Abstract
Purpose
The aim of this systematic review was to identify methods used to assess medication preferences in older adults and evaluate their advantages and disadvantages with respect to their applicability to the context of multimorbidity and polypharmacy.
Material and Methods
Three electronic databases (PubMed, Web of Science, PsycINFO) were searched. Eligible studies elicited individual treatment or outcome preferences in a context that involved long-term pharmacological treatment options. We included studies with a study population aged ≥ 65 years and/or with a mean or median age of ≥ 75 years. Qualitative studies, studies assessing preferences for only two different treatments, and studies targeting preferences for life-sustaining treatments were excluded. The identified preference measurement methods were evaluated based on four criteria (time budget, cognitive demand, variety of pharmacological aspects, and link with treatment strategies) judged to be relevant for the elicitation of patient preferences in polypharmacy.
Results
Sixty articles met the eligibility criteria and were included in the narrative synthesis. Fifty-five different instruments to assess patient preferences, based on 24 different elicitation methods, were identified. The most commonly applied preference measurement techniques were “medication willingness” (description of a specific medication with inquiry of the participant’s willingness to take it), discrete choice experiments, Likert scale-based questionnaires, and rank prioritization. The majority of the instruments were created for disease-specific or context-specific settings. Only three instruments (Outcome Prioritization Tool, a complex intervention, “MediMol” questionnaire) dealt with the broader issue of geriatric multimorbidity. Only seven of the identified tools showed somewhat favorable characteristics for a potential use of the respective method in the context of polypharmacy.
Conclusion
Up to now, few instruments have been specifically designed for the assessment of medication preferences in older patients with multimorbidity. To facilitate valid preference elicitation in the context of geriatric polypharmacy, future research should focus on suitable characteristics of existing techniques to develop new measurement approaches for this increasingly relevant population.
Introduction
Incorporating a patient’s individual preferences into medical decision-making has improved treatment adherence and patient satisfaction.Citation1–Citation3 Various medical disciplines, such as oncology, cardiology, or psychiatry, have examined preference-oriented approaches to deliver optimized care.Citation4–Citation6 Including the patient’s priorities seems particularly favorable in clinical settings where sufficient evidence regarding the most effective treatment strategy is lacking.Citation7
The pharmacotherapy of older patients with multiple morbidities is characterized by relevant knowledge gaps due to a paucity of age-related and context-related data.Citation8 An uncritical adherence to disease-specific clinical practice guidelines in these patients will result in pronounced polypharmacy.Citation9 In numerous Western societies, prevalence rates between 10.0% and 27.4% have been reported for the chronic intake of 10 and more medicines (“excessive polypharmacy”) in adults aged ≥ 65 years.Citation10−Citation13 Polypharmacy increases the risk of adverse drug reactionsCitation14 and drug-drug interactions.Citation15 It is independently linked with the number of incident falls experienced by older adultsCitation16 as well as a decline in functionality.Citation17
In order to prevent or even reverse the negative effects of polypharmacy, recent articles advocate ”deprescribing”,Citation18,Citation19 which has been defined as “the process of withdrawal of an inappropriate medication, supervised by a health care professional with the goal of managing polypharmacy and improving outcomes”.Citation20 Including the older patient’s treatment preferences has been proposed as a technique to facilitate deprescribing.Citation21
Employing reliable and valid preference measurement methods is the prerequisite for evaluating patient preferences and integrating them into long-term health care decision-making. Instruments used in the geriatric setting need to be intelligible and operable for the older and potentially frail adults questioned. A recent systematic review aimed to identify tools suitable for eliciting treatment preferences in aged primary care patients with multimorbidity.Citation22 The authors found only one tool to be potentially relevant for recording outcome priorities in this context, concluding that there was an urgent need to further develop clinically applicable assessment strategies.Citation22
Acting on this need, we carried out a systematic review to identify preference measurement techniques that have been employed to determine the treatment priorities of older patients across various disease and non-disease specific settings, assess both their advantages and disadvantages with respect to their applicability in individuals with polypharmacy, and give recommendations for the development of future preference-based prescribing tools.
Material and Methods
The findings of this systematic review are presented in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.Citation23
Search Strategy and Information Sources
We searched three electronic databases (PubMed, PsycINFO, and Web of Science) for studies reported in English or German. Abstracts published from the inception of the databases up to October 6, 2017 were considered for inclusion. We based our search strategy on modified versions of two published search strings for the identification of literature on geriatric medicine and patient preferences for treatment outcomes.Citation24,Citation25 Variations of terms relating to pharmacotherapy and health outcomes were added. The search strategy was adapted for each of the three databases. The complete search string for the PubMed database search is accessible in the Electronic Supplementary Material Table S1 . Reference lists of included articles were screened manually for potentially relevant articles.
Eligibility Criteria
Studies were eligible for inclusion if they aimed to identify individual treatment or outcome preferences or priorities of older adults by using a standardized, non-qualitative methodology. We defined preference as the relative “desirability” of a certain option.Citation26 The medical context of preference elicitation had to involve the pharmacological treatment of chronic conditions, either by directly evaluating preferences for specific medicines or their characteristics or by evaluating preferences for health outcomes potentially achieved with medicines. Studies were included if they addressed an aged population, defined by a cut-off for inclusion of ≥ 65 years. In case the eligibility criteria of individual studies did not exclude younger patients from participation, studies were suitable for inclusion if they reported a mean or median age of participants of ≥ 75 years. We also considered articles that focused on analyzing an aged population as a specific subgroup as stated in the abstract of the respective article. Full-text articles of abstracts not reporting age-related demographic data were only retrieved if the study context referred to diseases or medical problems characteristic of old age. We only included original research journal articles.
Owing to the particular nature and non-therapeutic quality of their setting, studies reporting patient preferences in the context of advance care planning and life-sustaining treatments were excluded. We also excluded studies that assessed preferences for merely two different treatments.
Study Selection
After removal of duplicates, one of the authors (AE) screened study titles and abstracts for eligibility. Full-text articles of potentially suitable abstracts were retrieved and assessed for inclusion independently by two reviewers (AE and AR). Disparities between the reviewing authors were resolved by discussion and through consultation with a third reviewer (SG).
In case of undetermined age-related eligibility, authors of the respective articles were contacted and asked to provide relevant age-related characteristics concerning the study population.
Data Collection Process and Analysis
Two authors (AE or AR) collected data from the included studies using a standardized data extraction form. Besides core study characteristics (year, location, study design, and sample size) extracted information comprised the following items: study population (age, cognitive status, and affective status), study context, preference measurement method used with, if applicable, the name of the specific instrument, and quality criteria including validity testing. Verification of all extracted data was carried out by the second reviewer (AE or AR).
Regarding the nomenclature of the identified preference elicitation techniques we relied primarily on the characterization of the method given by the respective authors. We coined the term “medication willingness” for all not otherwise specified instruments that gave a description of a certain medication and asked the study participants whether or not and, possibly, in what kind of circumstances they were willing to take that specific medication.
The data extraction form was complemented by a short assessment evaluating the adaptability of the identified preference measurement techniques to the context of aged patients with polypharmacy (“polypharmacy assessment”). We aimed at detecting a method that could be used in a routine clinical setting to individualize the medication of older adults with multiple chronic conditions based on the individual patient’s preferences. This method would have to reflect the complexity of the context by integrating the majority of the multitude of treatment outcomes and treatment-related considerations innate to geriatric polypharmacy without neglecting the characteristics of the often physically and cognitively frail older population. Therefore, the assessment consisted of four methodological characteristics judged to be of relevance for preference elicitation instruments used in this setting: (1) time budget needed for health care workers to assess patient preferences, (2) level of cognitive demand imposed on the older respondent, (3) representation of a variety of pharmacological aspects including treatment options, and (4) link of recorded preferences with specific pharmacotherapeutic strategies. Each item was evaluated on a three-point scale with the categories “high”, “intermediate”, “low” (time budget, cognitive demand, and variety of pharmacological aspects) or “distinct”, “moderate”, “indistinct” (link with treatment strategies). summarizes the criteria used to make the allocation to the respective categories. To assess the level of cognitive demand imposed on the respondent, we evaluated the number of cognitive steps needed as well as the assumed amount of time spent to reach an appropriate decision, which is an approach previously used in the optimization of experimental designs for conjoint analysis.Citation27 Two authors (AE and AR) independently completed the assessment for all 55 identified instruments, analyzing the preference methods as implemented by the respective instruments. Additionally, both reviewers evaluated if the respective preference measurement technique required the respondent to make trade-offs between competing medical problems. Disagreements were settled through consensus discussions. In the case of incomplete presentation of the preference elicitation instrument, authors of the respective articles were contacted and asked to provide additional information.
Table 1 Assessment Evaluating the Adaptability of the Identified Preference Measurement Instruments to the Context of Aged Patients with Polypharmacy (“Polypharmacy Assessment”)
Due to the heterogeneity and mostly observational design of the eligible studies we did not conduct a meta-analysis but opted to present the collected data as an in-depth narrative summary.
Quality Assessment
Owing to a lack of definite guidance on how to perform an appropriate quality assessment for preference-based studies,Citation28 the methodological quality of the included studies was evaluated by adapting four criteria from two previously published instruments for patient preference studies.Citation29,Citation30 Two reviewers (AE, AR) independently assessed whether (1) there was a well-defined question in relation to preferences, (2) the characteristics of the participants were clearly described, (3) the respective methods to assess preferences were clearly explained, and (4) the authors reported quality criteria in relation to the elicitation methods used. We expanded criterion number (2) (characteristics of the participants) to include data on the cognitive and affective status of the respondents. Any disparities were resolved by discussion (see Electronic Supplementary Material Tables S2 and S3).
Results
Study Selection
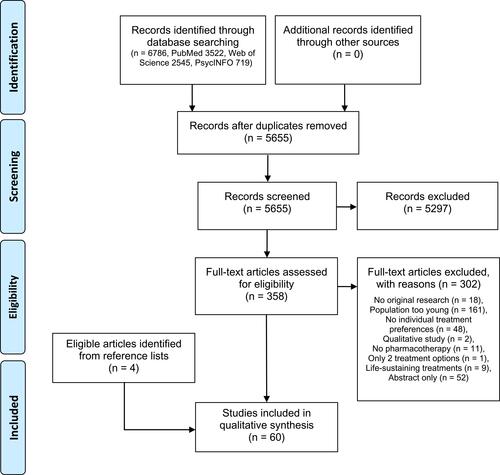
Our database search identified 6786 citations; 358 articles were retrieved for full-text screening and 56 of these articles met the eligibility criteria. Four additional studies were identified by manually searching the reference lists of eligible articles. depicts the PRISMA flow diagram of the screening and selection process.
Study Characteristics
All articles included are listed in . The majority (86.7 %) of the studies had a cross-sectional design. The research was performed in 12 different countries. Three (5.0%) of the eligible articles were published between 1994 and 1999,Citation31–Citation33 19 (31.7%) between 2000 and 2009,Citation34–Citation52 and 38 (63.3%) between 2010 and 2017 (). The respective sample size ranged from 13 to 2637 participants. In four of the considered studies, the elicitation of patient preferences was only of minor interest.Citation39,Citation53–Citation55 One studyCitation56 was a follow-up study of a previously published cross-sectional study.Citation57 Four studies presumably used the same study sample.Citation58–Citation61
Table 2 Characteristics of Included Studies
Study Populations
Thirty-nine studies met our age-related eligibility criteria by exclusively including adults aged 65 years and older,Citation32,Citation35,Citation37–Citation39,Citation41,Citation42,Citation44–Citation46,Citation49–Citation60,Citation62–Citation78 16 by reporting a mean or median age ≥ 75 years,Citation31,Citation36,Citation40,Citation43,Citation47,Citation48,Citation61,Citation79–Citation87 and 5 by systematically analyzing a subgroup within their overall study population aged 65 years or older.Citation33,Citation34,Citation88–Citation90 Eleven studies indicated a mean or median age of 80 years or older.Citation42,Citation47,Citation49,Citation51,Citation52,Citation54,Citation56,Citation57,Citation71,Citation74,Citation79 Thirty-seven studies reported data regarding the cognitive status of their participants.Citation31,Citation32,Citation35,Citation36,Citation41–Citation44,Citation47,Citation49,Citation51–Citation53,Citation55–Citation61,Citation63–Citation72,Citation74,Citation75,Citation78,Citation80,Citation83,Citation84,Citation90 In 83.8 % of these studies participants were ineligible if one of the following criteria was present: some form of cognitive impairment such as a formal diagnosis of dementia, the failure to meet a specific cut-off on a standardized cognitive assessment, or cognitive problems as suspected by the investigator (). Seventeen studies detailed the results of a standardized cognitive evaluation of their study population.Citation32,Citation36,Citation43,Citation51,Citation52,Citation55,Citation66,Citation68–Citation72,Citation74,Citation78,Citation80,Citation83,Citation84 The participants’ affective status was considered by 30 studies ().
Study Context
The majority of the identified 55 instruments were designed to assess patient preferences within a disease-specific or context-specific setting. The most prevalent contexts were various types of cancer (9 tools),Citation33,Citation41,Citation48,Citation55,Citation66,Citation72,Citation86,Citation90 mental health issues including depression (7 tools),Citation43,Citation45,Citation67,Citation71,Citation73,Citation75,Citation82,Citation83 cardiovascular prevention (7 tools),Citation46,Citation47,Citation60,Citation61,Citation63,Citation74,Citation88 stroke prevention in atrial fibrillation (5 tools),Citation32,Citation42,Citation44,Citation50,Citation80 and age-related macular degeneration (4 tools).Citation79,Citation81,Citation85,Citation87 Only three tools were designed for the greater context of multimorbidity: the Outcome Prioritization Tool,Citation56,Citation57,Citation59,Citation60,Citation65 the complex intervention “PrefCheck” combining a comprehensive geriatric assessment (“STEP assessment”) with a health priority evaluation,Citation68–Citation70,Citation78 and the “MediMol” questionnaire.Citation53 The latter, however, focuses on the monitoring of various medication-related problems and includes only a minor preference assessment. Four tools concentrated on a specific sub-context of multimorbidity, such as the trade-offs between present and future health (2 tools)Citation58,Citation59 or the trade-offs between a specific subset of competing health outcomes in multimorbidity (2 tools).Citation51,Citation52,Citation63 One instrument evaluated a set of general health outcomes for the medical care of older adults.Citation62
Methods to Assess Patient Preferences
The most prevalent preference elicitation methods among the 55 identified instruments were “medication willingness” (9 instruments), discrete choice experiments (7 instruments), and Likert scale-based questionnaires (6 instruments) ( and ). Six instruments used ranking exercises, two in combination with a preceding choice of the most relevant options by the participant and one in combination with a Likert scale.Citation43,Citation62,Citation71,Citation75,Citation77,Citation82,Citation83 A direct choice between different treatment options was employed by four instruments, one using an additional questionnaire.Citation48,Citation54,Citation66,Citation73 The time trade-off technique and questionnaires were used by three instruments respectively.Citation35,Citation37,Citation38,Citation53,Citation67,Citation72 Two instruments each used adaptive conjoint analysis,Citation76,Citation89 the probability trade-off technique,Citation46,Citation90 or an individualized decision analysis.Citation46,Citation50 Single instruments applied the analytical hierarchy process,Citation81 a complex intervention,Citation68–Citation70,Citation78 traditional conjoint analysis,Citation79 the format of a decision aid,Citation44 the feeling thermometer,Citation40 maximum difference scaling,Citation77 paired comparisons,Citation49 switch-point vignettes,Citation33 a visual analogue scale,Citation59,Citation60 and willingness to pay.Citation84 One specific health outcome prioritization tool was evaluated by five different studies.Citation56,Citation57,Citation59,Citation60,Citation65
Table 3 Results of the “Polypharmacy Assessment” (Assessment Evaluating the Adaptability of the Identified 55 Preference Measurement Instruments to the Context of Aged Patients with Polypharmacy)
Test Quality of the Instruments to Assess Patient Preferences
The column “quality criteria” in gives a short summary regarding the test quality for each of the different instruments as reported in the respective articles. Owing to the large number of studies using literature searches and expert opinions during the design of the preference tools, data on content validity were omitted in favor of further validity or reliability assessments. Five studies evaluated the construct validity of the respective tools by measuring their agreement with other preference instruments or with predefined hypotheses.Citation40,Citation58,Citation60,Citation65,Citation84 All seven discrete choice experiments and the traditional conjoint analysis used additional choice scenarios to test the participants’ understanding of the task as well as the consistency of their answers.Citation51,Citation64,Citation79,Citation80,Citation85–Citation88 One study targeted criterion validity by inquiring the patients’ satisfaction with the treatment that was selected based on the results of the preference elicitation.Citation48 Nine studies applied qualitative research to aid the interpretation of the quantitative results of the respective preference task.Citation42,Citation43,Citation57,Citation59,Citation65,Citation76,Citation81,Citation87,Citation90
Polypharmacy Assessment
The results of our “polypharmacy assessment” are detailed in . The majority of the instruments (70.9 %) were exclusively tested in interview mode and thus claimed a high amount of time of the respective health care worker. Eighteen instruments were judged to impose a low cognitive demand on the older respondent, 20 to impose an intermediate, and 15 to impose a high cognitive demand. Tools that were rated to present a low cognitive demand primarily used Likert scales, other questionnaires, a direct choice, or ranking exercises with few attributes as their methodological approach. Instruments with a high cognitive demand asked of the respondent to simultaneously trade-off between a large number of attributes or several probability calculations, for example within more complex conjoint analysis-based experiments or during time trade-off tasks. Only three tools evaluated more than 10 pharmacological attributes and were rated to represent a broad variety of aspects.Citation35,Citation62,Citation68–Citation70,Citation78 The majority of the instruments provided an either distinct (28 tools) or moderate (22 tools) link with a specific drug therapy. The advantages and disadvantages of the various methods showed opposing trends: no tool with a low cognitive demand and only one toolCitation35 that represented a high variety of pharmacological aspects was rated to provide a distinct link with a specific treatment strategy ().
No tool was given an ideal rating, and none of the instruments scored more than two positives out of the four ratings. Only seven tools scored at least one positive rating and no negative rating for the criteria “cognitive demand”, “variety of pharmacological aspects”, and “link with treatment strategies”: one analytical hierarchy process,Citation81 the complex intervention “PrefCheck”,Citation68–Citation70,Citation78 two discrete choice experiments,Citation80,Citation87 the maximum difference scaling,Citation77 one example of “medication willingness”,Citation42 and the “MediMol” questionnaireCitation53 (). The analytical hierarchy process, maximum difference scaling as well as discrete choice experiments are well-established methods to measure preferences.Citation77,Citation80,Citation81,Citation87 “PrefCheck” is a complex intervention, specifically designed to assess health priorities in older primary care patients.Citation68 It is based on a validated comprehensive geriatric assessment.Citation68 No data regarding the validity or reliability of the instrument have been reported for the “MediMol” questionnaire.Citation53
Methodological Quality of Included Studies
The results of the quality assessment are depicted in the Electronic Supplementary Material Table S3. Four studies failed to give a comprehensive description of the preference measurement method used.Citation36,Citation48,Citation66,Citation89
Discussion
To our knowledge, this is the first review to both systematically identify methods used to assess medication preferences in older adults and to rate their potential in regard to preference elicitation within the context of polypharmacy. We aimed at determining methods that could support an individualized prescribing process by including the patient’s medication preferences.
We identified 55 different instruments that have been applied to evaluate patient preferences in old age pharmacotherapy, based on 24 different methods to determine preferences. Apart from “medication willingness”, a term that we originally coined to specify a group of not otherwise characterized instruments that directly elicited the participant’s willingness for the use of a certain medication, we found that discrete choice experiments, Likert scale-based ratings, and ranking exercises were the most commonly employed elicitation methods in older adults. This finding is in line with data from a non-age-specific investigation on the integration of patient preferences in clinical decision-making that listed rating scales, ranking exercises as well as discrete choice experiments amongst the most prevalent methods.Citation91
Only a minority of the eligible instruments targeted the context of multimorbidity-related polypharmacy. In addition to the Outcome Prioritization Tool identified in a review on preference elicitation in older primary care patients with multiple conditions,Citation22 we found two further tools that were specifically designed to assess multimorbidity-related patient preferences. The complex intervention “PrefCheck” combines the geriatric “STEP assessment” with a Likert scale-based individual health priority evaluation by the patients and their general practitioners followed by a priority-setting consultation. It focusses on general health problems, with medication-related aspects being one part of an extensive evaluation including social and financial issues. Because it is based on a comprehensive geriatric assessment, this approach is time-consuming but might be practicable in settings routinely collecting the respective data. The reliability of the “PrefCheck”-related health priority evaluation is yet to be determined.Citation68 The second additional instrument identified, the “MediMol” questionnaire, does not primarily focus on measuring patient preferences but incorporates the elicitation of universal health priorities into the assessments of various medication-related issues. Neither the “PrefCheck” intervention nor the “MediMol” questionnaire allow for trade-offs between competing health outcomes.
To identify further preference measurement techniques that could serve as a basis for future tools to allow for preference-based individualized prescribing in polypharmacy, we conducted a thorough evaluation of all 55 identified preference elicitation instruments and analyzed their advantages and disadvantages with the help of four relevant characteristics (“time budget”, “cognitive demand”, “variety of pharmacological aspects”, and “link with treatment strategies”). In order to meet the time constraints imposed by routine medical practice, tools for the measurement of patient preferences should reduce the amount of time the physician needs to invest in preference elicitation. However, the vast majority of the instruments in our review were tested in interview mode and as such did not offer any time-saving benefits. To allow for large-scale application outside of separately funded study settings, future tools need to be simple and usable to be self-administered by the older patient and future research should particularly address the feasibility of self-administration. Alternatively, one might advocate integrating the standardized elicitation of patient preferences into the comprehensive geriatric assessment, thereby providing a time frame for this important task within the routine geriatric setting.
Besides the “PrefCheck” intervention and the “MediMol” questionnaire only five other instruments showed no negative rating and at least one positive rating for the remaining three criteria “cognitive demand”, “variety of pharmacological aspects”, and “link with treatment strategies”. Two discrete choice experiments were among these five favorable tools.Citation80,Citation87 Typical for this method, these two instruments were designed to directly link the measured patient preferences to a specific drug therapy, revealing one of the advantages of this approach. Both tools balanced the cognitive demand of their choice tasks and the number of attributes within the choice sets by defining simple attribute levels, refraining from confronting the participants with risk reduction percentages, and using visual aids such as pictographs or a clear questionnaire layout. The cognitive demand of a discrete choice experiment is expected to increase with the number of attributes included in the choice task and the maximum number of attributes to consider is generally given as six to seven.Citation92 This restricted number of health outcomes or other pharmacotherapy-related aspects that could be represented in the instrument seems to limit the profitable use of the method in multimorbidity-related polypharmacy. However, future tools aiming at preference-based individualized prescribing might combine the actual discrete choice experiment with an individual preselection of the relevant attributes by the patient. The indirect scenario-based approach of preference measurement is thought to be challenging for the respondent and none of the seven discrete choice experiments in this review was rated to impose a low cognitive demand on the participant. However, research indicates that simple discrete choice experiments with a reduced number of choice sets might be successfully completed even by older adults with mild cognitive impairment.Citation93 The cognitive demand for the older participant and its impact on the feasibility of the instruments need to be evaluated in more depth in order to clarify whether the potential of this popular method of preference elicitation is developable or limited in old-age polypharmacy. Similar considerations might apply to the analytical hierarchy processCitation81 and the maximum difference scalingCitation77 which, according to the standards of our assessment, impose an intermediate cognitive demand on the respondent. To date, little evidence in regard to the measurement of patient preferences exists for either of these methods. The last approach rated as possibly favorable in polypharmacy, a version of “medication willingness”,Citation42 differs from the aforementioned techniques in that it would require developing a reasoned algorithm of adding or altering variables of a medication scenario and repeatedly asking the patients whether or not they would be willing to take the specified drug therapy.
Interestingly, the Outcome Prioritization Tool, which was previously identified as potentially relevant for measuring treatment preferences in older patients with multiple conditions,Citation22 did not tend towards an overall favorable rating on our “polypharmacy assessment”. This evaluation was based on the time-consuming design as an interview tool, the intermediate level of cognitive demand owing to the need of multiple trade-offs, the limited number of health outcomes incorporated in the instrument, and a moderate to unclear link of the recorded priorities with a specific drug therapy. The latter evaluation is substantiated by the results of a recent study that suggested that primary care physicians might find it difficult to translate the patient’s preference for the general outcome “maintaining independence” to a certain change in his or her medication.Citation56
Several limitations of this systematic review should be addressed. Despite our comprehensive search strategy, potentially eligible articles might not have been identified. The four methodological criteria for the “polypharmacy assessment” were developed by our research group instead of being derived from a thorough literature review and accompanying expert interviews. Due to the unique characteristics of pharmacotherapy we limited our eligibility criteria to exclude studies without any long-term drug therapy-related aspects. It might be possible, that published methods assessing patient preferences within non-pharmacological contexts, eg surgery, could also offer some potential in relation to preference elicitation in polypharmacy.
Conclusion
To our knowledge, this is the first systematic review offering a comprehensive overview of instruments used to assess patient preferences in old age pharmacotherapy. No ideal method for practicable and valid elicitation of patient preferences in the context of geriatric polypharmacy could be identified. By evaluating the existing preference measurement instruments on four criteria salient for successful preference-based individualized prescribing in polypharmacy, the findings of this systematic review can guide future research in polypharmacy-related patient preferences and provide relevant information for the development of new and more appropriate measurement approaches.
Acknowledgments
This work was funded by the Baden-Württemberg Ministry of Science, Research and the Arts as part of the project “Medication and circumstances of life in old age” (Verbundprojekt “Medikation und Lebenssituation im Alter”). The funding source had no role in the design of this systematic review, the collection and interpretation of the data, and the writing of this manuscript. In addition, we acknowledge financial support by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg (Open Access Publishing Fund).
Disclosure
Dr Annette Eidam reports grants from the State Ministry of Baden-Wuerttemberg (Germany) for Sciences, Research and Arts, during the conduct of the study. Ms Anja Roth reports grants from the State Ministry of Baden-Wuerttemberg (Germany) for Sciences, Research and Arts, during the conduct of the study.
Dr André Lacroix reports grants from the State Ministry of Baden-Wuerttemberg (Germany) for Sciences, Research and Arts, during the conduct of the study.
Dr Sabine Goisser reports grants from the State Ministry of Baden-Wuerttemberg (Germany) for Sciences, Research and Art, during the conduct of the study.
Dr Hanna M. Seidling reports grants from the State Ministry of Baden-Wuerttemberg (Germany) for Sciences, Research and Arts, during the conduct of the study; non-financial support from VKliPha; AkdÄ; GSASA, APS e.V., NHS, ESCP, BAK, ÄZQ, SFPC, Dosing GmbH, Karolinska Institutet, University of Bonn, University Hospital Hamburg, personal fees from Universitätsklinikum Heidelberg IMBI, Govi Verlag, Deutscher Apotheker Verlag, Wissenschaftliche Verlagsgesellschaft Stuttgart, Bundesgesundheitsblatt, personal fees, non-financial support from ADKA e.V.; EAHP; Chamber of Pharmacists, Hessen; Chamber of Pharmacists, Baden-Württemberg, Chamber of Pharmacists, Westfalen-Lippe, Chamber of Pharmacists Nordrhein, Chamber of Pharmacists Bavaria, DPhG, AD REM TEAM München, ABDA - Bundesvereinigung Deutscher Apotheker e.V., Omnicell, Chamber of Pharmacists Niedersachsen, Chamber of Pharmacists Thüringen, grants from Chambers of Pharmacists Baden-Württemberg, Nordrhein, Hessen and Niedersachsen, Klaus Tschira Stiftung gGmbH, Dosing GmbH, ABDA - Bundesvereinigung Deutscher Apotheker e.V., g-BA, BMBF, European Commission Horizon 2020, outside the submitted work.
Prof. Dr. Walter E. Haefeli reports grants from the State Ministry of Baden-Wuerttemberg (Germany) for Sciences, Research and Arts, during the conduct of the study; grants from ADIR, travel expenses from AID Berlin, grants from AOK BW, personal fees from Apoth.kammer Schlesw., grants from Basilea Ltd., grants from Bayer AG, speaker fees and traveling expenses from Berlin-Chemie AG, grants from BMBF, speaker fees and traveling expenses from Boehringer GmbH, grants, speaker fees and traveling expenses from Bristol-Myers Squibb, grants from Chiesi GmbH, personal fees from COCS/DGD, grants, personal fees from Daiichi-Sankyo, grants from DFG No. 79, personal fees from DiakonissenKH MA, research funding from DKFZ, he is a shareholder of Dosing GmbH and his wife an employee of Dosing GmbH, personal fees from ESA Köln, grants from EU Projects, traveling expenses from Fresenius, research funding from Gem.Bundesausschuss, personal fees from GenPlus GmbH, personal fees, speaker fees and traveling expenses from Grünenthal GmbH, grants from GSK, grants from HDIT, grants from Hepatera Ltd., grants from IPMB, grants from Janssen GmbH, consultancy services and traveling expenses from LAK BW, personal fees from Ligatur Verlag, grants from MWK, grants from MYR GmbH, speaker fees and traveling expenses from Novartis, traveling expenses from Orphix Consulting, grants from PCI, speaker fees and traveling expenses from Pfizer, consultancy services and traveling expenses from PIQUR Basel, grants from QPS Netherlands B.V., grants from SFB 1389/1 TP C1.2, grants from Smooth Clin. Trial, grants from Sumaya Biotec, grants from K. Tschira Stiftung, consultancy and traveling expenses from Stiftung Warentest, personal fees from Thieme Verlag, traveling expenses from Uni Saarbrücken, grants from Vaximm GmbH, outside the submitted work.
Prof. Dr. Jürgen M. Bauer reports grants from the Ministry for Research, Baden-Württemberg, Germany, during the conduct of the study; personal fees from Nestlé, personal fees from Nutricia, personal fees from Novartis, personal fees from Fresenius, personal fees from Daiichi Sankyo, personal fees from Bayer, outside the submitted work.
The authors report no other conflicts of interest in this work.
References
- Umar N, Schaarschmidt M, Schmieder A, Peitsch WK, Schollgen I, Terris DD. Matching physicians’ treatment recommendations to patients’ treatment preferences is associated with improvement in treatment satisfaction. J Eur Acad Dermatol Venereol. 2013;27(6):763–770. doi:10.1111/jdv.2013.27.issue-6
- Wilder CM, Elbogen EB, Moser LL, Swanson JW, Swartz MS. Medication preferences and adherence among individuals with severe mental illness and psychiatric advance directives. Psychiatr Serv. 2010;61(4):380–385. doi:10.1176/ps.2010.61.4.380
- Abraham NS, Naik AD, Street RL Jr., et al. Complex antithrombotic therapy: determinants of patient preference and impact on medication adherence. Patient Prefer Adherence. 2015;9:1657–1668. doi:10.2147/PPA.S91553
- Currie A, Askari A, Nachiappan S, Sevdalis N, Faiz O, Kennedy R. A systematic review of patient preference elicitation methods in the treatment of colorectal cancer. Colorectal Dis. 2015;17(1):17–25. doi:10.1111/codi.12754
- Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R. Patient preferences for oral anticoagulation therapy in atrial fibrillation: a systematic literature review. Patient. 2017;10(1):17–37. doi:10.1007/s40271-016-0185-9
- McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595–602. doi:10.4088/JCP.12r07757
- Bowling A, Ebrahim S. Measuring patients’ preferences for treatment and perceptions of risk. Qual Health Care. 2001;10(Suppl 1):i2–8. doi:10.1136/qhc.0100002
- Fialova D, Onder G. Medication errors in elderly people: contributing factors and future perspectives. Br J Clin Pharmacol. 2009;67(6):641–645. doi:10.1111/bcp.2009.67.issue-6
- Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–724. doi:10.1001/jama.294.6.716
- Hovstadius B, Petersson G, Hellstrom L, Ericson L. Trends in inappropriate drug therapy prescription in the elderly in Sweden from 2006 to 2013: assessment using national indicators. Drugs Aging. 2014;31(5):379–386. doi:10.1007/s40266-014-0165-5
- Guerriero F, Orlando V, Tari DU, et al. How healthy is community-dwelling elderly population? Results from Southern Italy. Transl Med UniSa. 2015;13:59–64.
- Moriarty F, Hardy C, Bennett K, Smith SM, Fahey T. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross-sectional study. BMJ Open. 2015;5(9):e008656. doi:10.1136/bmjopen-2015-008656
- Herr M, Sirven N, Grondin H, Pichetti S, Sermet C. Frailty, polypharmacy, and potentially inappropriate medications in old people: findings in a representative sample of the French population. Eur J Clin Pharmacol. 2017;73(9):1165–1172. doi:10.1007/s00228-017-2276-5
- Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging. 2009;26(6):475–482. doi:10.2165/00002512-200926060-00004
- Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30(10):911–918. doi:10.2165/00002018-200730100-00009
- Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–2272. doi:10.1111/jgs.13153
- Saum KU, Schottker B, Meid AD, et al. Is polypharmacy associated with frailty in older people? Results from the ESTHER cohort study. J Am Geriatr Soc. 2017;65(2):e27–e32. doi:10.1111/jgs.14718
- Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–834. doi:10.1001/jamainternmed.2015.0324
- Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLoS One. 2016;11(3):e0149984. doi:10.1371/journal.pone.0149984
- Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254–1268. doi:10.1111/bcp.12732
- Holmes HM, Todd A. The role of patient preferences in deprescribing. Clin Geriatr Med. 2017;33(2):165–175. doi:10.1016/j.cger.2017.01.004
- Mangin D, Stephen G, Bismah V, Risdon C. Making patient values visible in healthcare: a systematic review of tools to assess patient treatment priorities and preferences in the context of multimorbidity. BMJ Open. 2016;6(6):e010903. doi:10.1136/bmjopen-2015-010903
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
- van Hoorn R, Kievit W, Booth A, et al. The development of PubMed search strategies for patient preferences for treatment outcomes. BMC Med Res Methodol. 2016;16:88. doi:10.1186/s12874-016-0192-5
- van de Glind EM, van Munster BC, Spijker R, Scholten RJ, Hooft L. Search filters to identify geriatric medicine in Medline. J Am Med Inform Assoc. 2012;19(3):468–472. doi:10.1136/amiajnl-2011-000319
- Dirksen CD, Utens CM, Joore MA, et al. Integrating evidence on patient preferences in healthcare policy decisions: protocol of the patient-VIP study. Implement Sci. 2013;8:64. doi:10.1186/1748-5908-8-64
- Huertas-García R, Nunez-Carballosa A, Miravitlles P. Statistical and cognitive optimization of experimental designs in conjoint analysis. Eur J Manage Bus Econ. 2016;25:142–149. doi:10.1016/j.redee.2015.10.001
- Yu T, Enkh-Amgalan N, Zorigt G. Methods to perform systematic reviews of patient preferences: a literature survey. BMC Med Res Methodol. 2017;17(1):166. doi:10.1186/s12874-017-0448-8
- Purnell TS, Joy S, Little E, Bridges JF, Maruthur N. Patient preferences for noninsulin diabetes medications: a systematic review. Diabetes Care. 2014;37(7):2055–2062. doi:10.2337/dc13-2527
- Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics. 2013;31(10):877–892. doi:10.1007/s40273-013-0089-7
- Miller DK, Morrison MJ, Blair SD, Miller JP, Morley JE. Predilection for frailty remedial strategies among black and white seniors. South Med J. 1998;91(4):375–380. doi:10.1097/00007611-199804000-00013
- Sudlow M, Thomson R, Kenny RA, Rodgers H. A community survey of patients with atrial fibrillation: associated disabilities and treatment preferences. Br J Gen Pract. 1998;48(436):1775–1778.
- Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. J Natl Cancer Inst. 1994;86(23):1766–1770. doi:10.1093/jnci/86.23.1766
- Bowling A, Reeves B, Rowe G. Patient preferences for treatment for angina: an overview of findings from three studies. J Health Serv Res Policy. 2008;13(Suppl 3):104–108. doi:10.1258/jhsrp.2008.008012
- Brown SE, Meltzer DO, Chin MH, Huang ES. Perceptions of quality-of-life effects of treatments for diabetes mellitus in vulnerable and nonvulnerable older patients. J Am Geriatr Soc. 2008;56(7):1183–1190. doi:10.1111/j.1532-5415.2008.01757.x
- Carpenter BD, Kissel EC, Lee MM. Preferences and life evaluations of older adults with and without dementia: reliability, stability, and proxy knowledge. Psychol Aging. 2007;22(3):650–655. doi:10.1037/0882-7974.22.3.650
- Cherniack EP, Ceron-Fuentes J, Florez H, Sandals L, Rodriguez O, Palacios JC. Influence of race and ethnicity on alternative medicine as a self-treatment preference for common medical conditions in a population of multi-ethnic urban elderly. Complement Ther Clin Pract. 2008;14(2):116–123. doi:10.1016/j.ctcp.2007.11.002
- Chin MH, Drum ML, Jin L, Shook ME, Huang ES, Meltzer DO. Variation in treatment preferences and care goals among older patients with diabetes and their physicians. Med Care. 2008;46(3):275–286. doi:10.1097/MLR.0b013e318158af40
- Cline RR, Mott DA. Use of antiresorptive drugs among older women: a case study in Wisconsin. Am J Health Syst Pharm. 2003;60(5):453–463. doi:10.1093/ajhp/60.5.453
- Cranney A, Coyle D, Pham BA, et al. The psychometric properties of patient preferences in osteoporosis. J Rheumatol. 2001;28(1):132–137.
- Extermann M, Albrand G, Chen H, et al. Are older French patients as willing as older American patients to undertake chemotherapy? J Clin Oncol. 2003;21(17):3214–3219. doi:10.1200/JCO.2003.08.091
- Fuller R, Dudley N, Blacktop J. Avoidance hierarchies and preferences for anticoagulation–semi-qualitative analysis of older patients’ views about stroke prevention and the use of warfarin. Age Ageing. 2004;33(6):608–611. doi:10.1093/ageing/afh202
- Fyffe DC, Brown EL, Sirey JA, Hill EG, Bruce ML. Older home-care patients’ preferred approaches to depression care: a pilot study. J Gerontol Nurs. 2008;34(8):17–22. doi:10.3928/00989134-20080801-06
- Holbrook A, Labiris R, Goldsmith CH, Ota K, Harb S, Sebaldt RJ. Influence of decision aids on patient preferences for anticoagulant therapy: a randomized trial. CMAJ. 2007;176(11):1583–1587. doi:10.1503/cmaj.060837
- Landreville P, Landry J, Baillargeon L, Guerette A, Matteau E. Older adults’ acceptance of psychological and pharmacological treatments for depression. J Gerontol B Psychol Sci Soc Sci. 2001;56(5):P285–P291. doi:10.1093/geronb/56.5.P285
- Man-Son-Hing M, Laupacis A, O’Connor AM, Coyle D, Berquist R, McAlister F. Patient preference-based treatment thresholds and recommendations: a comparison of decision-analytic modeling with the probability-tradeoff technique. Med Decis Making. 2000;20(4):394–403. doi:10.1177/0272989X0002000403
- Murphy DJ, Gahm GJ, Santilli S, North P, Oliver SC, Shapiro H. Seniors’ preferences for cancer screening and medication use based on absolute risk reduction. J Gerontol A Biol Sci Med Sci. 2002;57(2):M100–M105. doi:10.1093/gerona/57.2.M100
- Nyman CR, Andersen JT, Lodding P, Sandin T, Varenhorst E. The patient’s choice of androgen-deprivation therapy in locally advanced prostate cancer: bicalutamide, a gonadotrophin-releasing hormone analogue or orchidectomy. BJU Int. 2005;96(7):1014–1018. doi:10.1111/bju.2005.96.issue-7
- Pfisterer MH, Johnson TM 2nd, Jenetzky E, Hauer K, Oster P. Geriatric patients’ preferences for treatment of urinary incontinence: a study of hospitalized, cognitively competent adults aged 80 and older. J Am Geriatr Soc. 2007;55(12):2016–2022. doi:10.1111/j.1532-5415.2007.01457.x
- Protheroe J, Fahey T, Montgomery AA, Peters TJ. The impact of patients’ preferences on the treatment of atrial fibrillation: observational study of patient based decision analysis. BMJ. 2000;320(7246):1380–1384. doi:10.1136/bmj.320.7246.1380
- Tinetti ME, McAvay GJ, Fried TR, et al. Development of a tool for eliciting patient priority from among competing cardiovascular disease, medication-symptoms, and fall injury outcomes. J Am Geriatr Soc. 2008;56(4):730–736. doi:10.1111/jgs.2008.56.issue-4
- Tinetti ME, McAvay GJ, Fried TR, et al. Health outcome priorities among competing cardiovascular, fall injury, and medication-related symptom outcomes. J Am Geriatr Soc. 2008;56(8):1409–1416. doi:10.1111/jgs.2008.56.issue-8
- Muth C, Harder S, Uhlmann L, et al. Pilot study to test the feasibility of a trial design and complex intervention on PRIoritising MUltimedication in Multimorbidity in general practices (PRIMUMpilot). BMJ Open. 2016;6(7):e011613. doi:10.1136/bmjopen-2016-011613
- Schnabel K, Binting S, Witt CM, Teut M. Use of complementary and alternative medicine by older adults–a cross-sectional survey. BMC Geriatr. 2014;14:38. doi:10.1186/1471-2318-14-38
- Schonberg MA, Birdwell RL, Bychkovsky BL, et al. Older women’s experience with breast cancer treatment decisions. Breast Cancer Res Treat. 2014;145(1):211–223. doi:10.1007/s10549-014-2921-y
- van Summeren JJ, Schuling J, Haaijer-Ruskamp FM, Denig P. Outcome prioritisation tool for medication review in older patients with multimorbidity: a pilot study in general practice. Br J Gen Pract. 2017;67(660):e501–e506. doi:10.3399/bjgp17X690485
- van Summeren JJ, Haaijer-Ruskamp FM, Schuling J. Eliciting preferences of multimorbid elderly adults in family practice using an outcome prioritization tool. J Am Geriatr Soc. 2016;64(11):e143–e148. doi:10.1111/jgs.14415
- Case SM, Towle VR, Fried TR. Considering the balance: development of a scale to assess patient views on trade-offs in competing health outcomes. J Am Geriatr Soc. 2013;61(8):1331–1336. doi:10.1111/jgs.2013.61.issue-8
- Case SM, Fried TR, O’Leary J. How to ask: older adults’ preferred tools in health outcome prioritization. Patient Educ Couns. 2013;91(1):29–36. doi:10.1016/j.pec.2012.11.010
- Case SM, O’Leary J, Kim N, Tinetti ME, Fried TR. Relationship between universal health outcome priorities and willingness to take medication for primary prevention of myocardial infarction. J Am Geriatr Soc. 2014;62(9):1753–1758. doi:10.1111/jgs.12983
- Fried TR, Tinetti ME, Towle V, O’Leary JR, Iannone L. Effects of benefits and harms on older persons’ willingness to take medication for primary cardiovascular prevention. Arch Intern Med. 2011;171(10):923–928. doi:10.1001/archinternmed.2011.32
- Akishita M, Ishii S, Kojima T, et al. Priorities of health care outcomes for the elderly. J Am Med Dir Assoc. 2013;14(7):479–484. doi:10.1016/j.jamda.2013.01.009
- Caughey GE, Tait K, Vitry AI, Shakib S. Influence of medication risks and benefits on treatment preferences in older patients with multimorbidity. Patient Prefer Adherence. 2017;11:131–140. doi:10.2147/PPA
- Decalf VH, Huion AMJ, Benoit DF, Denys MA, Petrovic M, Everaert K. Older people’s preferences for side effects associated with antimuscarinic treatments of overactive bladder: a discrete-choice experiment. Drugs Aging. 2017;34(8):615–623. doi:10.1007/s40266-017-0474-6
- Fried TR, Tinetti M, Agostini J, Iannone L, Towle V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns. 2011;83(2):278–282. doi:10.1016/j.pec.2010.04.032
- Girones R, Torregrosa D, Gomez-Codina J, Maestu I, Tenias JM, Rosell R. Lung cancer chemotherapy decisions in older patients: the role of patient preference and interactions with physicians. Clin Transl Oncol. 2012;14(3):183–189. doi:10.1007/s12094-012-0782-6
- Jimenez DE, Bartels SJ, Cardenas V, Dhaliwal SS, Alegria M. Cultural beliefs and mental health treatment preferences of ethnically diverse older adult consumers in primary care. Am J Geriatr Psychiatry. 2012;20(6):533–542. doi:10.1097/JGP.0b013e318227f876
- Junius-Walker U, Stolberg D, Steinke P, Theile G, Hummers-Pradier E, Dierks ML. Health and treatment priorities of older patients and their general practitioners: a cross-sectional study. Qual Prim Care. 2011;19(2):67–76.
- Junius-Walker U, Wrede J, Voigt I, et al. Impact of a priority-setting consultation on doctor-patient agreement after a geriatric assessment: cluster randomised controlled trial in German general practices. Qual Prim Care. 2012;20(5):321–334.
- Junius-Walker U, Wiese B, Klaassen-Mielke R, Theile G, Muller CA, Hummers-Pradier E. Older patients’ perceived burdens of their health problems: a cross-sectional analysis in 74 German general practices. Patient Prefer Adherence. 2015;9:811–820. doi:10.2147/PPA.S81348
- Luck-Sikorski C, Stein J, Heilmann K, et al. Treatment preferences for depression in the elderly. Int Psychogeriatr. 2017;29(3):389–398. doi:10.1017/S1041610216001885
- Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physicians. J Clin Oncol. 2010;28(19):3146–3153. doi:10.1200/JCO.2009.24.3295
- Mohlman J. A community based survey of older adults’ preferences for treatment of anxiety. Psychol Aging. 2012;27(4):1182–1190. doi:10.1037/a0023126
- Perret-Guillaume C, Genet C, Herrmann FR, Benetos A, Hurst SA, Vischer UM. Attitudes and approaches to decision making about antihypertensive treatment in elderly patients. J Am Med Dir Assoc. 2011;12(2):121–128. doi:10.1016/j.jamda.2010.07.004
- Raue PJ, Weinberger MI, Sirey JA, Meyers BS, Bruce ML. Preferences for depression treatment among elderly home health care patients. Psychiatr Serv. 2011;62(5):532–537. doi:10.1176/ps.62.5.pss6205_0532
- Rochon D, Eberth JM, Fraenkel L, Volk RJ, Whitney SN. Elderly patients’ experiences using adaptive conjoint analysis software as a decision aid for osteoarthritis of the knee. Health Expect. 2014;17(6):840–851. doi:10.1111/hex.2014.17.issue-6
- Silverman S, Calderon A, Kaw K, et al. Patient weighting of osteoporosis medication attributes across racial and ethnic groups: a study of osteoporosis medication preferences using conjoint analysis. Osteoporos Int. 2013;24(7):2067–2077. doi:10.1007/s00198-012-2241-1
- Voigt I, Wrede J, Diederichs-Egidi H, Dierks ML, Junius-Walker U. Priority setting in general practice: health priorities of older patients differ from treatment priorities of their physicians. Croat Med J. 2010;51(6):483–492. doi:10.3325/cmj.2010.51.483
- Baxter JM, Fotheringham AJ, Foss AJ. Determining patient preferences in the management of neovascular age-related macular degeneration: a conjoint analysis. Eye (Lond). 2016;30(5):698–704. doi:10.1038/eye.2016.18
- Böttger B, Thate-Waschke IM, Bauersachs R, Kohlmann T, Wilke T. Preferences for anticoagulation therapy in atrial fibrillation: the patients’ view. J Thromb Thrombolysis. 2015;40(4):406–415. doi:10.1007/s11239-015-1263-x
- Danner M, Vennedey V, Hiligsmann M, Fauser S, Gross C, Stock S. How well can analytic hierarchy process be used to elicit individual preferences? Insights from a survey in patients suffering from age-related macular degeneration. Patient. 2016;9(5):481–492. doi:10.1007/s40271-016-0179-7
- Gum AM, Ayalon L, Greenberg JM, Palko B, Ruffo E, Arean PA. Preferences for professional assistance for distress in a diverse sample of older adults. Clin Gerontol. 2010;33(2):136–151. doi:10.1080/07317110903551901
- Gum AM, Iser L, Petkus A. Behavioral health service utilization and preferences of older adults receiving home-based aging services. Am J Geriatr Psychiatry. 2010;18(6):491–501. doi:10.1097/JGP.0b013e3181c29495
- König M, Pfarr C, Zweifel P. Mutual altruism: evidence from Alzheimer patients and their spouse caregivers. Adv Health Econ Health Serv Res. 2014;24:141–160.
- Mueller S, Agostini H, Ehlken C, Bauer-Steinhusen U, Hasanbasic Z, Wilke T. Patient preferences in the treatment of neovascular age-related macular degeneration: a discrete choice experiment. Ophthalmology. 2016;123(4):876–883. doi:10.1016/j.ophtha.2015.12.001
- Uemura H, Matsubara N, Kimura G, et al. Patient preferences for treatment of castration-resistant prostate cancer in Japan: a discrete-choice experiment. BMC Urol. 2016;16(1):63. doi:10.1186/s12894-016-0182-2
- Vennedey V, Danner M, Evers SM, et al. Using qualitative research to facilitate the interpretation of quantitative results from a discrete choice experiment: insights from a survey in elderly ophthalmologic patients. Patient Prefer Adherence. 2016;10:993–1002. doi:10.2147/PPA
- de Vries ST, de Vries FM, Dekker T, et al. The role of patients’ age on their preferences for choosing additional blood pressure-lowering drugs: a discrete choice experiment in patients with diabetes. PLoS One. 2015;10(10):e0139755. doi:10.1371/journal.pone.0139755
- Fraenkel L, Cunningham M, Peters E. Subjective numeracy and preference to stay with the status quo. Med Decis Making. 2015;35(1):6–11. doi:10.1177/0272989X14532531
- Hamelinck VC, Bastiaannet E, Pieterse AH, et al. A prospective comparison of younger and older patients’ preferences for adjuvant chemotherapy and hormonal therapy in early breast cancer. Clin Breast Cancer. 2016;16(5):379–388. doi:10.1016/j.clbc.2016.04.001
- Weernink MGM, Janus SIM, van Til JA, Raisch DW, van Manen JG, Ijzerman MJ. Systematic review to identify the use of preference elicitation methods in healthcare decision making. Pharm Med. 2014;28(4):175–185. doi:10.1007/s40290-014-0059-1
- Helter TM, Boehler CE. Developing attributes for discrete choice experiments in health: a systematic literature review and case study of alcohol misuse interventions. J Subst Use. 2016;21(6):662–668. doi:10.3109/14659891.2015.1118563
- Milte R, Ratcliffe J, Chen G, Lancsar E, Miller M, Crotty M. Cognitive overload? An exploration of the potential impact of cognitive functioning in discrete choice experiments with older people in health care. Value Health. 2014;17(5):655–659. doi:10.1016/j.jval.2014.05.005