Abstract
Background
Patients have evolved from mere objects of study to active contributors to drug research in recent decades. Since individual patient’s influence to change research processes effectively is limited, patient groups play an important role in the planning and conducting of pharmaceutical studies. Patient group engagement in drug research is usually seen as being beneficial from an ethical viewpoint as well as from the perspective of research practice, while potential disadvantages and risks have been discussed considerably less.
Purpose
A systematic review of reasons was conducted to allow for an overview of the reasons for and against involving patient groups in drug research.
Methods
The literature search was conducted in PubMed and Web of Science. Reasons concerning the influence of patient groups on drug research were extracted and synthesized using qualitative content analysis. The review’s main limitation arises from a lack of critical appraisal regarding the quality of the reasons.
Results
A total of 2271 references were retrieved, of which 97 were included in the analysis. Data extraction revealed 91 (73.4%) reasons for and 30 (24.2%) reasons against involving patient organizations in drug research, and 3 (2.4%) ambivalent reasons; amounting to 124 reasons. The main groups of reasons were clustered around the categories: quality of research, acquisition and allocation of resources, and the patient role in research.
Conclusion
This is the first systematic review of reasons concerning the influence of patient groups on drug research. It provides a basis for a continuing debate about the value as well as the limits of involving patient groups. Due to the diversity of research projects there can be no general recommendation for or against patient group involvement. More research is necessary to assess potential advantages and disadvantages of patient groups’ influence on other types of research (eg genetics).
Plain Language Summary
Patient groups play an important role in the planning and conducting of pharmaceutical studies. Therefore, their engagement in drug research is usually regarded as being beneficial from both an ethical and a scientific viewpoint. Meanwhile, potential disadvantages and risks of their involvement have received little attention.
For the first time, a systematic overview of the reasons for and against involving patient groups in drug research was created. After identifying relevant literature, reasons concerning the influence of patient groups on drug research were extracted. In total, 2271 references were retrieved, of which 97 contained reasons and were included in the analysis. Data extraction revealed 91 (73.4%) reasons for and 30 (24.2%) reasons against involving patient organizations in drug research, and 3 (2.4%) ambivalent reasons; amounting to 124 reasons.
By presenting all reasons concerning the involvement of patient groups in drug research, this review provides its readers with a basis to form an educated opinion for the continuing debate about the value and the limits of such an involvement.
Introduction
The involvement of patients and the public in science has become a major factor in the international research landscape.Citation1–Citation3 Provisions for adequate involvement of patient and public representatives, for example, have become increasingly important for researchers and scientific institutions as a precondition for research funding. In addition, regulatory institutions such as the US Food and Drug Administration increasingly emphasize the importance of patients’ input in drug research.Citation4 The variety of ways in which patientsCitation5 and the publicCitation6 can contribute to research has already been discussed in detail. It ranges from educating patients and the public and building a public opinion, to setting research agendas and supporting the conduct of studies.
The involvement of non-researchers in the research process has been given numerous names, for example, Patient and Public Involvement, patient engagement, public participation and Citizen Science. The way in which and the degree to which patient and public representatives influence the research process vary depending on the conceptual backgrounds. One of the most far-reaching approaches refers to the slogan: “Every participant is a PI”.Citation7 The key idea of this concept is to encourage patients to submit personal health data to an open data repository (like Open HumansCitation8) and afterwards to consistently involve them in every step of scientific knowledge production.
In the current literature, there is often no distinction between the involvement of patients and the involvement of the public. However, these differences between patients and the public are important, since each group seems to be driven by different interests.Citation9 The differing motives may even result in a paradox.Citation10 Patients can most notably contribute the experience of living with a certain disease – often called “experiential expertise” or “experiential knowledge”Citation11 – to the development of drugs, distinguishing them from healthy individuals. In addition, they usually have a personal incentive to get involved in drug research for a specific disease, whereas members of the public would rather work towards general improvements in health care.Citation12 Thus, both a conceptual as well as a practical distinction between the involvement of patients and the involvement of the public seems necessary regarding the epistemic backgrounds and interests of the groups involved.
Another point of controversy relates to the moral value of letting patients participate, for example, in the planning and design of a research project. Patient involvement is usually considered as ethically important in the current literature.Citation13,Citation14 Some authors see a “compelling ethical rationale [that] supports patient engagement in healthcare research”.Citation5 This “rationale” can, for example, be related to the idea of “epistemic justice”. Besides arguing for the inclusion of experiential expertise in knowledge production, “epistemic justice” sees a moral duty in involving patients’ perspectives in decisions that will affect primarily patients.Citation15
In contrast, discussions about critical aspects have been widely missing, although they deserve just as much attention, as in some cases, patient involvement can be unfavorable.Citation16,Citation17 A patient organization, for example, can fail to represent the patients’ perspective properly and, consequently, promote researchers’ rather than patients’ interests.Citation18,Citation19 Another example of a doubtful patient activity is demanding access to unproven and possibly harmful treatments. This creates the risks of resources being spent ineffectively and patient safety being at stake. This has been, for instance, the case with a breast cancer treatment in the 1990s.Citation20
Finally, many publications on Patient and Public Involvement are restricted to certain aspects of the phenomenon. Broader assessments of the status quo of functions performed by patients and the publicCitation5,Citation6 and several guidelines on how to implement their involvementCitation21–Citation23 exist. Seemingly, some researchers are still unsure how patient involvement can be included in their research.Citation24 A full picture of all reasons for and against patient group (PG) involvement in research has not yet been provided. This can only be achieved through systematic reviews (SRs). This article aims at giving researchers and healthcare decision-makers a comprehensive overview to form their opinions on involving patients in drug research. Due to the different epistemic and normative characters of the involvement of patients or the public respectively, this SR is restricted to patients, and more concretely to PGs. Since individual patient’s influence to change research processes effectively is limited, PGs usually function as the major stakeholders in pharmaceutical studies.
Materials and Methods
A SR of reasonsCitation25 with the objective of collecting all reasons regarding the involvement of PGs in drug research was conducted and is reported according to the PRISMA Statement to the extent to which it is applicable to SRs of reasons (see Additional file 1). SRs generally aim to systematically present all evidence-based knowledge (and lack of such) concerning a specific research question.Citation26 In recent years, the SR methodology has been adopted and further developed for the field of bioethics, which is characterized by a close connection between normative and empirical research questions.Citation27 When analyzing argumentative literature, adjustments need to be made to the “classic” SR methodology.Citation25 There are different types of SRs of argumentative literature, for example, SRs of (ethical) issues, conclusions, concepts, recommendations and reasons.Citation28 Even if SRs are a rather new methodological approach within the field of bioethics, there have been comprehensive publications on the value of such reviews,Citation29,Citation30 and several SRs of argumentative literature in generalCitation31 and specifically of SRs of reasons have been already conducted and published.Citation32–Citation34
Inclusion Criteria
Two key terms were defined for the search strategy to arrive at a systematic overview of reasons regarding our research objective: “patient groups” and “drug research”. We deliberately decided to use broad definitions of our key terms in order to avoid missing any relevant literature. Publications were only considered if they fitted both definitions.
“Patient group”, within this review, means any group consisting of patients and/or patient advocates which consistently promotes patients’ interests.Citation35 The activities of individual patients regarding their needs and interests were not included in the review.
Concerning the term “drug research”, the review considers all phases of research and development of a medicine product from target identification to clinical Phase III studies as described in the final report of the pharmaceutical sector inquiry of the European Commission.Citation36
Groups of patients may have various impacts on medical research. They may, for instance, highly influence the public acceptance and economic feasibility of research. They can also play an important political role or contribute scientifically to research.Citation37 All these types of impacts were considered in the review if they affected the research and development phases of a drug mentioned above. Only publications in English or German language were included, due to the authors’ language capabilities. The search was not limited to a certain time period.
Database Search
After gaining an overview of the existing literature by hand and exploratory database searches, two databases were selected for the systematic search: PubMed and Web of Science. A search strategy was built based on the two key terms – PGs and drug research – and their synonyms. The search term used in PubMed is presented in . The search was conducted in March 2019.
Box 1 Search Term for PubMed
Some of the relevant publications identified via hand search did not appear in the results of our database search, presumably due to their being parts of books. We decided to include them in our study sample to complement the database search results.
Study Selection
Publications which address both of our key terms were included. Two authors, CR and RM, screened the title and abstract of the publications identified via hand and database search and discarded publications not meeting the inclusion criteria. Any disagreement between the two authors was resolved through discourse.
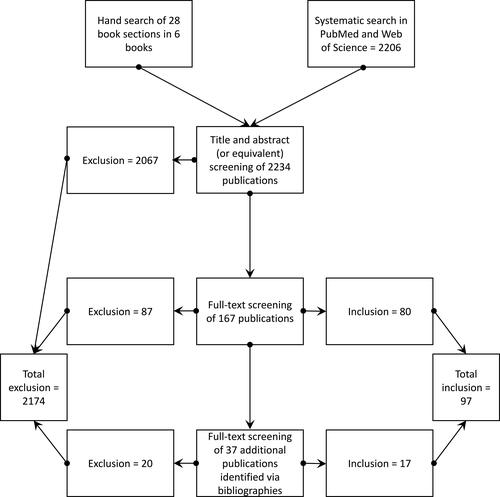
The full texts of the remaining publications were then analyzed regarding their relevance by CR and RM and the results were discussed in regular team meetings. Again, publications not meeting the inclusion criteria were discarded. The remaining publications were included in the review and their bibliographies were screened for additional relevant literature. This resulted in adding further 17 relevant publications to the finally included publications. A flow chart illustrating the study selection is shown in .
Data Extraction and Qualitative Synthesis
In this review, a reason is understood as the first part of an argument (in this context, often called a “premise”), the second being a conclusion. An argument can consist of multiple reasons/premises that may all lead to one conclusion (eg “the influence of PGs is favorable”).Citation38 This was the case in some of the publications included in the review, as they stated only an “all things considered”-conclusion, but many premises.
Publications were analyzed by two authors (CR and RM) using the method of qualitative text analysis proposed by Mayring,Citation39 supported by the software MAXQDA Standard 12. According to the research question, the authors screened the publications for reasons regarding the involvement of PGs in drug research. A code was assigned to each occurrence of a reason. Reasons extracted inductively from the material were labeled as narrow reason types. Deductively created categories that condense narrow reason types were labeled as broad reason types. Narrow reasons were analyzed for their alleged implications (pro, contra or ambivalent) regarding the involvement of PGs in drug research.Citation25 After all the publications had been analyzed once and theoretical saturation was reached, the code system was revised to eliminate doubling and overlapping reason types. All publications were analyzed a second time to ensure the assignment of the correct code from the revised code system for every reason occurrence. Publications were also analyzed for their publication type and their “all-things-considered”-conclusion, which is the final conclusion a publication comes to based on all mentioned reasons.Citation25
A quality appraisal of the extracted reasons was deliberately not conducted. Firstly, assessing the quality of a reason is a complex endeavor and can only be achieved by thorough discourse.Citation38 Methodological standards for quality assessment in SRs of reasons are not available so far.Citation28 Secondly, the results of such an endeavor depend partly on the context of the particular situation at hand. Therefore, it exceeds the limits of what can be provided in a systematic review of reasons. However, we encourage the readers to assess the quality of reasons presented within the context of their research projects.
Results
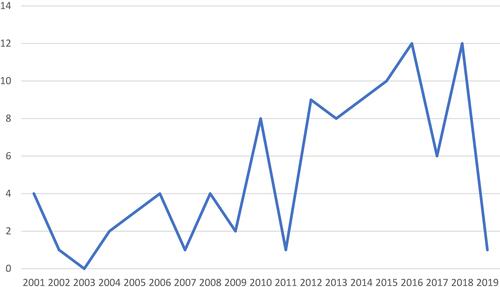
A total of 97 publications were finally included from 2271 identified publications during the systematic search. The study sample consists entirely of journal articles and book sections published between 2001 and 2019. shows the number of publications per year. Even though there is some fluctuation, the overall interest in the involvement of patients in drug research is gradually rising. The small number of publications from 2019 is mainly due to the database search being conducted in March 2019.
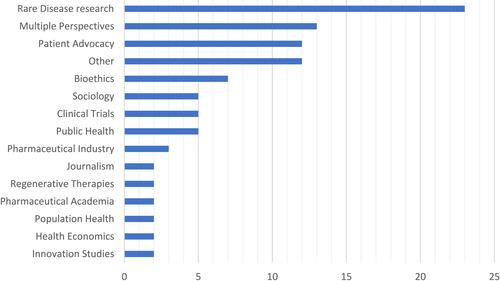
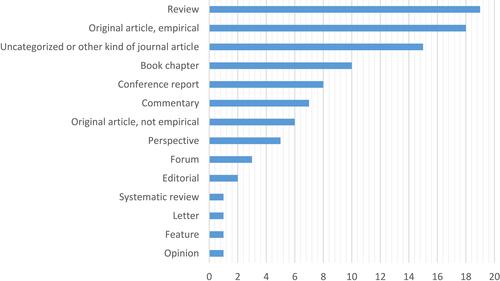
The study sample is very heterogeneous and shows a wide variety of perspectives of the authors and publication types. Most of the publications focused on rare diseases which leads to the assumption that research on rare diseases benefits greatly from patient involvement. Authors from the pharmaceutical industry were much less interested in patient involvement than patient advocates. The distribution of the authorship possibly contributed to the high number of reasons for the involvement of patients in drug research. The variety of author perspectives is shown in and the quantity of publication types in . All publications were written in English. A list of all publications included is part of the supplementary material of this article (see Additional file 2).
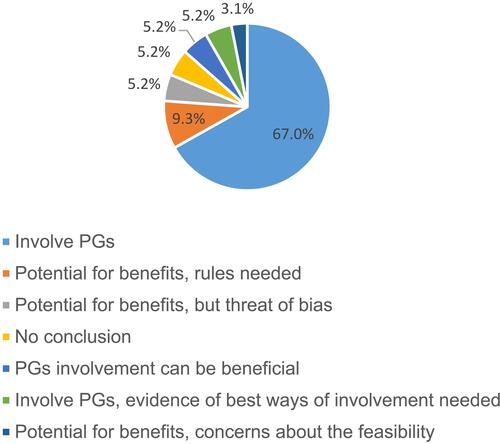
Despite the obvious heterogeneity of the study sample, the “all-things-considered”-conclusions were surprisingly consistent. Most publications drew the conclusion, that the involvement of PGs in drug research is or can be beneficial under certain circumstances. A minority of publications did not have a conclusion. No publication rejected the involvement of PGs entirely. However, publications with occurrences of reasons against the involvement of PGs often warned of risks and dangers, that should be avoided. A summary of the conclusions of all included publications is provided in .
Broad Reason Types and Narrow Reason Types
Reasons were categorized during the analysis of the study sample by assigning broad reason types (BRTs) and narrow reason types (NRTs). BRTs summarize NRTs that are closely linked in content. The following six BRTs were identified:
Resources: Since resources are limited, many reasons relate to the question whether PGs can acquire, distribute and use resources needed for the research process effectively. Resources discussed include financial investments, research samples, scientific data and time.
Collaboration: The creation of new acquaintances and connections between researchers and other stakeholders was generally rated highly for the research process. PGs play a key role in establishing these collaborations.
Science: This BRT deals with all reasons concerning quality, conditions, aims and conduct of scientific studies. There are ways in which PGs can influence these parameters either positively or negatively. Setting research agendas is one of the topics mentioned most frequently in this BRT.
Patient community: Reasons regarding the quality of patient representation by PGs can be found in this BRT. Possible contributions of patients based on their unique experiences and potential benefits and risks which affect patients directly are also discussed.
Ethics: Justification and fairness of research with the involvement of PGs are major reasons in this BRT. PGs’ handling of ethical issues is also considered.
Public relations: The ability of PGs to promote research-friendly political surroundings and shape the public perception of drug research is subject to reasons in this BRT.
All these six BRTs encompass reasons for and against the involvement of PGs in drug research. Ambivalent reasons can be found in the BRTs Resources and Science. shows a detailed list of all reasons, the number of publications each reason occurred in and how the reasons were used. An additional table reveals, which NRTs were found in each included publication (see Additional file 3).
Table 1 Reasons For and Against Involving PGs in Drug Research
Discussion
As expected, a broad variety of reasons which support the involvement of PGs in drug research was found (91; 73.4%). However, the same applies to reasons against involvement on a smaller scale (30; 24.2%), while only a few reasons were used ambivalently (3; 2.4%). The reason for the discrepancy between pro and contra reasons in this SR is possibly an accurate depiction of a real difference in numbers of respective reasons. However, contra reasons have been mentioned by far fewer publications than pro reasons. Many publications included in this review do not discuss the inclusion of PGs in drug research as their central topic. These articles might tend to address the issue rather superficially and advocate the inclusion of PGs without critical reflection. Publications that cover it as a central topic tend to be more balanced.Citation19,Citation40,Citation41 They also do not draw their arguments from individual experiences or single examples of good collaboration between PGs and researchers as many of the other publications do. A generalization of these positive experiences is not possible. These findings could indicate that the real cause of the discrepancy is an underrepresentation of contra reasons.
The often-unquestioned ethical rationale whether to involve patients in research is reflected in the NRTs “Patient perspective in research” and “Poor patient representation”. Indeed, there are arguments stressing that the status of being affected fundamentally distinguishes healthy people from ill people who, therefore, deserve representation.Citation42 While most authors agree that this is a desirable goal, some express concerns about whether and how this goal can be achieved by involving PGs. Strategies for addressing these concerns have been rarely discussed so far. One approach could be the analysis of representation and trust models applied by PGs.Citation43 The concept of a “collective agency”Citation44 examines the quality of representation in PGs more thoroughly and considers engaging other collective actors like, for example, families. In this concept, four characteristics of collective actors are identified, one of them being building “a shared practice of trust”.Citation44
The risk of a collaboration with PGs being misused by pharmaceutical companies for commercial purposes is reflected in the NRT “Risk of manipulation by other stakeholders”. This risk is especially evident when PGs are being sponsored by companies.Citation45,Citation46 On the other hand, industrial sponsoring offers opportunities for PGs. This leads to debates with good arguments on both sides.Citation47,Citation48 The results of this review show that this factor has been used rather rarely as a reason against the involvement of PGs in drug research. Furthermore, it has been acknowledged in every occurrence of the reason that the risk of manipulation can be alleviated by applying preventive measures as, for example, adequate disclosure practices.Citation49,Citation50
Limitations
The review is restricted to two databases and a small selection of book chapters identified during hand search. Any other databases, including Google Books, were not considered due to a lack of relevant results in the exploratory searches.
Another limitation is the neglect of literature written in languages other than English and German. One publication (written in Dutch) had to be excluded due to this limitation. The definition of the two key terms and the inclusion of publications and reasons based on them is a crucial point of this review. The definitions developed confine the variety of reasons collected. Moreover, the decision whether a publication or a reason deals with both key terms as part of qualitative data synthesis is subjective. We made these decisions as intersubjectively valid as possible by discussing relevant decisions within the disciplinary research team and solving disagreement by discourse.
Conclusion
The results of this review indicate that the inclusion of PGs in research can be fruitful. Nevertheless, due to the variety of PGs, no general recommendation to involve or not involve PGs in drug research can be made from this SR of reasons. The reasons presented should, however, be considered carefully when thinking about such a collaboration. Leaders of PGs, for example, can decide whether their PG should get involved in drug research or if patients’ interests can be promoted better if resources are spent on other PG activities. Similarly, leaders of pharmaceutical companies can decide whether engaging PGs in their specific research field is likely to favor the research process. Policy-makers can use this review to create new policies that will improve the conditions for research landscapes.
The reasons presented in this review refer specifically to PGs and drug research. Although they can certainly be adapted to other contexts, there is a need for more SRs assessing reasons for patient involvement relating to other fields of research as, for example, genetics research.
Abbreviations
PG, patient group; SR, systematic review; BRT, broad reason types; NRT, narrow reason types.
Availability of Data and Material
A list of all publications included in the SR and a detailed list of all reason type occurrences in each publication are part of the additional material. Further data and material can be requested from the first author.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work is part of the joint research project “PePPP” and is supported by the European Social Fund (ESF), reference: ESF/14-BM-A55-0050/16, ESF/14-BM-A55-0045/16 and ESF/14-BM-A55-0046/16, and the Ministry of Education, Science and Culture of Mecklenburg-Vorpommern, Germany. The funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge support for the Article Processing Charge from the DFG (German Research Foundation, 393148499) and the Open Access Publication Fund of the University of Greifswald.
Disclosure
All authors report grants from the European Social Fund during the conduct of the study. The authors declare that they have no other competing interests in this work.
References
- Dent M, Pahor M. Patient involvement in Europe–a comparative framework. J Health Organ Manag. 2015;29(5):546–555. doi:10.1108/JHOM-05-2015-0078
- The King’s Fund. Working with Patients, Service Users, Carers and the Public [Homepage on the Internet]. London: The King’s Fund; 2019. Available from: https://www.kingsfund.org.uk/about-us/what-we-do/patients-service-users-carers-public.Accessed May 12, 2019.
- About Involve [Homepage on the Internet]. London: The Involve Foundation; 2018. Available from: http://www.involve.org.uk/about-involve/. Accessed May 12, 2019..
- Mullin T, Vaidya P, Chalasani M. Recent US food and drug administration efforts to integrate the patient’s perspective in drug development and decision making. Clin Pharmacol Ther. 2019;105(4):789–791. doi:10.1002/cpt.2019.105.issue-4
- Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14(1):89. doi:10.1186/1472-6963-14-89
- Lander J, Hainz T, Hirschberg I, Strech D. Current practice of public involvement activities in biomedical research and innovation: a systematic qualitative review. PLoS One. 2014;9(12):e113274. doi:10.1371/journal.pone.0113274
- Buyx A, Del Savio L, Prainsack B, Volzke H. Every participant is a PI. Citizen science and participatory governance in population studies. Int J Epidemiol. 2017;46(2):377–384. doi:10.1093/ije/dyw204
- About Open Humans [Homepage on the Internet]. Boston: Open Humans Foundation. Available from: https://www.openhumans.org/about/. Accessed May 12, 2019.
- McCoy MS, Warsh J, Rand L, Parker M, Sheehan M. Patient and public involvement: two sides of the same coin or different coins altogether? Bioethics. 2019;33:708–715. doi:10.1111/bioe.2019.33.issue-6
- Ives J, Damery S, Redwod S. PPI, paradoxes and Plato: who’s sailing the ship? J Med Ethics. 2013;39(3):181–185. doi:10.1136/medethics-2011-100150
- Caron-Flinterman JF, Broerse JEW, Bunders JFG. The experiential knowledge of patients: a new resource for biomedical research? Soc Sci Med. 2005;60(11):2575–2584. doi:10.1016/j.socscimed.2004.11.023
- Fredriksson M, Tritter JQ. Disentangling patient and public involvement in healthcare decisions: why the difference matters. Sociol Health Illn. 2017;39(1):95–111. doi:10.1111/1467-9566.12483
- Bauer G, Abou-El-Enein M, Kent A, Poole B, Forte M. The path to successful commercialization of cell and gene therapies: empowering patient advocates. Cytotherapy. 2017;19(2):293–298. doi:10.1016/j.jcyt.2016.10.017
- Staley K, Minogue V. User involvement leads to more ethically sound research. Clin Ethics. 2006;1(2):95–100. doi:10.1258/147775006777254489
- Schicktanz S. The ethical legitimacy of patient organizations’ involvement in politics and knowledge production. In: Wehling P, Viehöver W, Koenen S, editors. The Public Shaping of Medical Research: Patient Associations, Health Movements and Biomedicine. Abingdon: Routledge; 2014:246–264.
- Cassidy J. Why patient representation might harm science? Breast Cancer Res. 2007;9(Suppl 2):S4. doi:10.1186/bcr1802
- Madden M, Speed E. Beware zombies and unicorns: toward critical patient and public involvement in health research in a neoliberal context. Front Sociol. 2017;2:7. doi:10.3389/fsoc.2017.00007
- Stockdale A. Waiting for the cure: mapping the social relations of human gene therapy research. Sociol Health Illn. 1999;21(5):579–596. doi:10.1111/1467-9566.00174
- Panofsky A. Generating sociability to drive science: patient advocacy organizations and genetics research. Soc Stud Sci. 2011;41(1):31–57. doi:10.1177/0306312710385852
- Mello MM, Brennan TA. The controversy over high-dose chemotherapy with autologous bone marrow transplant for breast cancer. Health Aff (Millwood). 2001;20(5):101–117. doi:10.1377/hlthaff.20.5.101
- Tritter J, McCallum A. The snakes and ladders of user involvement: moving beyond arnstein. Health Policy. 2006;76:156–168. doi:10.1016/j.healthpol.2005.05.008
- Wicks P, Lowe M, Gabriel S, Sikirica S, Sasane R, Arcona S. Increasing patient participation in drug development. Nat Biotechnol. 2015;33(2):134–135. doi:10.1038/nbt.3145
- Warner K, See W, Haerry D, Klingmann I, Hunter A, May M. EUPATI guidance for patient involvement in medicines research and development (R&D); guidance for pharmaceutical industry-led medicines R&D. Front Med. 2018;5:270. doi:10.3389/fmed.2018.00270
- Evans D, Bird E, Gibson A, et al. Extent, quality and impact of patient and public involvement in antimicrobial drug development research: a systematic review. Health Expect. 2018;21(1):75–81. doi:10.1111/hex.2018.21.issue-1
- Strech D, Sofaer N. How to write a systematic review of reasons. J Med Ethics. 2012;38(2):121–126. doi:10.1136/medethics-2011-100096
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26(2):91–108. doi:10.1111/j.1471-1842.2009.00848.x
- Strech D, Synofzik M, Marckmann G. Systematic reviews of empirical bioethics. J Med Ethics. 2008;34(6):472–477. doi:10.1136/jme.2007.021709
- Mertz M. How to tackle the conundrum of quality appraisal in systematic reviews of normative literature/information? Analysing the problems of three possible strategies (translation of a German paper). BMC Med Ethics. 2019;20(1):81. doi:10.1186/s12910-019-0423-5
- Sofaer N, Strech D. The need for systematic reviews of reasons. Bioethics. 2012;26(6):315–328. doi:10.1111/bioe.2012.26.issue-6
- Mertz M, Sofaer N, Strech D. Did we describe what you meant? Findings and methodological discussion of an empirical validation study for a systematic review of reasons. BMC Med Ethics. 2014;15:69. doi:10.1186/1472-6939-15-69
- Mertz M, Kahrass H, Strech D. Current state of ethics literature synthesis: a systematic review of reviews. BMC Med. 2016;14(1):152. doi:10.1186/s12916-016-0688-1
- Sofaer N, Strech D. Reasons why post-trial access to trial drugs should, or need not be ensured to research participants: a systematic review. Public Health Ethics. 2011;4(2):160–184. doi:10.1093/phe/phr013
- Mahieu L, Gastmans C. Sexuality in institutionalized elderly persons: a systematic review of argument-based ethics literature. Int Psychogeriatr. 2012;24(3):346–357. doi:10.1017/S1041610211001542
- Christenhusz GM, Devriendt K, Dierickx K. To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts. Eur J Hum Genet. 2013;21(3):248–255. doi:10.1038/ejhg.2012.130
- Epstein S. Patient Groups and Health Movements. In: Hackett EJ, Amsterdamska O, Lynch M, Wajcman J, editors. The Handbook of Science and Technology Studies. 3rd ed. Cambridge (MA): MIT Press; 2008:499–539.
- Pharmaceutical Sector Inquiry Final Report. European Comission. 2008. Available from: http://ec.europa.eu/competition/sectors/pharmaceuticals/inquiry/staff_working_paper_part1.pdf. Accessed May 12, 2019.
- Wehling P, Viehöver W, Koenen S. The Public Shaping of Medical Research: Patient Associations, Health Movements and Biomedicine. Abingdon: Routledge; 2014.
- Govier T. A Practical Study of Argument. 7th ed. Wadsworth; 2010.
- Mayring P. Qualitative Content Analysis [28 paragraphs]. Forum Qual Soc Res. 2000;1(2):20.
- Tranfaglia MR. The rise of rare disease foundations: how patient associations can drive the drug discovery process. In: Chackalamannil S, Rotella D, Ward S, editors. Comprehensive Medicinal Chemistry III, Volume . Amsterdam: Elsevier; 2017:549–559. doi:10.1016/B978-0-12-409547-2.12307-8t>
- Koay PP, Sharp RR. The role of patient advocacy organizations in shaping genomic science. Annu Rev Genomics Hum Genet. 2013;14:579–595. doi:10.1146/annurev-genom-091212-153525
- Schicktanz S, Schweda M, Franzen M. ‘In a completely different light’? The role of ‘being affected’ for the epistemic perspectives and moral attitudes of patients, relatives and lay people. Med Health Care Philos. 2008;11(1):57–72. doi:10.1007/s11019-007-9074-2
- Gerhards H, Jongsma K, Schicktanz S. The relevance of different trust models for representation in patient organizations: conceptual considerations. BMC Health Serv Res. 2017;17(1):474. doi:10.1186/s12913-017-2368-z
- Beier K, Jordan I, Wiesemann C, Schicktanz S. Understanding collective agency in bioethics. Med Health Care Philos. 2016;19(3):411–422. doi:10.1007/s11019-016-9695-4
- Rose SL, Highland J, Karafa MT, Joffe S. Patient advocacy organizations, industry funding, and conflicts of interest. JAMA Intern Med. 2017;177(3):344–350. doi:10.1001/jamainternmed.2016.8443
- McCoy MS, Carniol M, Chockley K, Urwin JW, Emanuel EJ, Schmidt H. Conflicts of interest for patient-advocacy organizations. N Engl J Med. 2017;376(9):880–885. doi:10.1056/NEJMsr1610625
- Kent A. Should patient groups accept money from drug companies? Yes. BMJ. 2007;334:934. doi:10.1136/bmj.39185.461968.AD
- Mintzes B. Should patient groups accept money from drug companies? No. BMJ. 2007;334:935. doi:10.1136/bmj.39185.394005.AD
- Rothman SM, Raveis VH, Friedman A, Rothman DJ. Health advocacy organizations and the pharmaceutical industry: an analysis of disclosure practices. Am J Public Health. 2011;101(4):602–609. doi:10.2105/AJPH.2010.300027
- Colombo C, Mosconi P, Villani W, Garattini S. Patient organizations’ funding from pharmaceutical companies: is disclosure clear, complete and accessible to the public? An italian survey. PLoS One. 2012;7(5):e34974. doi:10.1371/journal.pone.0034974