Abstract
Purpose
Patients who test positive on the fecal immunochemical test (FIT) for colorectal cancer (CRC) are referred for colonoscopy for further diagnostic evaluation. Colonoscopy is not a perfect method and may be a challenge for some FIT-positive patients. Computed tomographic colonography (CTC) is an alternative method that is less invasive and allows examination of the whole colon. The study objective was to evaluate the preference of FIT-positive patients for either colonoscopy or CTC for CRC examination.
Patients and Methods
Individuals older than 40 years with a positive FIT test at eight Japanese hospitals between December 2012 and July 2015 were invited to participate. Participants were given detailed information regarding colonoscopy and CTC before deciding on either examination. They completed questionnaires before the procedure regarding their preference and after the procedure regarding their experience.
Results
The pre- and post-questionnaires of 846 and 834 participants, respectively, were analyzed. Participants preferred colonoscopy over CTC (colonoscopy, 72%; CTC, 28%). The possibility of obtaining biopsy samples and removing colorectal polyps during the procedure was the main reason for colonoscopy selection. Patients selected CTC to reduce discomfort but reported that CTC bowel preparation was more burdensome than colonoscopy bowel preparation. The overall experience of the examination did not differ between the groups.
Conclusion
Colonoscopy is the standard examination for FIT-positive patients. However, when given a choice, almost one-third of participants chose CTC because they thought it would be a more “comfortable” examination. Clinicians should therefore be aware of patients’ potential preference for noninvasive colorectal examinations.
Introduction
Globally, colorectal cancer (CRC) ranks third in incidence and second in mortality among cancers, with an estimated 1.8 million cases and approximately 881,000 deaths occurring in 2018.Citation1 Screening methods for CRC have been developed to decrease incidence rates and overall mortality rates. Population-based screening programs for CRC, such as the guaiac fecal occult blood test (gFOBT) or fecal immunochemical test (FIT), have since been implemented in many parts of the world, and colonoscopy is widely recommended for diagnostic evaluation in those with a positive FIT result.Citation2,Citation3 In about 5.6% of gFOBT-positive individuals, CRC is subsequently detected by colonoscopy, whereas adenomatous polyps are detected in 25.6% of this patient population.Citation4
However, colonoscopy is not a perfect diagnostic method and may be a challenge for some FIT-positive patients. For example, a colonoscopy may be contraindicated or refused by some patients,Citation5 and results may be incomplete because colonoscopic procedures may not achieve the target 90% cecal intubation rate, and even if they do, 10% of patients will still not undergo an examination of the entire colon.Citation6 Colonoscopy can also result in complications, such as rectal bleeding or perforation of the colon.Citation6 Additionally, in many countries, colonoscopic resources are inadequate for diagnostic workup of patients with a positive FIT result.Citation3,Citation7
Computed tomographic colonography (CTC) is safe, well-tolerated, and accurate for evaluation of cancer and advanced adenoma.Citation8,Citation9 In the United States, for patients at average or moderate risk for colorectal cancer, CTC is usually appropriate for colorectal cancer detection following a FIT-positive result.Citation10 However, in Japan and Europe, CTC is currently not recommended as a triage examination prior to colonoscopy in patients with a FIT-positive result.Citation11,Citation12 CTC is only performed as an alternative examination for those patients who cannot undergo colonoscopy or for those in which the colonoscopy procedure did not result in examination of the entire colon.Citation11,Citation12
Several studies have reported on patient preference between colonoscopy and CTC by conducting questionnaires after the examinations. Many of these studies revealed CTC to be chosen by patients as the preferred method for potential future examination.Citation9,Citation13,Citation14 However, these results do not reflect patients’ pre-examination preferences because they were mainly based on the experience of the procedures obtained post-examination. To determine whether CTC is an acceptable test for FIT-positive patients, it is necessary to investigate why the patient being tested chose CTC instead of colonoscopy.
The purpose of this study was to evaluate the preference for either colonoscopy or CTC as the first examination for CRC in patients with FIT-positive results who do not have a contraindication and who have not previously refused either of these two types of diagnostic exams. In addition, we evaluated the patients’ experience of the examination after it was performed.
Methods
Participants
A total of eight hospitals in Japan participated in this survey-based study, and institutional review board approval for the study was obtained from the ethical committees of Hokkaido Gastroenterology Hospital and all participating institutes. This study was registered with the UMIN Clinical Trials Registry (UMIN000009456; www.umin.ac.jp/ctr/index.htm; registered on 2012/12/04). Between December 2012 and July 2015, all individuals older than 40 years with a positive FIT result were invited to participate, and all voluntarily visited the participating sites for colorectal examination. The trial was performed in accordance with the Declaration of Helsinki. Participants were excluded if they presented any of the following conditions: serious medical conditions associated with an increased risk of complications from bowel preparation and colonoscopy or CTC; history of inflammatory bowel disease, Lynch syndrome, familial polyposis, or colorectal surgery; pregnancy; or severe psychiatric symptoms that would affect their ability to understand and complete a questionnaire. Participants were registered after providing written informed consent for prospective enrollment in the study.
Participants were given detailed information about colonoscopy and CTC in the form of a written document (Table S1 in Supplementary Material) that described all essential clinical information in plain language, including information on bowel preparation; sensitivity, acceptability, and length of procedure; sedation; cost of the examination; and information about the advantages and disadvantages of both examinations. In addition, all information was arranged side by side in a table to make it easy to understand the difference between the two examinations. After providing an explanation of both examinations, any remaining questions were answered by a physician. Subsequently, each participant was allowed to choose either examination procedure. If CTC was selected, a full-laxative bowel preparation methodCitation8 or a reduced-laxative methodCitation9 could be chosen by the participant based on the same written document (Table S1 in Supplementary Material).
Questionnaires
After the participants selected one of the two colonic examinations, they completed the first questionnaire before the colorectal examination was conducted to determine the reason for their choice of colorectal examination type (Table S2 in Supplementary Material). The questionnaire included nine response options describing possible reasons for their choice of examination type. The items and response options for choosing between colonoscopy and CTC are described in Table S2 of Supplementary Material.
To assess participants’ experiences during the preparations and procedures, a second three-item questionnaire developed by the authors was completed by participants on the day of the examination (Table S3 in Supplementary Material). Participants who received sedation during colonoscopy were given questionnaires after they recovered from sedation. The first two items asked participants to rate their discomfort related to bowel preparation and to the examination itself using a five-point Likert scale (where 1 indicated very easy; 2, somewhat easy; 3, neutral; 4, somewhat difficult; and 5, very difficult). The third item asked which part of the examination generated the most discomfort. Participants were allowed to select one or more of the seven responses.
Colonoscopy Procedure
Colonoscopy was performed at each participating site. For bowel preparation, participants received 2 L of polyethylene glycol (PEG) solution, 1.8 L of magnesium citrate solution, or 1 L of polyethylene glycol electrolyte solution with ascorbic acid (MoviPrep®). The use of antispasmodics and sedatives during colonoscopy was based on the judgment and responsibility of the physician in charge of the examination. The type of gas used for colonic insufflation during colonoscopy was not specified in this study. Polyps discovered during colonoscopy could be removed endoscopically, depending on the judgment of the endoscopist.
CTC Procedure
For bowel preparation before examination by CTC, participants received either a full laxative preparation methodCitation8 or a reduced-laxative preparation method.Citation9 Details of these methods are described in previous studies.Citation8,Citation9 In summary, the full laxative method consisted of 1,620 mL of PEG over the course of 2 h, followed by 400 mL solution consisting of 380 mL of PEG plus 20 mL of sodium diatrizoate for tagging of residual fluid. The reduced-laxative method consisted of 760 mL PEG and 40 mL sodium diatrizoate for tagging, which was administered one day before CTC. Colonic distention was obtained with an automatic CO2 insufflator using a rectal catheter. CTC examinations were performed with either 64- or 16-channel multi-detector row CT scanners, used single-breath-hold supine and prone positioning, and did not use intravenous contrast medium or sedation.
Statistical Analysis
For the a priori power calculation, we assumed that the difference between the selection ratio of colonoscopy and CTC would be at least 10% based on the results of previous studies,Citation13 and we assumed that the dropout or withdrawal rate would be 5%. Based on these assumptions, we determined that 863 participants would be necessary for sufficient statistical power (80%), with an alpha of 0.05 (two-tailed).Citation15
We used the binomial exact test to evaluate whether participants preferred CTC or colonoscopy and full laxative or reduced-laxative preparation for CTC. In addition, non-normally distributed data between CTC and colonoscopy were analyzed using the Wilcoxon rank-sum test or the Wilcoxon rank-sum test with continuity correction. The chi-squared tests or the chi-squared test with Yates continuity correction were used for categorical data. All confidence intervals were within 95%. All P values involved a hypothesis test against a two-sided alternative, and P< 0.05 was considered to indicate statistical significance. These analyses were performed using R (The R Foundation for Statistical Computing Platform).
Results
A total of 881 participants were included in this study. Nineteen participants withdrew their informed consent, and 15 participants were excluded based on the exclusion criteria. Data from the questionnaires of the remaining 847 participants (502 men, 345 women; mean age, 60.5 years) were analyzed ().
Table 1 Participant Characteristics
Participant’s Examination Preference
Colonoscopy was preferred over CTC; however, almost 1/3 of the participants chose CTC over colonoscopy (n= 607 participants [72%] vs n= 240 participants [28%], p<0.001) ().
Between participants who chose colonoscopy (colonoscopy group) and those who chose CTC (CTC group), there were no significant differences in age, sex, the use of antithrombotic medicine, or the percentage of participants who had previous experience with colonic examinations (). In the CTC group, reduced-laxative preparation was selected by 157 participants (65%) and the full-laxative preparation by 83 participants (35%) (). Reduced-laxative preparation was selected significantly more often by female participants than by male participants (p= 0.0158) ().
Table 2 Characteristics of Participants That Chose CTC
The Reasons for Choosing a Specific Type of Examination
The first questionnaire was completed by 846 of the 847 participants ( and ). The reason for choosing colonic examination was shown by the response option “the possibility of obtaining biopsy samples and removing colorectal polyps if detected during procedure” being selected most often (86% (522/606)) in the colonoscopy group (). The response option “CTC is comfortable” was most often selected by the CTC group (76% [63/83]) and 80% (125/157) (). In the reduced-laxative preparation CTC group, the response option “reduced bowel preparation method” was selected by 59% (93/157) of participants (), while this response was not relevant for the standard laxative preparation group.
Table 3 Reasons Participants Chose Colonoscopy
Table 4 Reasons Participants Chose CTC
The Burden of the Preparation and Examination Procedures
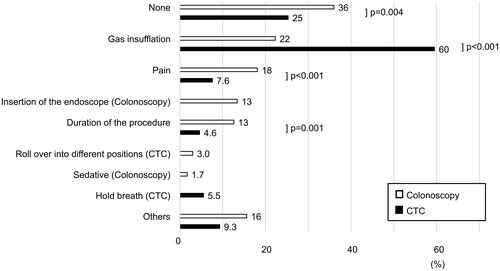
The second questionnaire was completed by 834 of the 847 participants (). In preparation experience, bowel preparation for CTC was found to be significantly more burdensome than that for colonoscopy, with 10% (24/237) in the CTC group and 5.7% (34/597) in the colonoscopy group, reporting that it was very difficult to drink the laxative and 15% (36/237) in the CTC group and 23% (138/597) in the colonoscopy group, which reported that it was easy (p=0.003) (). The overall experience of the examination itself did not differ between the groups, with little difference in the responses on the Likert scale (p=0.582) (). Regarding part of the examination that caused most discomfort, “pain” and “duration of the procedure” were experienced significantly more often in the colonoscopy group (18% (108/597) and 13% (75/597), respectively) than in the CTC group (7.6% (18/237) and 4.6% (11/237), respectively) (p<0.001and p=0.001, respectively) (). The response option “injection of the gas” was the most common cause of discomfort in the CTC group (60% [141/237]), and this was experienced more often than in the colonoscopy group (22% [133/597]) (p<0.001) ().
Table 5 Results on the Experiences of Colonic Examinations
Figure 1 Experience of preparation and examination for colonoscopy and CTC. Graph of the responses on the survey regarding patients’ experiences on preparation (A) and examination (B) for colonoscopy and CTC, Based on surveys of 834 people. The Wilcoxon rank-sum test with continuity correction was used to calculate the p values.
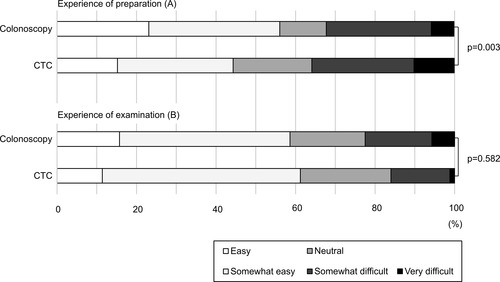
Figure 2 Discomfort during the colonic examination. Response to the survey regarding discomfort during colonic examination based on surveys of 834 people. They were allowed to select one or more answers to each question; hence, the total percentage was more than 100. The chi-squared test with Yates´ continuity correction was used to calculate the p values.
Discussion
We investigated the factors that FIT-positive patients consider when choosing a colorectal examination by investigating the exam preference of FIT-positive patients before the colon examination. A systematic review of studies on stated preference for cancer screening tests showed that attributes, such as efficacy, process, and cost, are significant determinants of choice.Citation16 The process includes information provided to patients. However, FIT-positive patients do not provide sufficient information on examination modalities other than colonoscopy in clinical settings. Therefore, there was a dearth of studies that considered patients’ decision-making process of selecting the preferred choice of procedure during the assessment. Colonoscopy has the advantage of detecting, diagnosing, and treating lesions in a single examination. In contrast, CTC only detects lesions, and if lesions are found, colonoscopy is required for diagnostic evaluation or treatment. Therefore, colonoscopy is considered to have an advantage over CTC for FIT-positive patients who are more likely to have lesions in the large intestine. In our study, the most common reason for selection given by patients who chose colonoscopy was “obtaining biopsy samples and removing colorectal polyps,” and the second most common reason was “higher diagnostic accuracy.” The diagnostic accuracy of CTC is considered equivalent to that of colonoscopy for detecting polyps and cancer with a diameter of ≥10 mm,Citation8,Citation9 but colonoscopy is superior to CTC in diagnostic accuracy for flat lesions.Citation8 According to a discrete choice study investigating patients’ preference for colonoscopy or CTC in 130 patients who underwent both examinations because of suspected CRC, the patients preferred colonoscopy to CTC because of the need for a second procedure after CTC, the higher likelihood of missing cancers or polyps with CTC, and higher costs of a CTC test.Citation17 Another study reported that the lay public (n=100), who were presented with information about three types of colorectal examinations, chose colonoscopy, CTC, and colon capsule endoscopy in 71%, 22%, and 7% of cases, respectively, if the investigation of a positive fecal occult blood test (FOBT) was needed.Citation18 The results of these studies are consistent with our results, suggesting that FIT-positive individuals are more likely to opt for colonoscopy after receiving sufficient explanation about both tests. However, the proportion of choices for each examination was not adequately investigated for FIT-positive participants actively scheduled for examination. Our results from a large-scale study showed that 72% of well-informed participants chose colonoscopy. Interestingly, a considerable proportion of patients (28%) chose CTC after fully understanding the advantages of colonoscopy.
The reason that many participants chose CTC was “I think CTC is comfortable.” In a multicenter study on patients’ experience, it was found that participants assigned a high satisfaction level with CTC, and those with experience of both modalities preferred CTC over optical colonoscopy.Citation19 In another study, it was reported that patient acceptance of CTC was significantly higher than that of colonoscopy, and 62% of patients would choose CT colonography as the first examination if they have a positive FIT result in the future.Citation9 Approximately 40% of those who chose CTC had previously undergone colonoscopy, but few had undergone CTC. Thus, it seems more appropriate to consider that participants avoided colonoscopy rather than actively chose CTC. Several reports indicated that patients consider colonoscopy distressing or burdensome.Citation18,Citation20 It was found that the most decisive reason not to participate in screening by colonoscopy was the unpleasantness of the examination.Citation21 In addition, many patients consider the burden of the preparation to be greater than that of the colorectal examination itself.Citation22,Citation23 In comparative studies of full-laxative colonoscopy and reduced or no laxative CTC, CTC was chosen as the preferred test by the majority of participants against colonoscopy.Citation9,Citation24 Our results also showed that 65% of the participants who underwent CTC chose reduced-laxative preparation. The participants who chose full-laxative preparation appeared to value the higher diagnostic accuracy of the procedure with full-laxative preparation compared to reduced-laxative preparation. Our study highlights the fact that although colonoscopy is often considered as a preferred method, there might be finer aspects to patients’ preference. To reinforce the patient’s decision-making process to a more informed one, efforts should be made to include discussions on the benefits and uncertainties associated with all the options. Our experimental approach for providing detailed information sheet addressed this concern.
Currently, the criteria for active recommendation of CTC in place of colonoscopy as the first examination are only the patient’s refusal of colonoscopy or the presence of a contraindication for colonoscopy.Citation11 A report of a national screening program in the UK revealed that in 52,202 patients with a positive FOBT result, colonoscopy was performed in 50,975 patients (97.6%), whereas CTC was performed in only 1970 patients (3.7%).Citation25 If FIT-positive patients can freely choose the examination method as they did in our study, CTC may be performed as a detailed examination more often, which may reduce the number of colonoscopies.
In evaluating the post-examination experience, the proportion of participants who responded that bowel preparation was burdensome in the CTC group was significantly higher than in the colonoscopy group, although 65% of the participants underwent CTC with reduced-laxative preparation. No significant difference was observed between the colonoscopy and CTC groups in the overall experience of the procedure itself. Many reports based on surveys conducted in patients who underwent both types of examinations showed a higher preference for CTC.Citation13,Citation14 However, these results are not consistent with a questionnaire conducted among patients who underwent only one of the examinations. Based on the questionnaire by Plumb et al,Citation25 many patients unexpectedly found CTC to present greater discomfort than colonoscopy. The patients chose CTC because they expected it to be easier and more comfortable. Thus, the patients might feel more burdened than expected in the actual examination. According to our results, almost 60% of the participants who underwent CTC responded that the gas injection generated the most discomfort. Before the procedure, patients should be informed that gas injection can be burdensome.
Our study has several limitations. The study participants were all visiting a medical institution for diagnostic examination after receiving a positive FIT result, while those with a positive FIT result that did not visit a medical institution for examination were not included. Fear of colonoscopy is reported as one of the main reasons for lack of follow-up colonoscopy among persons with a positive FOBT result.Citation26 Another study is needed to determine the selectivity and acceptability of CTC in patients with positive FOBT results that did not visit a medical institution for follow-up examination. In addition, our study did not include a comprehensive account of factors that can influence patients’ perspectives of treatment procedures.
Since the diagnostic performance of CTC for superficial lesions differs among reports,Citation8,Citation9,Citation27,Citation28 the actual diagnostic performance was not described in the document that patients were given before making their decision. However, it was noted that CTC has a reduced capacity for diagnosing superficial lesions. In addition, the actual percentage of patients who require colonoscopy after undergoing CTC was not presented in the document because the indications for endoscopic treatment for colorectal polyps differ among institutions. The possibility that the lack of information about these factors affected the rate of selection of the type of examination cannot be ruled out. The patient’s knowledge of the actual percentage might have affected their decision. During the preparation of the information documents about both procedures, a statement that CTC is less uncomfortable was included. CTC was less uncomfortable than colonoscopy in a Japanese multicenter study.Citation9 However, the opposite results have also been published,Citation29 and it must therefore be noted that these opposing results were reported from another country. Furthermore, it is not possible to rule out the possibility that inclusion of this statement will affect selection rates. Cost has been reported as a barrier to colonoscopy.Citation30 We found no major differences between the costs of CTC and colonoscopy in our country. However, conditions are different overseas, and there may therefore be differences in the selection rates due to cost.
Conclusions
Currently, the majority of those with positive FIT results undergo detailed examination via colonoscopy. However, a fraction of individuals with positive FIT results will attempt to avoid colonoscopy at all costs. We found that when FIT-positive individuals were given a choice, almost one-third of the participants chose CTC, as they thought it would be a more “comfortable” examination. Clinicians should therefore be aware of patients’ potential preference for noninvasive colorectal examinations. In addition, CTC can serve a supplementary role for the ever-increasing demand for colonoscopy, and this may lead to more efficient use of CTC.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Editorial support, in the form of medical writing, assembling tables and creating high-resolution images based on authors’ detailed directions, collating author comments, copyediting, fact checking, and referencing, was provided by Editage, Cactus Communications.
Disclosure
Junta Yamamichi reports personal fees from Canon Inc. and Mitsubishi Chemical Corp., outside the submitted work. The authors report no other potential conflicts of interest in this work.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
- Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi:10.1136/gutjnl-2014-309086
- Blom J, Kilpelainen S, Hultcrantz R, Tornberg S. Five-year experience of organized colorectal cancer screening in a Swedish population - increased compliance with age, female gender, and subsequent screening round. J Med Screen. 2014;21(3):144–150. doi:10.1177/0969141314545555
- Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med. 2011;171(3):249–256. doi:10.1001/archinternmed.2010.372
- Bowles CJ, Leicester R, Romaya C, Swarbrick E, Williams CB, Epstein O. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53(2):277–283. doi:10.1136/gut.2003.016436
- Macfarlane M, Leicester L, Romaya R, Epstein E. Colonoscopy services in the United Kingdom. Endoscopy. 1999;31(6):409–411. doi:10.1055/s-1999-8040
- Nagata K, Endo S, Honda T, et al. Accuracy of CT colonography for detection of polypoid and nonpolypoid neoplasia by gastroenterologists and radiologists: a nationwide multicenter study in Japan. Am J Gastroenterol. 2017;112(1):163–171. doi:10.1038/ajg.2016.478
- Utano K, Nagata K, Honda T, et al. Diagnostic performance and patient acceptance of reduced laxative CT colonography for the detection of polypoid and non-polypoid neoplasms: a multicenter prospective trial. Radiology. 2017;282(2):399–407. doi:10.1148/radiol.2016160320
- Moreno C, Kim DH, et al. ACR appropriateness criteria((r)) colorectal cancer screening. J Am Coll Radiol. 2018;15(5S):S56–S68. doi:10.1016/j.jacr.2018.03.014
- Sali L, Grazzini G, Mascalchi M. CT colonography: role in FOBT-based screening programs for colorectal cancer. Clin J Gastroenterol. 2017;10(4):312–319. doi:10.1007/s12328-017-0744-1
- Saito H, Kanaoka S, Shimada T, et al. The placement of large intestinal CT exam as a method of detailed checkup, as well as its necessary conditions and issues. J Gastroenterol Cancer Screen. 2016;54(3):425–441.
- Lin OS, Kozarek RA, Gluck M, et al. Preference for colonoscopy versus computerized tomographic colonography: a systematic review and meta-analysis of observational studies. J Gen Intern Med. 2012;27(10):1349–1360. doi:10.1007/s11606-012-2115-4
- Gareen IF, Siewert B, Vanness DJ, Herman B, Johnson CD, Gatsonis C. Patient willingness for repeat screening and preference for CT colonography and optical colonoscopy in ACRIN 6664: the National CT Colonography trial. Patient Prefer Adherence. 2015;9:1043–1051. doi:10.2147/PPA.S81901
- Chow S-C, Shao J, Wang H. Sample Size Calculations in Clinical Research. 2nd ed. 2008.
- Mansfield C, Tangka FK, Ekwueme DU, et al. Stated preference for cancer screening: a systematic review of the literature, 1990–2013. Prev Chronic Dis. 2016;13:E27. doi:10.5888/pcd13.150433
- Howard K, Salkeld G, Pignone M, et al. Preferences for CT colonography and colonoscopy as diagnostic tests for colorectal cancer: a discrete choice experiment. Value Health. 2011;14(8):1146–1152. doi:10.1016/j.jval.2011.07.012
- Ojidu H, Palmer H, Lewandowski J, et al. Patient tolerance and acceptance of different colonic imaging modalities: an observational cohort study. Eur J Gastroenterol Hepatol. 2018;30(5):520–525. doi:10.1097/MEG.0000000000001090
- Pooler BD, Baumel MJ, Cash BD, et al. Screening CT colonography: multicenter survey of patient experience, preference, and potential impact on adherence. AJR Am J Roentgenol. 2012;198(6):1361–1366. doi:10.2214/AJR.11.7671
- Denters MJ, Deutekom M, Bossuyt PM, Fockens P, Dekker E. Patient burden of colonoscopy after positive fecal immunochemical testing for colorectal cancer screening. Endoscopy. 2013;45(5):342–349. doi:10.1055/s-0032-1326238
- de Wijkerslooth TR, de Haan MC, Stoop EM, et al. Reasons for participation and nonparticipation in colorectal cancer screening: a randomized trial of colonoscopy and CT colonography. Am J Gastroenterol. 2012;107(12):1777–1783. doi:10.1038/ajg.2012.140
- van Gelder RE, Birnie E, Florie J, et al. CT Colonography and colonoscopy assessment of patient preference in a 5-week follow-up study. Radiology. 2004;233(2):328–337. doi:10.1148/radiol.2331031208
- Ristvedt SL, McFarland EG, Weinstock LB, Thyssen EP. Patient preferences for CT colonography, conventional colonoscopy, and bowel preparation. Am J Gastroenterol. 2003;98(3):578–585. doi:10.1111/j.1572-0241.2003.07302.x
- Zalis ME, Blake MA, Cai W, et al. Diagnostic accuracy of laxative-free computed tomographic colonography for detection of adenomatous polyps in asymptomatic adults: a prospective evaluation. Ann Intern Med. 2012;156(10):692–702. doi:10.7326/0003-4819-156-10-201205150-00005
- Plumb AA, Ghanouni A, Rees CJ, et al. Patient experience of CT colonography and colonoscopy after fecal occult blood test in a national screening programme. Eur Radiol. 2017;27(3):1052–1063. doi:10.1007/s00330-016-4428-x
- Llovet D, Serenity M, Conn LG, et al. Reasons for lack of follow-up colonoscopy among persons with a positive fecal occult blood test result: a qualitative study. Am J Gastroenterol. 2018;113(12):1872–1880. doi:10.1038/s41395-018-0381-4
- Suzuki N, Ignjatovic A, Burling D, Taylor SA. CT colonography and non-polypoid colorectal neoplasms. Gastrointest Endosc Clin N Am. 2010;20(3):565–572. doi:10.1016/j.giec.2010.03.011
- Utano K, Katsuki S, Matsuda T, et al. Colon capsule endoscopy versus CT colonography in patients with large non-polypoid tumours: a multicentre prospective comparative study (4CN Study). Digestion. 2019;1–9.
- Akerkar GA, Yee J, Hung R, McQuaid K. Patient experience and preferences toward colon cancer screening: a comparison of virtual colonoscopy and conventional colonoscopy. Gastrointest Endosc. 2001;54(3):310–315. doi:10.1067/mge.2001.117595
- Decruz GM, Ng CH, Lim KT, et al. Afterthoughts on colonoscopy was it that bad? . J Med Screen. 2020;969141320923381.
