Abstract
Purpose
Multiple sclerosis (MS) prognosis is often uncertain. This literature review considers patients’ understanding of, and perspectives on, MS progression to better comprehend the unmet needs of people with MS (PwMS), in order to improve treatment adherence and quality of life (QoL).
Methods
Literature searches for peer-reviewed papers concerning patient perspectives on the progression of MS and comparable conditions, published between January 2000 and January 2020, were conducted.
Results
Little qualitative evidence exists that examines PwMS’ perspectives on MS progression. The understanding and meaning ascribed to terms such as “disease progression” vary. Some PwMS find disease labels stigmatizing, confusing, and disconnected from reality. The lack of a clear definition of progression and discrepancies between PwMS and healthcare professional (HCP) perspectives may contribute to misunderstanding and poor communication. Patient descriptions of progression and relapses include symptoms in addition to those evaluated by standard severity and disability measures. Compared with HCPs, PwMS are still focused on relapse prevention but place higher priority on QoL and ascribe different relative importance to the causes of poor adherence to treatment plans. PwMS want to discuss progression and likely prognosis. Such communication needs to be personalized and delivered with sensitivity, at an appropriate time. Poor treatment adherence may arise from a lack of understanding and poor communication, particularly around treatment goals. The few studies that directly considered patient perspectives on the progression of comparable conditions supported and extended the perspectives of PwMS. Lack of adequate communication by HCPs was the most common theme.
Conclusion
Patient perspectives on disease progression in MS and other chronic progressive conditions are under-investigated and under-reported. The limited evidence available highlights the importance of providing adequate information and effective HCP communication. While further studies are needed, the current evidence base offers information and insights that may help HCPs to enhance patient care, well-being, and treatment adherence.
Plain Language Summary
Disease progression occurs in most people with multiple sclerosis (PwMS). However, patients’ views of what MS progression means are largely unknown. This study aimed to explore patients’ understanding of, and perspectives on, MS progression through the review of journal articles published between January 2000 to January 2020.
The study found that:
Understanding of the term “disease progression” varied among PwMS, their caregivers and healthcare professionals (HCPs). PwMS’ descriptions of progression included symptoms that are not evaluated by standard measures of MS, such as mobility scales.
Emotional responses to disease progression conversations varied. Some patients were not concerned and wanted to talk about how their disease may change, while others were more distressed by the topic.
Poor communication with HCPs about MS progression was common. While many PwMS reported a desire to discuss long-term prognosis, there was little opportunity for this. Conversations on MS progression need to occur at an appropriate time, be delivered with sensitivity and be personalized to the patient.
Understanding of MS progression may help patients stick to their treatment schedule. However, the treatment goals of patients and HCPs can differ. Compared to HCPs, PwMS are more focused on improving symptoms that impact their quality of life and are less focused on preventing relapse.
Overall, this review found that few studies have examined views on MS progression in PwMS. Based on the studies reported, this review highlights the importance of effective communication between HCPs and PwMS, leading to improved MS management and treatment results.
Introduction
Disease progression in multiple sclerosis (MS) occurs in most people with MS (PwMS).Citation1–Citation3 Disease progression in MS can be highly variable, the prognosis is often uncertain and there is no universal consensus regarding terminology or management.Citation4,Citation5 Bodily systems in which MS progression can be detected continue to be elucidated.Citation6 Lublin et al suggest using “worsening” rather than “progressing” for patients with relapsing MS, reserving “progression” for those with clinically defined progressive diseaseCitation2 ‒ a recommendation adopted here.
PwMS, therefore, have to contend with an uncertain future as their MS worsens or progresses, alongside, in many cases, an accumulating symptomatic burden and increasing levels of disability. In addition, drug treatments, their associated side effects, and the impact of regular monitoring add to the disease burden. Not surprisingly, exacerbations and progressive MS can disrupt many aspects of a PwMS’s daily life, including employment, daily activities, relationships, establishing a family, and their sense of self.Citation7,Citation8 Continuing stigmatization of MS may exacerbate the difficultiesCitation9,Citation10 and may worsen the disease, which can result in a vicious cycle.Citation11,Citation12
Collaborations between PwMS and healthcare professionals (HCPs) show that enhanced patient understanding of MS disease progression and engagement in care can contribute to improved disease management and quality of life (QoL).Citation13,Citation14 However, there is little qualitative evidence regarding patient perspectives on MS disease progression.Citation13
Methods
Literature searches for peer-reviewed journal articles concerning patient perspectives on MS disease progression, published between January 2000 and January 2020, were conducted in PubMed using eight keyword search strings (). Bibliographies of included papers were further searched for relevant articles. Exclusion criteria were determined and results falling under the following categories removed: proceedings, conference abstracts, papers not published in English, and papers concerned with drug efficacy.
Table 1 MeSH Term Search Strings and Results from PubMed
The methodology selected has certain limitations. The date range restriction was chosen based on the focus and interests of the MS in the 21st Century initiative as a whole, namely the changing landscape of MS care post-2000. However, the exclusion of earlier publications must be acknowledged as a potential limitation. Similarly, restricting results to articles published in English was a decision made to ensure the accuracy of the authors' interpretations, but likely excluded publications relevant to the overall focus. Finally, the majority of excluded publications were removed because of a focus on drug efficacy. The decision to avoid clinical trial data and other treatment investigations was taken to restrict results to publications whose primary focus was understanding the perspectives of PwMS and not evaluating medical interventions. However, it is probable that some of the excluded studies would have used endpoints that may be relevant to the topic.
A parallel narrative, rather than a systematic literature review, concerning patient perspectives on disease progression in other chronic or significantly life-altering conditions was conducted using the same search strings with “chronic condition” replacing “multiple sclerosis”. Papers covering the same themes identified in the MS results were selected with a view to contrasting, supporting or expanding the findings of the MS review.
Results
The initial searches using PubMed MeSH terms identified 757 results across the eight search terms which consisted of 683 publications (). Following the application of exclusion criteria based on abstract content, 7 publications were identified for full text review and inclusion. Searches were then expanded by removing MeSH term restrictions and resulting in 2332 results across the eight searches which consisted of 2077 publications (). Following the application of exclusion criteria to these expanded results and additional 7 publications for review and inclusion. Exploration of the bibliographies of these 14 publications identified a further 13 papers of relevance to the topic and meeting the criteria for inclusion. shows the PRISMA flow diagram of the combined results.
Table 2 Expanded Search Strings and Results from PubMed
Figure 1 PRISMA flow diagram of combined results across both stages of methodology taking into account duplicates in each individual stage and between stages.
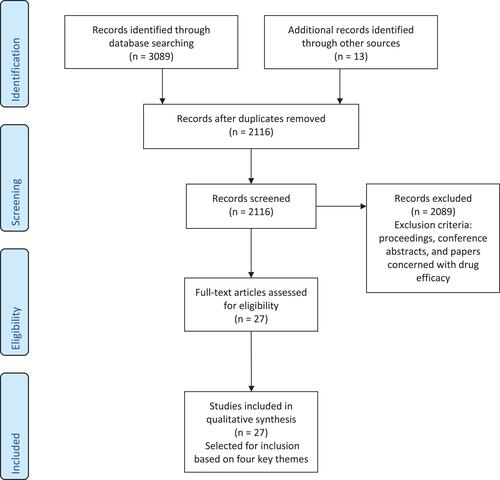
Full text review of the 27 papers selected for inclusion led to the identification of four key themes which the results of the included papers were categorized into:
Knowledge and understanding of MS disease progression
Emotional responses and attitudes to MS disease progression
Communication with healthcare professionals regarding MS disease progression
Disease progression and treatment adherence
Following additional searching of papers from non-MS chronic and progressive conditions, 21 papers were selected for full text review. Discussion of the results of these studies is included in a later section of this paper that looks at how the patient perspectives, of disease progression, in these other conditions compare with those of PwMS.
Knowledge and Understanding of MS Disease Progression
The understanding of terms such as “disease progression” varies considerably among PwMS and their caregivers.Citation15,Citation16 Some patients find disease labels stigmatizing, confusing, and divorced from the reality of their lives with MS.Citation15 Cognitive symptoms may interfere with their ability to understand information.Citation17 Moreover, there may be wide variation in the meanings that PwMS ascribe to disease progression.Citation16
A lack of clarity on the definition of MS progression among neurologists may contribute to the variation in the understanding of disease progression terminology among PwMS. There is no universally accepted definition and no clear relationship between different clinical metrics, such as magnetic resonance imaging (MRI) results and Expanded Disability Status Scale (EDSS) disability-based metrics and physical findings.Citation18,Citation19 Discrepancies in what disease progression means for PwMS and HCPs may further hinder shared understanding. Patient descriptions of progression and MS relapses are practical and include symptoms such as fatigue, pain, and cognitive impairment, in addition to those evaluated by standard severity and disability measures of MS.Citation20
There is also confusion among some PwMS about MS classification. Findings from an online survey of 215 adults with secondary progressive MS (SPMS) of ≥5 years’ duration in Italy and Germany showed that 57% were aware of their diagnosis in Italy compared with 77% in Germany. Moreover, 48% and 56%, respectively, reported not receiving information on SPMS, indicating a need to improve patient-physician communication.Citation21 Likewise, a French study of patient perceptions of MS and its treatment reported that more than half of PwMS (57.4%; n=202) did not consider themselves well informed about their disease.Citation22 Similarly, in a small-scale UK study designed to explore PwMS (n=20) and caregiver (n=13) experiences during patients’ transition to SPMS, some were surprised to discover that the disease had progressed to SPMS and did not understand how the diagnosis had been reached, which increased their confusion. On the other hand, other patients had accepted their disease progression and found the label of little relevance.Citation16
Emotional Responses and Attitudes to MS Disease Progression
Emotional responses of PwMS to a diagnosis of SPMS also varied considerably. Some PwMS were unperturbed by the label, while others had strong emotional reactions, which, in some cases, required additional psychological support. Some PwMS may be distressed that an SPMS diagnosis limits the disease-modifying therapy (DMT) options. Some view the news that further follow-ups with the MS multidisciplinary team will be reduced with a “sense of abandonment”.Citation16 Disbelief, hopelessness and a sense of loss are common responses.Citation16,Citation23,Citation24 Such negative reactions can be severe and often persist long term.Citation25 Patients’ levels of hopefulness are extremely individual and variable, though an attitude of hope is important in order for PwMS to accept and live with MS.Citation22
Indeed, suicide rates in PwMS are approximately double those in the general population.Citation26 In an analysis of 28 interviews involving 14 patients with primary progressive MS (PPMS) or SPMS, Frost et al found that disease progression may be associated with thoughts of suicide, suggesting a need for improved dialog between patients and professionals to ensure that PwMS are adequately supported throughout the disease course.Citation8 In common with the above studies, Frost et al also reported that PwMS experience loss of self-identity. Some patients also experienced reductions in their ability to manage, resilience, and hope.Citation8
A patient’s cultural background may influence their responses to MS progression. One study found that UK patients of Black Caribbean descent were more likely to experience extremes of frustration, loss, and confusion due to more rapid disease progression than their white counterparts.Citation24
Successful coping by PwMS, in general, requires accepting the diagnosis and progression of MS, awareness that its effects can often be managed and that this management must be integrated into daily life.Citation27 An attitude of hope seems to help PwMS overcome the challenges of MS and accept the illness and its prognosisCitation23 taking into account that levels of hopefulness can be extremely individual and variable. In a study involving 54 patients with RRMS, Król et al show that focusing on a positive future, rather than dwelling on negative past events, can enable disease acceptance and conclude that psychological support may benefit PwMS in the early stages of disease progression.Citation28
Communication with HCPs Regarding MS Disease Progression
Poor communication regarding MS disease progression was a consistent finding, which may partly arise from differences in the perspectives of PwMS and HCPs. A survey of 3175 UK MS Register members found that most PwMS wanted to know their long-term prognosis. However, there was a lack of opportunity for such discussions. As a result, this need frequently remained unmet.Citation29 A survey by the German MS Society of 573 PwMS found that more than three-quarters felt it was important to discuss disease progression with their doctor.Citation29 The study also found that doctors who PwMS perceived as communicating poorly were considered less empathetic.Citation30 An interview-based study of 25 PwMS in Iran also found that patients want to discuss disease progression with HCPs and most participants looked at this as a chance to receive counselling from their doctor or nurse. However, most were dissatisfied with their HCPs, especially their doctors, because they lacked empathy, which undermined trust.Citation7 In a series of 15 in-depth semi-structured interviews, PwMS in the UK reported having minimal communication with HCPs about their prognosis and complained of a lack of opportunity for such discussions.Citation31 Some communication issues may be due to the work conditions of HCPs. In particular, an Italian interview-based study of 105 HCPs and nurses showed that compassion fatigue and burnout were common due to intensive working practices, under-resourcing of medical personnel, and anxiety caused by employment conditions.Citation32
The importance of sensitive communication with patients transitioning from RRMS to SPMS was highlighted in a qualitative study involving PwMS (n=9) and specialist MS HCPs (n=7) in the UK.Citation33 The transition to SPMS came as a shock to some PwMS. Several PwMS suggested that sufficient information provision and support were lacking at this stage compared with their initial diagnosis, and the investigators inferred careful consideration of the timing of such information is paramount, given the potential distress and the fact that not all PwMS progress to SPMS.Citation33 Many reported feeling abandoned by their MS-specialist HCPs.Citation33 A lack of understanding of disease progression can make PwMS reluctant to discuss this with their HCP. This highlights the importance of developing and using patient-friendly language to describe MS progression.Citation15
The timing of information delivery is an important consideration. Davies et al describe how some PwMS with worsening MS gradually become aware of their increasing disability. Some PwMS report feeling frustrated that their neurologist does not start conversations about disease progression.Citation16 In addition, Methley et al concluded that continuity of care and patient-centered care (ie, HCPs working collaboratively with patients to support them in developing the confidence, knowledge, and skills to effectively manage their health and healthcare and make informed decisionsCitation34) are pivotal to positive healthcare experiences for both PwMS and professionals.Citation35
Nevertheless, determining elements of optimal communication between HCPs and PwMS about disease progression is challenging and no consensus exists.Citation19,Citation29,Citation32,Citation36 The studies reviewed in this paper suggest that communication on disease progression with PwMS need to be delivered with sensitivity, at an appropriate time, and personalized to each patient’s disease, treatments, experiences, and understanding.
Disease Progression and Treatment Adherence
The review suggested that PwMS’ understanding of disease progression and its treatment, along with effective communication, improves treatment adherence. Conversely, poor adherence may arise from a lack of understanding and poor communications, particularly around treatment goals. In a French cross-sectional observational study 89% of PwMS (n=202) totally or partly agreed that treatment would prevent relapses, and 85% totally or partly agreed that treatment would help to delay disease progression. Adherence was significantly higher in well-informed patients.Citation22 However, patients often feel that neurologists do not discuss treatment goals, which play an important role in patients’ willingness to adhere to treatment. In a questionnaire-based study of 107 PwMS and 18 neurologists in the Netherlands, 69% of PwMS indicated that they had discussed the treatment goal of a “reduction in disease progression” with their neurologist, whereas 94% of the neurologists reported the same discussion having taken place. More than a quarter of the PwMS (27%) said they would prefer to have more information regarding their treatment.Citation37
Furthermore, another study found PwMS are less focused on the prevention of relapses than their physicians, and more focused on QoL.Citation25 Patients’ treatment goals tend to focus on improving specific symptoms that impact their daily lives, whereas providers’ goals focus on slowing disease progression.Citation38 These findings report the limitations of using only treatment goals defined by HCPs and investigators and suggest that a more patient-centred approach could be used to maximise the relevance to PwMS.Citation38 PwMS may view pharmacologic treatment with feelings of resignation and discomfort, and have to learn to integrate the treatment into their life and manage side effects.Citation39
Patients and HCPs also report different reasons for poor treatment adherence. A survey conducted in seven countries found that 82% of HCPs (n=280) thought side effects were the main reason for patients taking a break from or stopping treatment, followed by “no sign of disease declining” (54%). In contrast, 42% of patients (n=331) cited side effects as being the main reason, followed by “being emotionally drained/fed up with treatment” (13%), “practical issues in taking treatment” and “treatment not working” (9% each).Citation40 Lastly, a study of patient perspectives on using DMTs for MS found that considering and adhering to treatment are not primarily determined by individual, rational deliberation. Rather, constantly being confronted with the disease, managing an inevitable decline, hopes of delaying disease progression and the value of social support strongly influenced choice.Citation41
Comparison of PwMS’ Perspectives on Disease Progression with Patients with Other Chronic Progressive Conditions
Literature searches on comparative conditions identified few studies that directly considered patient perspectives on disease progression, as was the case with MS. However, those identified as relevant supported and extended the perspectives of PwMS.
People with long-term conditions consistently say that they want to be listened to, involved in decisions regarding their care and given access to information that will help them make these decisions. However, many challenges lie in achieving accurate prognostication due to PwMS individual variability. Essentially, these people want support with understanding their condition and managing it, and they want to be treated holistically.Citation42 In a prospective longitudinal survey from Norway, providing cancer patients with satisfactory information improved the patients’ knowledge about their disease and its treatment.Citation43 A study from the Czech Republic of the impact of information provision and delivery on patients with progressive neurological disease (including MS, Parkinson’s and atypical Parkinson’s disease, and Huntington’s disease) employed in-depth interviews with 20 participants (patients, family members, professionals). Participants often felt that the information provided and the delivery of this information were inadequate, especially regarding disease transitions and progression.Citation44 Such a lack of disease awareness and understanding could have negative impacts on patients’ family members and caregivers. Indeed, the sufficient provision of information for family members is one of the key unmet needs in the care of patients with chronic conditions.Citation44
Lack of adequate communication by HCPs was the most common recurring theme. As with MS, this theme is reflected in studies of breaking bad news to patients with other potentially life-limiting conditions, revealing room for improvement and providing indications of potentially beneficial ways forward. A questionnaire survey of UK patient preferences regarding the delivery of a cancer diagnosis (n=244) found that patients who were dissatisfied commented on the pessimistic or unsympathetic manner of the doctor.Citation45
A large-scale survey of Canadian patients with life-changing diagnosis (n=1337; including cancer, lupus, amyotrophic lateral sclerosis (ALS), MS, HIV/AIDS, and Parkinson’s disease) was used to assess one of the most widely used guidelines for breaking bad news ‒ SPIKES. The SPIKES guideline largely reflected the perspectives of the different patient groups. The four most important components of SPIKES from patients’ perspectives were physicians demonstrating empathy, explaining their diagnosis and its implications, taking their time rather than rushing patients, and asking patients if they understood.Citation46 A much smaller-scale study (n=30 patients) from Sweden found that the doctor’s character and their facility in creating personal relationships affected patients’ ability to cope with communications regarding the transition from curative to palliative cancer care.Citation47
A survey of patients from the USA with amyotrophic lateral sclerosis (n=144) and their caregivers (n=123) found that 56% of patients rated their HCP’s breaking of the news as “average”, “below average” or “poor”. Better communication assessed using the SPIKES protocol, along with more time discussing the diagnosis correlated with increased patient and caregiver satisfaction.Citation48 Likewise, a UK study of the perceptions and interpretations of patients (n=13) receiving a lung cancer diagnosis identified communication as a key issue. Patients cited, for example, doctors’ use of the words “tumor” and “growth” without regard to patients’ understanding.Citation49 The patients’ reactions and attitudes to their illness and its treatment varied widely, underlining the need for a personalized approach to care.Citation49
As with MS, some patients with other chronic progressive conditions may be ambivalent about HCP communications regarding disease progression.Citation50 Although most patients want to receive details of their prognosis,Citation45,Citation51 they may prefer the communication of this information to be tailored to them regarding the format, extent and timing of prognostic information delivered by doctors.Citation51
The papers examining other chronic progressive conditions confirm that diverse factors influence adherence and nonadherence to treatment, including patients’ health beliefs, motivation and perception of illness control, and relationships and communication with HCPs.Citation52,Citation53 Patients’ sense of responsibility, fear of complications and continuity of care can also influence treatment adherence.Citation54 Indeed, poor patient understanding can be a key factor in inadequate adherence and management of chronic progressive conditions. A study of patient perspectives on diabetes and its management in 12 patients with type 2 diabetes in Bangladesh found numerous misconceptions regarding the disease, poor knowledge of diabetes medications, their use and side effects, and noncompliance with their physicians’ advice on diet and physical activity.Citation55 Drug costs, concerns around side effects and forgetfulness may be further factors in nonadherence.Citation55
In a longitudinal study of patients with incurable cancer, the distress associated with fluctuations in the disease diminished over time, and patients grew accustomed to the disease, describing it as part of themselves. Most talked about their disease in a neutral or optimistic way, while HCPs typically encouraged hopefulness and optimism.Citation56
Maintaining hope is an important coping strategy in patients with incurable cancer.Citation57 A series of focus groups and interviews explored attitudes among patients with terminal cancer, caregivers from palliative care services and HCPs. Most participants believed that there were ways of maintaining hope and fostering coping, and that HCPs can help to facilitate this with patients and their caregivers.Citation57 Similar results were reported in a large study of older adults (>60 years; n=2293) in Sweden, showing that high life satisfaction and a positive health outlook were associated with lower rates of accumulation and progression of multi-morbidity and disability.Citation58 Again, good patient knowledge and understanding of chronic conditions are pivotal. Lack of understanding and participation in self-care behaviours limits the effectiveness of treatment, while, conversely, progressive levels of health literacy can gradually improve patients’ ability to become active participants in their care.Citation59 Encouraging such patient participation may prove invaluable both individually and collectively. When asked, in a citizen science study of adults with long-term conditions in France, patients provided a wealth of ideas on improving their standards of care across the piece, from HCP-patient discussions to the coordination and collaboration of inpatient and outpatient care.Citation60
Discussion
This review captured the perspectives of PwMS on disease progression from a variety of multi-patient studies (survey, interview-based, thematic analysis of personal narratives, focus group discussions, etc.) and considered findings on patient perspectives from other chronic progressive conditions. The findings build on two recent reports from a series of collaborative workshops with PwMS and HCPs, hosted by the MS in the 21st Century initiative, and designed to further the aims of shared decision-making and management. The first report highlighted key unmet needs in the care of PwMS, including the need for improved communication on complex issues such as disease progression, along with practical actions that could be undertaken to address these.Citation13 The second report considered the risks and benefits of open communication about disease progression and potential ways of optimizing such conversations.Citation61 The results of this review support the views highlighted in these two reports.
The aim of this literature review was to identify the number and relevance of publications that relate specifically to patients’ understanding of MS disease progression. The results demonstrated only a small number of publications fall into this category, despite the importance of this topic. It also demonstrated that greater PwMS’ understanding of their condition improved communication between patients and HCPs, and shared decision-making will, in general, decrease patients’ distress, help them adapt to MS, and potentially improve treatment adherence and overall outcomes. The findings from this review indicate that further studies could be done to understand if these factors correlate with objective measures of adherence and changes in clinical metrics. Patient preference to treatment options can be affected by the attribute-based communication used by HCPs.Citation62 Reducing patients’ distress is particularly important, since negative emotional responses in PwMS can persist over the long termCitation25 and potentially causes a range of negative impacts, including on QoL,Citation12,Citation25,Citation63 patients’ sense of self-identity, and adding to the disruption to other aspects of their lives.Citation8,Citation23,Citation64 Chronic illness has long been known to cause biographical disruption (the way that illness disrupts the imaged future that a patient had previously pictured).Citation65 Indeed, there are numerous parallels between PwMS’ experience of disease progression and those of patients with other chronic progressive conditions. Further studies would be interesting to directly compare attitudes on disease progression and self-management in PwMS and those with other chronic progressive conditions and any impact on treatment adherence.
The importance of good communication between PwMS and HCPs is well known.Citation22,Citation66–Citation69 Research on the breaking of bad news to patients with cancer and (to a lesser extent) with ALS consistently shows the critical importance of effective communication, and that patient satisfaction is largely determined by the HCP’s interpersonal skills. However, discrepancies in expectations of HCPs and patients can have many negative effects, including treatment delays and diminishing patient trust.Citation70 Similarly, a thematic analysis of first-person meta-narratives created from a series of systematic reviews and qualitative focus-group-derived data representing seven brain disorders (MS, Parkinson’s disease, alcohol dependence, depression, epilepsy, schizophrenia, stroke) showed strong commonalities in both the psychosocial difficulties and the factors influencing these across different disorders. Difficulties in communication were frequently identified, while stigma and social exclusion negatively impacted on these across the different disorders. Conversely, access to work and supportive relationships with family and friends, as well as self-help groups, had a considerable beneficial effect.Citation71
The prognosis of MS has improved considerably in recent decades, due in part to the availability of more effective DMTsCitation72 ‒ including one approved for PPMSCitation73 and several for SPMS. There has also been a marked improvement in the symptomatic management and treatment of MS. The change in treatment paradigms has the potential to significantly alter the landscape of MS communication by engendering positivity in both PwMS and HCPs. Yet as long as the prospect of progression remains, the communication skills of neurology consultants will be critical in facilitating patient acceptance and maintaining hope during conversations about the future.Citation19,Citation74 This reflects increasing evidence that good patient-HCP communication is, in general, associated with greater patient and doctor satisfaction and improved patient outcomes compared with poor communication.Citation75 Adequate healthcare resources, including training in communication skills for neurologists and other MS-specialist HCPs, is also fundamental to good communication.Citation13,Citation76 Newly diagnosed patient education initiatives may also play a role in enabling improved communication with MS-specialist HCPs, and in encouraging patients to self-manage.Citation61
Acceptance, adaptation and self-management strategies can be invaluable in helping PwMS to cope psychologically and emotionally.Citation7,Citation19,Citation77 Indeed, positive perceptions of MS by patients have been found to correlate more strongly with disease self-management than clinical variables, such as the severity, duration and type of MS.Citation78 Inclusion of patient perspectives and engagement may even improve the clinical utility of disease progression assessments.Citation79 Online communication is increasingly changing the patient-HCP dynamic by empowering patients to become more active participants in joint decision-making and may present an opportunity for open and early conversations on progression.Citation80,Citation81 Looking forward, technological developments, such as mobile phone apps, could aid self-management, although these will need to be validated.Citation82,Citation83
In conclusion, patient perspectives on progression in MS and chronic progressive conditions reveal further opportunities for exploration and areas where MS care could improve. The limited evidence available, summarized in this review, highlights the importance of good communication between HCPs and PwMS to improve PwMS’ experience and outcomes. While further studies are needed, the current evidence base offers information and insights that may help HCPs to enhance patient care, well-being, and adherence.
Data Sharing Statement
The database query/search strings and analyzed datasets from this study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study did not involve human subjects and/or animals or the use of identifiable patient data; therefore, ethical approval and informed consent were not required.
Author Contributions
All named authors had full access to all of the content within this manuscript and take complete responsibility for the integrity and accuracy of the content. All named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Acknowledgment
The MS in the 21st Century initiative is financially supported by Merck KGaA, Darmstadt, Germany. Secretariat support and editorial input were provided by Cello Health Communications. Search result sorting and medical writing assistance was provided by Owen Webb, Cello Health Communications. Secretariat support, editorial input and medical writing assistance were funded by Merck KGaA, Darmstadt, Germany.
Disclosure
Elisabeth G Celius has received honoraria for advisory boards and/or speaker honoraria from Almirall, Biogen, Merck KGaA, Roche, Novartis, Genzyme and Teva, and unrestricted research grants from Novartis and Genzyme, and reports personal fees from Biogen, Sanofi, and Novartis, and personal fees from Roche and Merck KGaA, outside the submitted work. Heidi Thompson has received honoraria for advisory board participation and travel grants from Merck KGaA and Biogen. Dawn Langdon has received research grants from Bayer, Merck KGaA, Novartis, and Biogen, all paid to her institution and has participated in speakers’ bureaus for Bayer, Merck KGaA, Almirall, Excemed, Teva, Roche, Novartis, Biogen, and Sanofi, has received consultancy honoraria from Novartis, Bayer, Merck KGaA, Biogen, Teva, and Sanofi, and reports grants and personal fees from Merck KGaA and Novartis, and personal fees from TEVA, Bayer, and Biogen, outside the submitted work. Alice Laroni has received personal compensation from Novartis, Sanofi Genzyme, Biogen, Merck KGaA, Roche and Teva for public speaking and advisory boards and has received research grants from the Italian Ministry of Health, the Italian Ministry of University, and The Italian MS Foundation, and reports personal fees from Biogen, Merck KGaA and Novartis, non-financial support from Roche, grants from Fondazione Italiana sclerosi multiplaoutside the submitted work. Trishna Bharadia reports personal fees from Merck KGaA, Actelion, Roche, Sanofi-Genzyme, Envision Pharma, Teva, Biogen, Novartis, Blue Latitude Health, and talkHealth, outside the submitted work, and is Ambassador for MS Society UK (voluntary position), Patron for Chilterns MS Centre (voluntary position), and Patron for Cambs Therapy Centre (voluntary position). Maija Pontaga, Stanca Potra, David Yeandle, Jane Shanahan, and Jürg Kesselring have received honoraria from Merck KGaA for MS in the 21st Century activities. Pieter van Galen has received honoraria from Merck KGaA for MS in the 21st Century activities and has received consulting and speaking fees from Novartis, Merck KGaA, Celgene R&D Sarl, F. Hoffman-La Roche, NV Roche SA, Mylan GMBH and Excemed. Nektaria Alexandri is an employee of Merck KGaA. The authors report no other potential conflicts of interest for this work.
References
- Multiple Sclerosis International Federation. What is MS? Multiple sclerosis (MS) is a progressive disease of the nervous system; 2019. Available from: https://www.msif.org/about-ms/what-is-ms/. Accessed December 5, 2020.
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis. Neurology. 2014;83(3):278–286. doi:10.1212/WNL.0000000000000560
- Chataway J. Tackling progression in multiple sclerosis. Lancet Neurol. 2018;17(6):489–491. doi:10.1016/S1474-4422(18)30158-3
- Faissner S, Gold R. Progressive multiple sclerosis: latest therapeutic developments and future directions. Ther Adv Neurol Disord. 2019;12:1756286419878323. doi:10.1177/1756286419878323
- Stankiewicz JM, Weiner HL. An argument for broad use of high efficacy treatments in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2020;7(1):e636. doi:10.1212/NXI.0000000000000636
- Filippi M, Preziosa P, Langdon D, et al. Identifying progression in multiple sclerosis: new perspectives. Ann Neurol. 2020;88(3):438–452. doi:10.1002/ana.25808
- Ghafari S, Fallahi-Khoshknab M, Nourozi K, Mohammadi E. Patients’ experiences of adapting to multiple sclerosis: a qualitative study. Contemporary Nurse. 2015;50(1):36–49. doi:10.1080/10376178.2015.1010252
- Frost J, Grose J, Britten N. A qualitative investigation of lay perspectives of diagnosis and self-management strategies employed by people with progressive multiple sclerosis. Health. 2017;21(3):316–336. doi:10.1177/1363459316674787
- Cadden MH, Arnett PA, Tyry TM, Cook JE. Judgment hurts: the psychological consequences of experiencing stigma in multiple sclerosis. Soc Sci Med. 2018;208:158–164. doi:10.1016/j.socscimed.2018.01.015
- Pérez-Miralles F, Prefasi D, García-Merino A, et al. Perception of stigma in patients with primary progressive multiple sclerosis. Mult Scler J Exp Transl Clin. April 2019. doi:10.1177/2055217319852717
- Greenberg B, Fan Y, Carriere L, Sullivan A. Depression and age at first neurology appointment associated with receipt of behavioral medicine services within 1 year in a multiple sclerosis population. Int J MS Care. 2017;19(4):199–207. doi:10.7224/1537-2073.2016-012
- Butler E, Thomas R, Carolan A, Silber E, Chalder T. ‘It’s the unknown’ – understanding anxiety: from the perspective of people with multiple sclerosis. Psychol Health. 2019;34(3):368–383. doi:10.1080/08870446.2018.1541989
- Rieckmann P, Centonze D, Elovaara I, et al. Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: a combined perspective from the MS in the 21st Century Steering Group. Mult Scler Relat Disord. 2018;19:153–160. doi:10.1016/j.msard.2017.11.013
- Colligan E, Metzler A, Tiryaki E. Shared decision-making in multiple sclerosis. Mult Scler. 2017;23(2):185–190. doi:10.1177/1352458516671204
- Burtchell J, Fetty K, Miller K, Minden K, Kantor D. Two sides to every story: perspectives from four patients and a healthcare professional on multiple sclerosis disease progression. Neurol Ther. 2019;8(2):185–205. doi:10.1007/s40120-019-0141-4
- Davies F, Edwards A, Brain K, et al. ‘You are just left to get on with it’: qualitative study of patient and carer experiences of the transition to secondary progressive multiple sclerosis. BMJ Open. 2015;5(7):e007674. doi:10.1136/bmjopen-2015-007674
- Goretti B, Portaccio E, Zipoli V, Razzolini L, Amato MP. Coping strategies, cognitive impairment, psychological variables and their relationship with quality of life in multiple sclerosis. Neurol Sci. 2010;31(Suppl 2):S227–230. doi:10.1007/s10072-010-0372-8
- Dahdaleh M, Alroughani R, Aljumah M, et al. Intervening to reduce the risk of future disability from multiple sclerosis: are we there yet? Int J Neurosci. 2017;127(10):944–951. doi:10.1080/00207454.2016.1277424
- Inojosa H, Proschmann U, Akgün K, Ziemssen T. A focus on secondary progressive multiple sclerosis (SPMS): challenges in diagnosis and definition. J Neurol. 2019. Online ahead of print. doi:10.1007/s00415-019-09489-5
- Matza LS, Kim K, Phillips G, et al. Multiple sclerosis relapse: qualitative findings from clinician and patient interviews. Mult Scler Relat Disord. 2019;27:139–146. doi:10.1016/j.msard.2018.09.029
- Solari A, Giovannetti AM, Giordano A, et al. Conversion to secondary progressive multiple sclerosis: patient awareness and needs. Results from an online survey in Italy and Germany. Front Neurol. 2019;10:916. doi:10.3389/fneur.2019.00916
- de Seze J, Borgel F, Brudon F. Patient perceptions of multiple sclerosis and its treatment. Patient Prefer Adherence. 2012;6:263–273. doi:10.2147/PPA.S27038
- Soundy A, Benson J, Dawes H, Smith B, Collett J, Meaney A. Understanding hope in patients with multiple sclerosis. Physiotherapy. 2012;98(4):349–355. doi:10.1016/j.physio.2011.05.003
- Koffman J, Gao W, Goddard C, et al. Progression, symptoms and psychosocial concerns among those severely affected by multiple sclerosis: a mixed-methods cross-sectional study of black Caribbean and white British people. PLoS One. 2013;8(10):e75431. doi:10.1371/journal.pone.0075431
- Lee Mortensen G, Rasmussen PV. The impact of quality of life on treatment preferences in multiple sclerosis patients. Patient Prefer Adherence. 2017;11:1789–1796. doi:10.2147/PPA.S142373
- Feinstein A, Pavisian B. Multiple sclerosis and suicide. Mult Scler J. 2017;23(7):923–927. doi:10.1177/1352458517702553
- Malcomson KS, Lowe-Strong AS, Dunwoody L. What can we learn from the personal insights of individuals living and coping with multiple sclerosis? Disabil Rehabil. 2008;30(9):662–674. doi:10.1080/09638280701400730
- Król J, Szcześniak M, Koziarska D, Rzepa T. Time perception and illness acceptance among remitting-relapsing multiple sclerosis patients under treatment. Psychiatr Pol. 2015;49(5):911–920. doi:10.12740/PP/38740
- Dennison L, Brown M, Kirby S, Galea I. Do people with multiple sclerosis want to know their prognosis? A UK nationwide study. PLoS One. 2018;13(2):e0193407. doi:10.1371/journal.pone.0193407
- Buecken R, Galushko M, Golla H, et al. Patients feeling severely affected by multiple sclerosis: how do patients want to communicate about end-of-life issues? Patient Educ Couns. 2012;88(2):318–324. doi:10.1016/j.pec.2012.03.010
- Dennison L, McCloy Smith E, Bradbury K,Galea I. How do people with multiple sclerosis experience prognostic uncertainty and prognosis communication? A qualitative study. PLoS One. 2016;11(7):e0158982. doi:10.1371/journal.pone.0158982
- Chesi P, Marini MG, Mancardi GL, et al. Listening to the neurological teams for multiple sclerosis: the SMART project. Neurol Sci. 2020;2231–2240. doi:10.1007/s10072-020-04301-z
- O’Loughlin E, Hourihan S, Chataway J, Playford ED, Riazi A. The experience of transitioning from relapsing remitting to secondary progressive multiple sclerosis: views of patients and health professionals. Disabil Rehabil. 2017;39(18):1821–1828. doi:10.1080/09638288.2016.1211760
- The Health Foundation. Person-Centred Care Made Simple: What Everyone Should Know About Person-Centred Care. Available from: https://www.health.org.uk/publications/person-centred-care-made-simple. Accessed December 17, 2020. 2014.
- Methley AM, Chew-Graham CA, Cheraghi-Sohi S, Campbell SM. A qualitative study of patient and professional perspectives of healthcare services for multiple sclerosis: implications for service development and policy. Health Soc Care Community. 2017;25(3):848–857. doi:10.1111/hsc.12369
- National Institute for Health and Care Excellence. Multiple sclerosis in adults: management. Clinical guideline [CG186]. Available from: https://www.nice.org.uk/guidance/cg186. Accessed December 5, 2020. 2019.
- Visser LH, Heerings MA, Jongen PJ, van der Hiele K. Perspectives and experiences of Dutch multiple sclerosis patients and multiple sclerosis-specialized neurologists on injectable disease-modifying treatment. Patient Prefer Adherence. 2016;10:659–667. doi:10.2147/PPA.S106155
- Col NF, Solomon AJ, Springmann V, et al. Whose preferences matter? A patient-centered approach for eliciting treatment goals. Med Decis Mak. 2018;38(1):44–55. doi:10.1177/0272989X17724434
- Buesa-Estelléz A, Cano-de-la-Cuerda R, Ortiz-Gutiérrez RM, Palacios-Ceña D. The impact of pharmacological treatment on patients with multiple sclerosis. Disabil Health J. 2019;12(4):615–621. doi:10.1016/j.dhjo.2019.05.005
- Riñon A, Buch M, Holley D, Verdun E. The MS Choices Survey: findings of a study assessing physician and patient perspectives on living with and managing multiple sclerosis. Patient Prefer Adherence. 2011;5:629–643. doi:10.2147/PPA.S26479.
- de Ceuninck van Capelle A, van der Meide H, Vosman FJH, Visser LH. A qualitative study assessing patient perspectives in the process of decision-making on disease modifying therapies (DMT’s) in multiple sclerosis (MS). PLoS One. 2017;12(8):e0182806. doi:10.1371/journal.pone.0182806
- Department of Health and Social Care. Long term conditions compendium of information: third edition. Avialble from: https://www.gov.uk/government/publications/long-term-conditions-compendium-of-information-third-edition. Accessed December 5, 2020.2012.
- Berger O, Grønberg BH, Loge JH, Kaasa S, Sand K. Cancer patients’ knowledge about their disease and treatment before, during and after treatment: a prospective, longitudinal study. BMC Cancer. 2018;18(1):381. doi:10.1186/s12885-018-4164-5
- Bužgová R, Kozáková R. Informing patients with progressive neurological disease of their health status, and their adaptation to the disease. BMC Neurol. 2019;19(1):250. doi:10.1186/s12883-019-1488-y
- Brown VA, Parker PA, Furber L, Thomas AL. Patient preferences for the delivery of bad news - the experience of a UK Cancer Centre. Eur J Cancer Care (Engl). 2011;20(1):56–61. doi:10.1111/j.1365-2354.2009.01156.x
- Mirza RD, Ren M, Agarwal A, Guyatt GH. Assessing patient perspectives on receiving bad news: a survey of 1337 patients with life-changing diagnoses. AJOB Empir Bioeth. 2019;10(1):36–43. doi:10.1080/23294515.2018.1543218
- Friedrichsen MJ, Strang PM, Carlsson ME. Breaking bad news in the transition from curative to palliative cancer care – patient’s view of the doctor giving the information. Support Care Cancer. 2000;8(6):472–478.
- McCluskey L, Casarett D, Siderowf A. Breaking the news: a survey of ALS patients and their caregivers. Amyotrophic Lateral Sclerosis Motor Neuron Dis. 2004;5(3):131–135. doi:10.1080/14660820410020772
- Yardley SJ, Davis CL, Sheldon F. Receiving a diagnosis of lung cancer: patients‘ interpretations, perceptions and perspectives. Palliat Med. 2001;15(5):379–386. doi:10.1191/026921601680419429
- Selman LE, Bristowe K, Higginson IJ, Murtagh FEM. The views and experiences of older people with conservatively managed renal failure: a qualitative study of communication, information and decision-making. BMC Nephrology. 2019;20(1):38. doi:10.1186/s12882-019-1230-4
- Hagerty RG, Butow PN, Ellis PA, et al. Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol. 2004;22(9):1721–1730. doi:10.1200/JCO.2004.04.095
- Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91. doi:10.3389/fphar.2013.00091
- Pagès-Puigdemont N, Mangues MA, Masip M, et al. Patients’ perspective of medication adherence in chronic conditions: a qualitative study. Adv Ther. 2016;33(10):1740–1754. doi:10.1007/s12325-016-0394-6
- Kähkönen O, Kyngäs H, Saaranen T, Kankkunen P, Miettinen H, Oikarinen A. Support from next of kin and nurses are significant predictors of long-term adherence to treatment in post-PCI patients. Eur J Cardiovasc Nurs. 2020;19(4):339–350. doi:10.1177/1474515119887851
- Islam SMS, Biswas T, Bhuiyan FA, Mustafa K, Islam A. Patients’ perspective of disease and medication adherence for type 2 diabetes in an urban area in Bangladesh: a qualitative study. BMC Res Notes. 2017;10(1):131. doi:10.1186/s13104-017-2454-7
- Buiting HM, van Ark MAC, Dethmers O, Maats EPE, Stoker JA, Sonke GS. Complex challenges for patients with protracted incurable cancer: an ethnographic study in a comprehensive cancer centre in the Netherlands. BMJ Open. 2019;9(3):e024450. doi:10.1136/bmjopen-2018-024450
- Clayton JM, Butow PN, Arnold RM, Tattersall MHN. Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers. Cancer. 2005;103(9):1965–1975. doi:10.1002/cncr.21011
- Calderón-Larrañaga A, Vetrano DL, Welmer A-K, Grande G, Fratiglioni L, Dekhtyar S. Psychological correlates of multimorbidity and disability accumulation in older adults. Age Ageing. 2019;48(6):789–796. doi:10.1093/ageing/afz117
- Dunn P, Conard S. Improving health literacy in patients with chronic conditions: a call to action. Int J Cardiol. 2018;273:249–251. doi:10.1016/j.ijcard.2018.08.090
- Tran V-T, Riveros C, Péan C, Czarnobroda A, Ravaud P. Patients’ perspective on how to improve the care of people with chronic conditions in France: a citizen science study within the ComPaRe e-cohort. BMJ Quality Safety. 2019;28(11):875-886. doi:10.1136/bmjqs-2018-008593
- Vermersch P, Shanahan J, Langdon D, Yeandle D, Alexandri N, Knowledgeis power, but is ignorance bliss? Optimising conversations about disease progression in multiple sclerosis. Neurol Ther. 2020;9(1):1–10. doi:10.1007/s40120-019-00170-7
- Webb EJD, Meads D, Eskyte I, et al. A systematic review of discrete-choice experiments and conjoint analysis studies in people with multiple sclerosis. Patient. 2018;11(4):391–402. doi:10.1007/s40271-017-0296-y
- Hayter AL, Salkovskis PM, Silber E, Morris RG. The impact of health anxiety in patients with relapsing remitting multiple sclerosis: misperception, misattribution and quality of life. Br J Clin Psychol. 2016;55(4):371–386. doi:10.1111/bjc.12106
- Kirk S, Hinton D. “I’m not what I used to be”: a qualitative study exploring how young people experience being diagnosed with a chronic illness. Child Care Health Dev. 2019;45(2):216–226. doi:10.1111/cch.12638
- Bury M. Chronic illness as biographical disruption. Sociol Health Illn. 1982;4(2):167–182. doi:10.1111/1467-9566.ep11339939
- Hickey JV. Good communication with healthcare providers helped patients with multiple sclerosis to cope and adapt. Evid Based Nurs. 2004;7(4):124. doi:10.1136/ebn.7.4.124
- Alroughani RA. Improving communication with multiple sclerosis patients. Neurosciences. 2015;20(2):95–97. doi:10.17712/nsj.2015.2.20140441
- Tintoré M, Alexander M, Costello K, et al. The state of multiple sclerosis: current insight into the patient/health care provider relationship, treatment challenges, and satisfaction. Patient Prefer Adherence. 2016;11:33–45. doi:10.2147/PPA.S115090
- Schlegel V, Leray E. From medical prescription to patient compliance: a qualitative insight into the neurologist–patient relationship in multiple sclerosis. Int J MS Care. 2018;20(6):279–286. doi:10.7224/1537-2073.2017-043
- Chiò A, Domenico Borasio G. Breaking the news in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(4):195–201. doi:10.1080/14660820310017326
- Watz H, Barnacle H, Hartley BF, Chan R. Efficacy and safety of the p38 MAPK inhibitor losmapimod for patients with chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(1):63–72. doi:10.1016/S2213-2600(13)70200-5
- Beiki O, Frumento P, Bottai M, Manouchehrinia A, Hillert J. Changes in the risk of reaching multiple sclerosis disability milestones in recent decades: a nationwide population-based cohort study in Sweden. JAMA Neurol. 2019;76(6):665–671. doi:10.1001/jamaneurol.2019.0330
- Adler AI, Knight H. Ocrelizumab for primary progressive multiple sclerosis. Lancet Neurol. 2019;18(9):816–817. doi:10.1016/S1474-4422(19)30245-5
- Manzano A, Eskytė I, Ford HL, et al. Impact of communication on first treatment decisions in people with relapsing-remitting multiple sclerosis. Patient Educ Couns. 2020;103(12):2540–2547. doi:10.1016/j.pec.2020.05.014
- Maskrey N. Shared decision making: why the slow progress? An essay by Neal Maskrey. BMJ. 2019;367:l6762. doi:10.1136/bmj.l6762
- Brashear A, Vickrey BG. Burnout in neurology. Extinguishing the embers and rekindling the joy in practice. Neurology. 2018;91(20):907-908. doi:10.1212/WNL.0000000000006520
- Ploughman M, Austin MW, Murdoch M, et al. Factors influencing healthy aging with multiple sclerosis: a qualitative study. Disabil Rehabil. 2012;34(1):26–33. doi:10.3109/09638288.2011.585212
- Wilski M, Tasiemski T. Illness perception, treatment beliefs, self-esteem, and self-efficacy as correlates of self-management in multiple sclerosis. Acta Neurol Scand. 2016;133(5):338–345. doi:10.1111/ane.12465
- Kragt JJ, Nielsen JM, van der Linden FAH, Polman CH, Uitdehaag BMJ. Disease progression in multiple sclerosis: combining physicians’ and patients’ perspectives? Mult Scler J. 2010;17(2):234–240. doi:10.1177/1352458510385505
- Synnot AJ, Hill SJ, Garner KA, et al. Online health information seeking: how people with multiple sclerosis find, assess and integrate treatment information to manage their health. Heal Expect. 2016;19(3):727–737. doi:10.1111/hex.12253
- Kantor D, Bright JR, Burtchell J. Perspectives from the patient and the healthcare professional in multiple sclerosis: social media and participatory medicine. Neurol Ther. 2018;7(1):37–49. doi:10.1007/s40120-017-0088-2
- Marziniak M, Brichetto G, Feys P, Meyding-Lamadé U, Vernon K, Meuth SG. The use of digital and remote communication technologies as a tool for multiple sclerosis management: narrative review. JMIR Rehabil Assist Technol. 2018;5(1):e5. doi:10.2196/rehab.7805
- Salimzadeh Z, Damanabi S, Kalankesh LR, Ferdousi R. Mobile applications for multiple sclerosis: a focus on self-management. Acta Inform Med. 2019;27(1):12–18. doi:10.5455/aim.2019.27.12-18
- Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). doi:10.1136/bmj.b2700
