity levels and prevent bleeding, prophylaxis is initiated at the onset of bleeding, usually between 2 and 4 years, and continued for life. As soon as possible, parents are taught to perform intravenous injections at home. Using disposable needles, the injections are performed manually in 2–3 mins and can be performed in a home setting. Just before puberty, around the age of 13 years, boys are taught to infuse themselves.Citation5 This continuous intensive intravenous treatment is very demanding for both parents and children.
Despite this treatment are AYAs with severe hemophilia continuously at risk for bleeding in their joints, muscles, and soft tissue.Citation6 Such bleeding can occur spontaneously and lead to irreversible damage, especially in the joints. Multiple instances of bleeding in the joints can lead to severe arthropathy and disability at a young age.Citation4,Citation7 To prevent this bleeding, AYAs with severe hemophilia require continuous and life-long intravenous prophylactic replacement therapy.Citation6 A high level of adherence is crucial to maintain the factor levels and prevent bleeding.Citation7 During adolescence, adherence drops in hemophilia (range 13% to 17% non-adherenceCitation8,Citation9), which is comparable to that of other chronic diseases.Citation10,Citation11 Non-adherence in hemophilia leads to significantly more breakthrough bleedings, target joints, more days missed at school and work, and subsequently a lower quality of life.Citation12,Citation13
Little remains known about the underlying reasons for non-adherence to prophylaxis from the AYA perspective. In adults, adherence to prophylaxis was associated with acceptance, feeling and fearing symptoms, as well as understanding and planning and infusion skills.Citation14 Brand et alCitation9 studied the reasons for non-adherence during the transition from pediatric care to adult services. Notably, they defined social (eg lower family and peer support), emotional and developmental (eg rebellion against prescribed treatment), practical (eg lack of time) and educational (eg lack of knowledge) issues.Citation9 We hypothesized that the underlying reason for (non)adherence in AYAs depends on factors other than care transition.
Understanding the underlying reasons for non-adherence, as experienced by AYAs, could help healthcare providers to begin patient-centered conversations and provide patient-tailored care. Therefore, the present study aimed to explore the underlying reasons for (non)adherence to prophylaxis in hemophilia from the perspective of AYAs.
Materials and Methods
This present study comprises a qualitative exploration using a grounded theory approach.Citation15 This approach is designed for developing a theoretical understanding of a subjective process that continues over a known period of time.Citation15 Data was collected using focus group interviews and individual interviews. Institutional review board (IRB) of the University Medical Centre Utrecht approved this study. The approval was obtained before start, written consent was obtained from participants before the interviews and the COREQ guidelineCitation16 was used for reporting qualitative research.
Sampling
Dutch AYAs (age 14–25 years) with hemophilia that were prescribed prophylactic treatment for at least two consecutive years at a minimum frequency of two times a week were invited to participate in the present study. To ensure sufficient experience and variability with decision making regarding the administration of routine prophylaxis in daily life, it was decided to include only patients who used prophylaxis for a minimum of two years. AYAs were excluded when they were unable to read or understand Dutch. Under qualitative sampling strategies, purposeful sampling was applied to create a diverse sample. Therefore, maximum variation was sought for age, adherence levels and bleeding frequency in the sample.Citation17 Predefined definitions were used to assess adherence levels,Citation18 bleeding frequency and education level (). Eligible AYAs, were informed about the study by phone. If they were interested in participating, they received an information letter by e-mail. The information letter contained information about the reason for this study and research aim. After providing potential participants with one week to consider participation (and their parents of young adults between the age of 14–18 years old), they were called to determine whether they were interested in participating. AYA’s, and if they were younger than 18 years both parents and AYA, signed written informed consent.
Table 1 Pre-specified definitions used for sampling variation
Data Collection and Study Procedures
A total of three face-to-face focus groups were conducted. The focus group interviews included a fun activity in advance (an “escape room”) to create a relaxed atmosphere between participating AYAs and to stimulate discussions among them. Individual interviews were subsequently performed to generate more in-depth exploration about barriers, motivators and facilitators of prophylaxis and decision making regarding adherence. Three respondents were then interviewed a second time to present them with the emerged conceptual figure and to accomplish this (member checking). All interviews were face-to-face and each interview took between 30–90 mins in total. According to the AYAs’ preferences, interviews were conducted either in their homes or at the hemophilia treatment center (HTC). Following interviews, AYAs answered three questions to determine their adherence level and checked with diaries or pharmacy data.
The interviews were guided by a topic list based on the literatureCitation14,Citation19 and the clinical expertise of the research team (JH, MK, KF, LS). The topics were converted to open questions and adapted (when necessary) after each round of interviewing (details in and Appendix 2). Interview topics included perceptions regarding hemophilia, self-monitoring and decision-making, barriers, motivators and facilitators of adherence, and the integration of prophylaxis in daily life. All interviews were audiotaped, transcribed verbatim and anonymized. Transcriptions were not returned to the respondents for comments, yet the conceptual figure was discussed and verified by respondents (member checking). The focus group interviews were executed by two (female) hemophilia nurses (JH and LS), both with formal interviewing training. Memos were made during the focus group by the second interviewer (LS) and used to evaluate the focus group. The individual interviews were conducted by one interviewer (JH or LS). Both interviewers had no current healthcare provider relationship with the respondents. The interviewers attempted to create a non-judgmental atmosphere and they emphasized the importance of learning from the AYAs. Further medical and treatment baseline characteristics (age diagnose and prescribed medication) were extracted from the medical record.
Box 1 Topic list
Qualitative Data Analysis
According to qualitative guidelines,Citation20 data were analyzed using open, axial, and selective coding.Citation17,Citation21 After each interview, the process started with a thorough reading of the interview followed by summarizing and conceptualizing the content.Citation20 Open coding of meaningful fragments was performed and categorized guided by their content into a more conceptual category (axial coding). Codes and themes were derived from the data and not specified in advance.
Interviews were coded by JH and verified by LS After each round of approximately 3 to 4 interviews, the (new) results were discussed among the study team (JH, MK, KF, and LS). Selective coding was used to compare new data with existing themes, leading to the strengthening of existing themes or the establishment of new themes (Appendix 1; additional details can be provided on request). The analysis process was supported by the qualitative software package NVivo 11®.
Results
Overall, 18 of the 38 AYAs approached agreed to participate in the present study (response rate: 47%), with 21 interviews being obtained (three respondents were interviewed twice). The main reasons for refusal included limited time and lack of interest. A total of nine AYAs joined one of the three focus groups (n=2, n=4, n=3 per group), while nine AYAs were interviewed individually. Data saturation was reached on the main components, meaning that no new themes or meaningful fragments were identified for addition into the current structure of the model.Citation17,Citation22
The majority of AYAs was interviewed at home in the absence of family members, though one was interviewed in the presence of some family members by their request. Patient characteristics are presented in . The median age was 18 years (range 14–24 years). A total of 11 AYAs were classified as adherent, while four were sub-optimally adherent and three were non-adherent to their prophylactic regimen.Citation18
Table 2 Demographic and background characteristics (N=18 patients with severe hemophilia)
Adherence behavior in AYAs: Varying treatment responsibility and estimating risk
Based on the obtained data, the present study revealed that adherence behavior was dependent on 1) the level of treatment responsibility and 2) the risk estimation of prophylaxis per activity. The first underlying reason was explained by the varying levels of parental support regarding treatment responsibility that AYAs experienced. Notably, three consecutive phases related to growing up with hemophilia were observed. In these three consecutive phases, the treatment responsibilities increased while the parental support decreased and the risk of non-adherence increased.
The second underlying reason was explained by AYAs weighing their potential bleeding risk by day and activity. They explained that this risk assessment was based on the desire to do what they prefer while simultaneously feeling safe. Feeling safe was described by AYAs as the absence of bleeding impacting on their (daily) life. Doing what they prefer was described as acting like their healthy peers. A schematic overview of this process is shown in , while presents the underlying themes.
Figure 1 Decision making concerning prophylaxis adherence among AYAs: Treatment responsibility and estimating bleeding risk.
Notes: Phase 1: parents took responsibility for their prophylactic treatment and performed bleeding management phase 2 and 3 increased self-management causing considerations concerning adherence
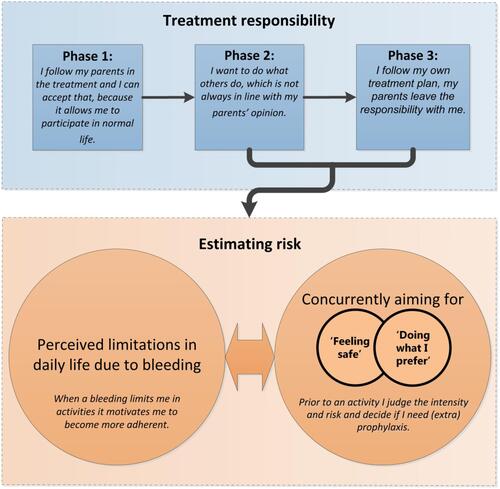
Table 3 Quotes explaining adherence to prophylactic treatment in adolescents with hemophilia
Varying treatment responsibility
AYAs mentioned varying levels of parental support, which resulted in various responsibilities for each AYA regarding prophylaxis. With increasing age, we identified three consecutive phases in the AYAs’ responsibility for treatment, with concomitant changes in adherence (see for a conceptualization of this description).
First phase of treatment responsibility
In the first phase, AYAs felt able to infuse themselves; however, parents performed the infusions most of the time. AYAs mentioned that their parents took responsibility for their prophylactic treatment and performed bleeding management. Most of the AYAs were unaware of their treatment schedule. They told us that their parents never skipped or missed prophylaxis, which enabled them to rely on their parents. AYAs explained that they felt comfortable with the support of their parents. AYAs in this phase informed us they experienced minimal or no bleeding at all. Moreover, AYAs described short discussions with their parents regarding the necessity of prophylaxis, and they mostly agreed with the opinion of their parents. Due to the high level of parental support, most AYAs in this phase adhered to the prescribed dose and frequency of prophylaxis.
Second phase of treatment responsibility
During the second phase, most AYAs explained that they prepared and infused the prophylaxis independently. Sometimes they skipped or forgot their infusion despite reminders from their parents. AYAs in this phase mentioned that they sometimes experienced bleeding. While they informed us that they recognized bleedings, decision making concerning bleedings varied per AYA. Some AYAs made their own decisions, while others asked their parents for advice. AYAs mentioned that they gradually became more responsible for their treatment by taking over the preparation and infusion, and eventually remembered and performed infusions independently. It was unclear whether the AYAs or their parents initiated the process of becoming more responsible. AYAs explained that they sometimes estimated the need for prophylaxis, which resulted in discussions between parents and the AYAs. Notably, AYAs experienced their parents being too careful. In this second phase, the combination of more responsibilities and estimated the need for prophylaxis resulted in a variety of adherence levels.
Third phase of treatment responsibility
In the third phase, AYAs felt entirely responsible for preparing and administering prophylaxis, as their parents were no longer involved. Some AYAs mentioned that they would rarely forget their prophylaxis, and, if they did, they took it as soon as possible. On the other hand, some AYAs told us that they purposefully skipped prophylaxis because they did not feel the need to take it. AYAs explained that they were weighing arguments in favor of and against prophylaxis. As such, AYAs were making their own decisions concerning their prophylactic treatment. Nearly all AYAs had experience with bleeding. AYAs told us that they only contacted their parents for advice in the case of an emergency and that discussions about this with their parents were out of the question. In this phase, the increased independence and weighing arguments related to prophylaxis resulted in various adherence levels.
Estimating risk
All AYAs mentioned that they were weighing treatment decisions based on the desire to do what they prefer while simultaneously feeling safe. These decisions were affected by the estimated bleeding risk. All AYAs stressed the importance of wanting to live like their healthy peers, without the presence of bleeding. Some AYAs reported that they felt safe when no bleeding occurred or when bleeding did not affect their daily activities. Other AYAs weighed the perceived need for prophylaxis per activity (“Is this activity safe without prophylaxis?”). Meanwhile, all AYAs mentioned skipping activities when they did not feel safe (even with prophylaxis). Therefore, AYAs mentioned that feeling safe was more important than doing what they preferred.
The impact of bleeding influenced the balance between feeling safe and doing what they preferred. AYAs mentioned that bleeding with a substantial impact on daily life motivated them to behave more adherently. When this bleeding occurred after a missed infusion, it was more motivating to take prophylaxis the next time. On the other hand, AYAs who experienced minimal bleeding or minimal bleeding impact were more non-adherent.
Discussion
The present study aimed to explore the underlying reasons for non-adherence to prophylaxis in hemophilia from an AYA perspective. The results revealed that adherence behavior was dependent on the 1) level of treatment responsibility and 2) AYAs risk estimation of prophylaxis per activity. We identified three consecutive phases in growing up with hemophilia that reflected changes in treatment responsibility: the treatment responsibilities increased, parental support decreased and adherence levels decreased. Notably, AYAs were estimated the need for prophylaxis by activity (risk estimation). They explained that this risk estimation was based on their desire to feel safe and do what they prefer.
Various strategies were used to optimize internal validity and minimize bias.Citation23 Interviewer bias was reduced by using formally trained interviewers specializing in hemophilia that did not have a treatment relationship with the patients. Three interview methods (focus group, individual and respondent validation) were used to establish general knowledge and more in-depth insights as well as to verify themes that emerged. Purposeful samplingCitation21 was used to improve external validity, while data saturationCitation24 for the main components was reached during the final two interviews. The distribution between adherence and non-adherence to prophylaxis among AYAs was approximately equal, although the inclusion of more non-adherent AYAs could create a more detailed understanding. All eligible AYAs were approached, and a total of 47% were willing to participate. We experienced that AYAs (especially non-adherent AYAs) were difficult to recruit due to their busy lives and lack of interest in research. Other qualitative studies reported comparable difficulties in recruiting AYAs.Citation19,Citation25 This probably resulted in sampling bias. The results of the present study are supported by previous studies in the literature. Namely, our findings concerning treatment responsibility are supported by two studiesCitation26,Citation27 that both described a gradual process of adolescents achieving self-management in three consecutive phases. Both studies mentioned AYAs taking over illness-related activities during adolescence (eg remembering medication, taking medication, conversations with clinicians) and decreased parental support between early, middle and late adolescence.Citation26,Citation27 In our opinion, this development is in line with the normal psychological development of puberty. This is supported by Casey at al. in their statement that “adolescence is the period in which independence skills are acquired to increase the success of separating from the protective influence of the family”.Citation28 In 2015, a comparable qualitative study in adults with hemophilia was performed,Citation14 which identified four factors influencing adherence to prophylaxis: 1) acceptance of hemophilia, 2) feeling and fearing symptoms, 3) understanding hemophilia and prophylaxis, and 4) planning and infusing skills. The “feeling and fearing symptoms” in adult corresponds with “feeling safe”, which was identified in the present study. In both adults and AYAs, this factor affects treatment decisions concerning adherence. AYAs did not report difficulties concerning understanding hemophilia and prophylaxis, or any difficulties in planning and infusing. This might be explained by the fact that their parents served a major role in administering the prophylaxis during the first two phases of adolescence and that these two factors are more expected in elderly people.
In the present study, the identified barriers to adherence resonate with findings in AYAs with cystic fibrosis (CF-AYA). Sawicki et alCitation29 performed a qualitative study exploring barriers to adherence among adolescents with cystic fibrosis. One of the barriers reported was interpreted as CF-AYA wishing to be “normal” instead of different or disabled. This is comparable to our findings (“to do what I prefer”). The second reported barrier was a lack of perceived consequences, which was explained as not recognizing the impact or not seeing the need for treatment. CF-AYA stated that the therapy “makes no difference”, which was congruent with hemophiliac AYAs that experienced minimal or no bleeding.
The present study has implications for clinical practice. Several interventions to improve adherence in AYAs with a chronic disease were studied. Most interventions were focused on reminders,Citation30 interactive smartphone apps,Citation30,Citation31,Citation32 text messagingCitation30,Citation31 and motivational interviewing (MI).Citation33 Two reviews on implementing reminders or interactive applications showed only short-term effects.Citation33,Citation34 In the present study, AYAs reported that they learned from bleeding events and did not mention using reminders. Motivational interviewing is a patient-centered communication techniqueCitation33 that has shown promising results in 11 out of 12 studies in AYAs with asthma, diabetes and human immunodeficiency virus (HIV). For example, the adherence levels of AYAs with asthma increased from 32% to 62% after using MI.Citation33,Citation35 In the present and previous studies, most boys with hemophilia learned through experiential learning instead of individualized education or expert patient programs.Citation36 AYAs did not consider the route of administration as a reason for non-adherence, our data may be compared with asthma, diabetes and human immunodeficiency virus (HIV). As such, we believe that patient education combined with MI should be applied during a bleeding assessment visit rather than a regular visit since the AYA could is more open to learning. Future research should be focused on individualized patient education per phase (as defined in this study) as well as interventions using MI in AYAs with hemophilia to improve adherence.
Conclusion
This study explored the underlying reasons for adherence to prophylaxis in hemophilia from an AYA perspective. The level of treatment responsibility and (short-term) risk estimation per activity influenced adherence positively or negatively.
Acknowledgment
We thank all AYAs that participated for their time and openness.
Disclosure
This work was supported by a non-restricted BHAP grant. This work was part of the PhD of J.W. Hoefnagels. Miss J.W. Hoefnagels reports grants from Bayer Hemophilia Awards Program (BHAP), during the conduct of the study. Dr Kathelijn Fischer reports grants from Bayer during the conduct of the study; and grants from Bayer, Pfizer, NovoNordisk, and Baxter, outside the submitted work. Dr L.H. Schrijvers reports grants from Bayer Pharmaceutics during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- Suris JC, Michaud PA, Viner R. The adolescent with a chronic condition. Part I: developmental issues. Arch Dis Child. 2004;89(10):938–942. doi:10.1136/adc.2003.045369
- Kynga H. Patient education: perspective of adolescents with a chronic disease. J Clin Nurs. 2003;2003:744–751. doi:10.1046/j.1365-2702.2003.00788.x
- Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48:177–187. doi:10.1016/S0738-3991(02)00032-0
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. doi:10.1111/j.1365-2516.2012.02909.x
- Schrijvers LH, Beijlevelt-van der Zande M, Peters M, Schuurmans MJ, Fischer K. Learning intravenous infusion in haemophilia: experience from the Netherlands. Haemophilia. 2012;18:516–520. doi:10.1111/j.1365-2516.2012.02752.x
- Fischer K, Ljung R. Primary prophylaxis in haemophilia care: guideline update 2016. Blood Cells Mol Dis. 2017;67:81–85. doi:10.1016/j.bcmd.2017.02.004
- Fischer K, Van Der Bom JG, Prejs R, et al. Discontinuation of prophylactic therapy in severe haemophilia: incidence and effects on outcome. Haemophilia. 2001;7:544–550. doi:10.1046/j.1365-2516.2001.00560.x
- Witkop ML, McLaughlin JM, Anderson TL, Munn JE, Lambing A, Tortella B. Predictors of non-adherence to prescribed prophylactic clotting-factor treatment regimens among adolescent and young adults with a bleeding disorder. Haemophilia. 2016;22:e245–e250. doi:10.1111/hae.12951
- Brand B, Dunn S, Kulkarni R. Challenges in the management of haemophilia on transition from adolescence to adulthood. Eur J Haematol. 2015;81:30–35. doi:10.1111/ejh.12582
- Schrijvers LH, Beijlevelt-van der Zande M, Peters M, et al. Adherence to prophylaxis and bleeding outcome in haemophilia: a multicentre study. Br J Haematol. 2016;174:454–460. doi:10.1111/bjh.14072
- Kyngäs HA, Kroll T, Duffy ME. Compliance in adolescents with chronic diseases: a review. J Adolesc Health. 2000;26(6):379–388. doi:10.1016/S1054-139X(99)00042-7
- Krishnan S, Vietri J, Furlan R, Duncan N. Adherence to prophylaxis is associated with better outcomes in moderate and severe haemophilia: results of a patient survey. Haemophilia. 2015;21:64–70. doi:10.1111/hae.12533
- Oladapo AO, Epstein JD, Williams E, Ito D, Gringeri A, Valentino LA. Health-related quality of life assessment in haemophilia patients on prophylaxis therapy: a systematic review of results from prospective clinical trials. Haemophilia. 2015;21(5):e344–e358. doi:10.1111/hae.12759
- Schrijvers LH, Kars MC, Beijlevelt-van der Zande M, Peters M, Schuurmans MJ, Fischer K. Unravelling adherence to prophylaxis in haemophilia: a patients’ perspective. Haemophilia. 2015;21:612–621. doi:10.1111/hae.12660
- Ruppel PS, Mey G. Grounded theory methodology—narrativity revisited. Integr Psychol Behav Sci. 2015;49(2):174–186. doi:10.1007/s12124-015-9301-y
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi:10.1093/intqhc/mzm042
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. 2nd ed. Londen: SAGA publications; 2006.
- Schrijvers LH, Cnossen MH, Beijlevelt-van der Zande M, Peters M, Schuurmans MJ, Fischer K. Defining adherence to propylaxis in haemophilia. Haemophilia. 2016;22:e301–e348. doi:10.1111/hae.12935
- Limperg P, Peters M, Gibbons E, et al. Themes in daily life of adolescents and young adults with congenital bleeding disorders: a qualitative study. Haemophilia. 2016;22(4):e330–e333. doi:10.1111/hae.12961
- Dierckx de Casterle B, Gastmans C, Bryon E, Denier Y. QUAGOL: a guide for qualitative data analysis. Int J Nurs Stud. 2012;49(3):360–371. doi:10.1016/j.ijnurstu.2011.09.012
- Boeije H. Analysis in Qualitative Research. first ed. Thousand Oaks, Calafornia: SAGE Publications; 2010.
- Creswell JW. Qualitative Inquiry & Research Design - Choosing Among Five Approaches. 2nd ed. Thousand Oaks: Sage; 2007.
- Wynder EL. Inverstigator bias and interviewer bias: the problem of reporting systematic error in epdidemiology. J Clin Epidemiol. 1994;47(8):825–827. doi:10.1016/0895-4356(94)90184-8
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–1907. doi:10.1007/s11135-017-0574-8
- Van Staa A, Jedeloo S, van der Stege H. What we want ”: chronically ill adolescents’ preferences and priorities for improving health care. Patient Prefer Adherence. 2011;5:291–305. doi:10.2147/PPA.S17184
- Schrijvers LH, Beijlevelt-van der Zande M, Peters M, et al. Achieving self-management of prophylactic treatment in adolescents: the case of haemophilia. Patient Educ Couns. 2016;99(7):1179–1183. doi:10.1016/j.pec.2016.01.016
- Keough L, Sullivan-Bolyai S, Crawford S, Schilling L, Dixon J. Self-management of Type 1 diabetes across adolescence. Diabetes Educ. 2011;37(4):486–500. doi:10.1177/0145721711406140
- Casey BJ, Jones RMJ, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;126:111–126. doi:10.1196/annals.1440.010
- Sawicki GS, Heller KS, Demars N, Robinson WM. Motivating adherence among adolescents with cystic fibrosis: youth and parent perspectives. Pediatr Pulmonol. 2015;50(2):127–136. doi:10.1002/ppul.23017
- Bain-Brickley D, Butler L, Kennedy G, Rutherford G. Interventions to improve adherence to antiretroviral therapy in children with HIV infection (Review). Cochrane Libr. 2011;12:1–32. doi:10.1002/14651858.CD009513.www.cochranelibrary.com
- Kosse R, Kaptein A, Geers C, van Dijk L. mHealth intervention to support asthma self- management in adolescents: the ADAPT study. Patient Prefer Adherence. 2017;11:571–577. doi:10.2147/PPA.S124615
- Qian W, Lam TT, Hon H, Lam W, Li C, Cheung YT. Telehealth interventions for improving self-management in patients with hemophilia: scoping review of clinical studies corresponding author. J Med Internet Res. 2019;21:1–15. doi:10.2196/12340
- Schaefer MR, Kavookjian J. Patient education and counseling the impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190–2199. doi:10.1016/j.pec.2017.05.037
- Linn AJ, Vervloet M, Van Dijk L, Smit EG, Van Weert JCM. Effects of eHealth interventions on medication adherence: a systematic review of the literature corresponding author. J Med Internet Res. 2011;13:1–16. doi:10.2196/jmir.1738
- Riekert KA, Borrelli B, Bilderback A, Rand CS. Patient education and counseling the development of a motivational interviewing intervention to promote medication adherence among inner-city, African-American adolescents with asthma. Patient Educ Couns. 2011;82(1):117–122. doi:10.1016/j.pec.2010.03.005
- Khair K, Hons L, Gibson F, Ed C. Self-management and skills acquisition in boys with haemophilia. Heal Expect. 2013;18:1105–1113. doi:10.1111/hex.12083
