Abstract
Purpose
Treatment for chronic lymphocytic leukemia (CLL) has changed dramatically with the approval of novel agents. Information regarding how patients and oncologists make trade-offs across attributes of novel therapies is limited. The purpose of this study was to understand how variations in attributes impact treatment choice among patients and oncologists.
Patients and Methods
In this study, 371 participants (patients [n=220] and oncologists [n=151]) completed an online discrete choice experiment (DCE) to quantify preferences for first-line (1L) CLL treatment with novel agents; participants chose between hypothetical treatment profiles consisting of eight attributes with varying levels taken from published literature. Hierarchical Bayesian models were used to estimate attribute level preference weights. The weights were used to compute relative importance, a measure of how influential an attribute is to treatment choice.
Results
Increasing 2-year progression-free survival (PFS) from 75% to 95% had the greatest impact on preferences in 1L CLL treatment, accounting for 40% and 30% of the variation in preferences among patients and oncologists, respectively. Risk differences in atrial fibrillation (AF), infection, and discontinuation due to adverse events (AEs) were also important to patients and oncologists. Among both groups, risk differences in tumor lysis syndrome (TLS) and bleeding were least influential in treatment choice. Oncologists required 2–4 times higher increases in 2-year PFS than patients to accept increased risks of AF, discontinuation due to AEs, bleeding, TLS, and arthralgia/myalgia.
Conclusion
Patient–oncologist communication may be improved by a more focused discussion on the risks of AEs, relative to treatment outcomes, with patient goals in mind.
Data Availability Statement
The data that support the findings of this study are available for noncommercial use from the corresponding author upon reasonable request.
Results
Of the 220 patients who completed the survey, 12 had 2 or more illogical responses on the very bad/very good rating scales, 8 completed the survey in less than 50% of the median time, and 3 did both. We estimated the HB model with and without these 17 respondents. Their inclusion did not affect the HB estimates, so we decided to include their data. Of the 167 oncologists who completed the survey, 16 had 2 or more illogical responses on the very bad/very good rating scales, 19 completed the survey in less than 50% of the median time, and 7 did both. We checked the HB model estimates with and without the data from the 16 oncologists who had 2 or more illogical responses on the very bad/very good rating scales, and the preference weights estimates were impacted; thus, we decided to exclude their data from the further analysis.
Sample Characteristics
A total of 151 oncologists completed the DCE and were included in the analysis. As per recruitment quotas, most oncologists practiced in a community setting (n=108, 71.5%). Most reported hematology/oncology as their primary medical specialty (n=117, 77.5%). Oncologists reported a mean of 16.3 (±7.0) years in practice. On average, oncologists managed 65.2 (±64.1) patients with CLL in the past 12 months of which 16.6 (±23.2) were WW, 26.6 (±29.3) were 1L treatment, and 22.1 (±21.7) were R/R.
A total of 220 patients completed the DCE and were included in the analysis. Patients had a mean age of 56.4 (±10.5) years, and three-quarters (n=163, 74.1%) self-identified as Caucasian. On average, patients had received a CLL diagnosis for 2.0 (±3.1) years at the time of study completion. As per recruitment quotas, the majority (n=150, 68.2%) had received CLL treatment (1L: n=80, 36.4%; R/R: n=70, 31.8%), with just under one-third (n=70, 31.8%) being WW ().
Table 2 Patient Sample Characteristics
Oncologist and Patient Preferences
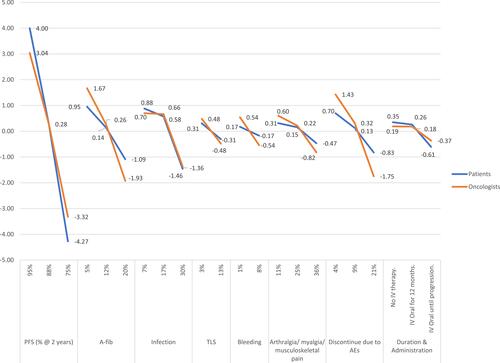
Attribute level preference weights are shown in . For oncologists, a change in 2-year PFS from 75% to 95% had the highest difference in preference weights (6.36). A change in the risks of AF (3.60) and treatment discontinuation due to AEs (3.18) had the next highest difference in preference weights. For patients, the same change in 2-year PFS also had the highest difference in preference weights (8.27). A change in 2-year PFS was nearly 4 times more influential to treatment preferences than changes in the risks of infection (8.27 vs 2.34) or AF (8.27 vs 2.04), the next two highest preference weights for patients.
Figure 1 Attribute level preference weights for oncologists and patients. Preference weights measure relative preference, which means that only changes between attribute level estimates and the relative size of those changes across attributes have meaningful interpretations.
Attribute relative importance was calculated to estimate the unique contribution to treatment choice made by each attribute across the range of preference weights. Oncologists and patients rated increasing the chance of 2-year PFS from 75% to 95% as significantly more important than improvements in any of the other attributes included in the DCE. Specifically, increasing PFS from 75% to 95% accounted for 29.5% and 40.4% of the variation in preferences among oncologists and patients, respectively. Among oncologists, decreasing the risk of AF from 20% to 5% accounted for 17.4% of the variation followed by decreasing the risk of treatment discontinuation due to AEs from 21% to 4% (RI=16.4%) were the next most important attributes driving treatment choice. Least important were decreasing the risks of TLS (RI=4.5%) and bleeding (RI=5.3%) Among patients, decreasing the risks of infection from 30% to 7% (RI=12.6%) and AF from 20% to 5% (RI=11.0%) were the next most important attributes driving treatment preferences. Least important were reducing the risks of bleeding from 8% to 1% (RI=4.7%) and TLS from 13% to 3% (RI=5.7%) ().
Figure 2 Relative importance of treatment attributes for oncologists and patients. Relative importance estimates are ratio scaled, so that an attribute with a relative importance of 40% is twice as important as an attribute with a relative importance of 20%; 95% confidence intervals are shown in the error bars. Symbols represent a statistically significant difference between groups at (*)P<0.01 and (†)P<0.001, two-tailed.
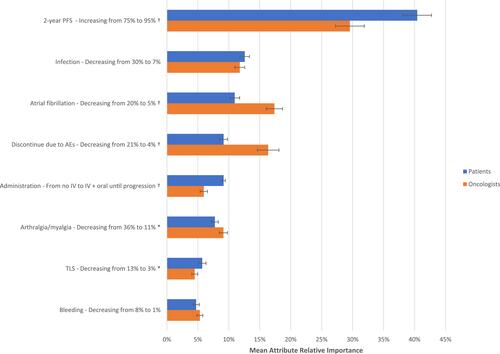
Comparing oncologists and patients, statistically significant differences in attribute RI were observed across 2-year PFS, route of administration, and risks of AF, TLS, discontinuation due to AEs, and arthralgia/myalgia (all P<0.01). The RI of risks of bleeding and infection did not vary significantly between groups ().
Subgroup Analysis
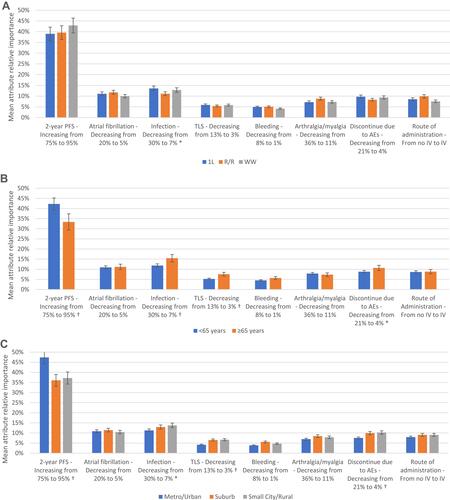
There were no statistically meaningful differences between oncologists by practice setting, years in practice, or patient volume (Appendix 2). However, subgroup differences were observed among patients. The most clear, consistent trends were observed for age and community type. Patients <65 years old and those living in urban/metropolitan areas ranked 2-year PFS as more important and AE-related risks as less important than patients ≥65 years old and those living in other community types, respectively ().
Figure 3 Relative importance of treatment attributes by patient subgroup. Relative importance estimates are ratio scaled, so that an attribute with a relative importance of 40% is twice as important as an attribute with a relative importance of 20%; 95% confidence intervals are shown in the error bars. Symbols represent a statistically significant difference between groups at (*)P<0.05 and (†)P<0.01, two-tailed. (A) Relative importance by patient treatment status. (B) Relative importance by patient age. (C) Relative importance by patient community type.
Trade-Offs in Relation to PFS Improvement
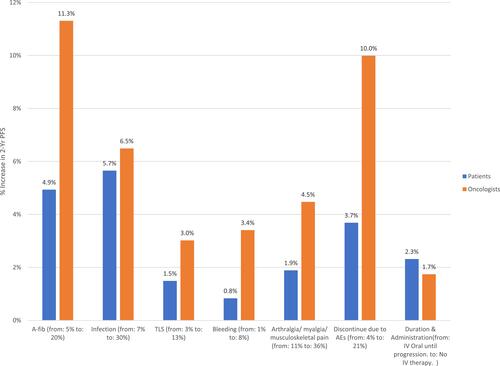
Trade-offs between 2-year PFS and each of the other attributes are shown in . The graph shows the additional percentage increase in 2-year PFS over the base level tested in the DCE (ie, 75%) that was necessary for oncologists and patients to accept a change from the best to the worst level in each of the other attributes. Oncologists required the largest percentage increase in 2-year PFS (11.3%) to compensate for an increased risk of AF, whereas patients required the largest percentage increase in 2-year PFS (5.7%) to compensate for an increased risk of infection. Oncologists required 1.1 to 4.1 times greater percentage increases in 2-year PFS than patients to compensate for increases in risks across all AE-related attributes, whereas patients required a 1.3 times greater percentage increase than oncologists in 2-year PFS in exchange for a change from receiving oral therapy to progression (no IV) to receiving oral therapy to progression plus IV therapy for 6 months.
Discussion
The current study provides unique insights into treatment preferences in the 1L treatment setting among patients with CLL and oncologists in the US. The highest value was placed on improvements in 2-year PFS by both oncologists and patients, but they also valued safety, especially decreased risks of AF, infection, and discontinuation due to AEs. Indeed, the largest increases in the percentage change of 2-year PFS would be required to compensate for an increased risk of these AEs. Notably, patients placed greater weight than oncologists on an increase in 2-year PFS when selecting a novel therapy for CLL. While both patients and oncologists were willing to make trade-offs to obtain incremental 2-year PFS benefits, oncologists required much greater improvements in efficacy than patients to offset the higher risks of AEs or treatment discontinuation due to AEs.
In the in-depth qualitative interviews conducted in the initial phase of the study, all of the attributes selected for the DCE were perceived as being important to treatment decision-making by patients and oncologists.Citation5 Although route of administration and risks of TLS and bleeding were mentioned spontaneously as being of high importance in the qualitative interviews, these attributes were ranked lower than the others included in the DCE choice tasks. This discrepancy may potentially be because the DCE choice task forces the respondent to consider the hypothetical treatment regimen as a collective set of attributes and levels, rather than evaluating attributes in isolation of other factors that may influence treatment choice.
Major bleeding was one of the least important attributes to patients and oncologists in the current study. While this AE is associated with BTK inhibitors,Citation9,Citation10 results may reflect perceptions that major bleeding has a low likelihood of occurrence, which is consistent with the range of the levels (1–8%) included for this attribute in the DCE. Oncologists perceived TLS to be the least important attribute, overall. This result may be a function of the infrequent occurrence of TLS in routine clinical practice or owing to the success of TLS risk-stratification and dose ramp-up strategies at preventing serious TLS.Citation11
In a previous systematic review, patients with cancer preferred oral treatment over IV therapy in 85% of the studies evaluated,Citation12 which was consistent with the in-depth qualitative research we initially conducted.Citation5 Our study found similar results with patients and oncologists preferring regimens without IV components. However, preferences for route of administration were superseded in the DCE by preferences for higher efficacy and lower risks of AEs. Route of administration may be more highly prioritized by patients and oncologists in light of the COVID-19 pandemic, although additional research is needed to confirm this possibility.
Subgroup analyses showed that patients <65 years old and those living in urban/metropolitan areas ranked 2-year PFS as more important and AE-related risks as less important than patients ≥65 years old and those living in other community types, respectively. Prior research has indicated that, among rural adults with cancer, greater travel times to the hospital or specialty cancer center are associated with receiving less medical care.Citation13 Accordingly, patients residing in small cities/rural areas may put greater emphasis on avoiding AEs when selecting CLL treatment because of difficulties in accessing the healthcare resources that are more readily available in urban locales. Our results are supportive of this hypothesis, although further research is required to validate this theory for patients with CLL. Younger patients also placed more value on 2-year PFS. It is possible that younger patients are more interested in achieving greater longevity, whereas, for older patients, an additional year of life may be less valuable to them than it is to a younger patient, thus prioritizing quality of life over quantity. Given that results for both urban community type and younger age were aligned, there may be a potential confounding effect due to demographic trends in which a disproportionate share of US adults aged ≥65 years reside in rural communities.Citation14
The results of the current study are consistent with past research by Mansfield regarding the primary importance of PFS to patients,Citation15 with the present study confirming a similar level of importance for this attribute among patients and oncologists. The present study is unique in its assessment and comparison of both patients and oncologists in the same study in a US sample. Whereas overall survival was found to be the most important factor in a previous European study of providers, patients, and the lay public,Citation16 the current study did not assess this attribute, as it was found to be of lesser importance than PFS to CLL treatment decisions in the initial qualitative phase of this study.Citation5 Importantly, in a study by Landfeldt and colleagues,Citation16 route of administration was found to be more important to patients than providers, a finding that is consistent with the results of the current study and reinforces the need to consider the preferences of all stakeholders in discussions of treatment options in CLL.
The current study addressed key gaps in the limited prior literature on stakeholder preferences in CLL. Specifically, prior studies, which were published in 2016–2017,Citation15–Citation17 did not include attributes and levels representing a broader array of novel therapies, as only ibrutinib was approved at that time in the 1L treatment setting. Instead, these earlier studies included choice tasks with chemotherapy and focused on the R/R setting. In addition, the few DCE studies conducted on treatment preferences in CLL have focused on the treatment preferences of multiple European stakeholders,Citation16,Citation17 and the sole US-based study only evaluated the preferences of patients.Citation15 As such, the perspectives of a broader array of key US stakeholders had been missing. The present study makes important contributions to the understanding of US patient and oncologist preferences in the context of the most current standards of care in 1L treatment for CLL.
Prior research has shown that when treatment decisions are aligned with patient preferences, patients report greater treatment satisfaction and adherence and have better clinical outcomes.Citation18 Furthermore, patients who take a more active role in decisions about their own healthcare have fewer emergency room visits and hospitalizations.Citation19 As a consequence, although there was considerable alignment in oncologist and patient preferences in the current study, the areas where they diverge warrant closer attention. It is important for oncologists to consider those aspects of treatment that are most valued by their patients when making treatment recommendations, and to recognize that patients’ preferences may differ from oncologists’ own preferences. As we increasingly follow a shared decision-making model in clinical practice, eliciting patients’ values and preferences is particularly important.
Limitations
The current study provides novel insights into the preferences of patients and oncologists; however, the following limitations should be noted. Our study used a convenience sampling technique, which may have led to a sample that is not generalizable to the broader CLL population. It is possible that younger, healthier patients or those interested in research may have been more likely to participate in the study, resulting in selection bias. Of note, the mean age of our study population was below the mean age of diagnosis for CLL. To address that limitation, subgroup analyses were conducted to evaluate the potential impact of age and other patient characteristics on preferences. Further, study variables were self-reported and could not be independently verified; self-reported data may also be subject to response bias, which can increase measurement error. Due to small sample sizes, oncologist subgroup comparisons may have been insufficiently powered to detect statistically significant differences. In addition, treatment preferences could differ for patients who have previously participated in a clinical trial, although this possibility will need to be examined in a future study with a larger sample of patients with this experience. Additionally, concerns about polypharmacy and potential interactions of novel therapies with medications for comorbid health conditions may influence treatment preferences with respect to different patient subpopulations than the ones included in the current study.
In addition to biases pertaining to study sampling, the DCE choice tasks may not reflect the same clinical, economic, or personal consequences of real-world treatment decisions. Notably, self-reported preferences may diverge from actual treatment decisions, and the DCE cannot include all possible factors that may underlie individuals’ treatment preferences. For instance, costs may influence preferences, and we cannot exclude the possibility that preferences may have varied if treatment costs had been considered among the attributes presented, particularly given the high costs of novel therapies. However, treatment cost was not considered in this study, as our primary focus was on how efficacy-, safety-, and dosing-related attributes influence preferences. Furthermore, grouping together administration form (IV vs oral) and frequency/duration of therapy (treat to progression vs treat for 12 months vs treat for 24 months) into a single attribute may have masked the unique contribution of each of these factors to treatment preferences. This is an area that would benefit from further research, given the increased number of treatment options available for CLL. Although choice tasks are not the same as making an individual treatment selection for a patient, the DCE was carefully designed to include the feedback of the two stakeholder groups of interest, to mimic realistic 1L treatment options for CLL, and to closely align with the clinical evidence available at the time that the study was conducted.
Conclusions
This study elucidates the importance of PFS to oncologists and patients with CLL in the era of novel targeted therapies. It adds to existing literature by quantifying the trade-offs that these groups are willing to make to avoid potential risks of AEs and associated treatment discontinuation. Important differences between the two groups emerged, with oncologists requiring much higher increases in PFS than patients to accept increased risks of AEs and treatment discontinuation. These data suggest that patients and oncologists may perceive the risks and benefits associated with novel agents differently. Patient–oncologist communication could be enhanced through a discussion of the risks of AEs, relative to treatment outcomes, with a focus on available novel therapies.
Abbreviations
1L, first-line; AE, adverse event; AF, atrial fibrillation; BCL-2, B-cell lymphoma-2; BTK, Bruton’s tyrosine kinase; CLL, chronic lymphocytic leukemia; DCE, discrete choice experiment; PFS, progression-free survival; RI, relative importance; TLS, tumor lysis syndrome; US, United States.
Acknowledgments
The study was funded by AstraZeneca. The authors acknowledge Thomas Campbell, PhD, for his input on the study design, Kathleen Beusterien, MPH, for her input on interpretation of results, and Errol J. Philip, PhD, for his assistance with manuscript preparation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal, which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
HL, KR, and SKW: Employees of AstraZeneca. MCM, EM, and OW: Employees of Kantar, which received funding from AstraZeneca to conduct this study. TWL: Personal fees for consulting or advisory boards: AbbVie, Agios, Amgen, AstraZeneca, BMS/Celgene, CareVive, Daiichi–Sankyo, Otsuka, Pfizer, Seattle Genetics; Royalties: UpToDate; Speakers bureaus/honoraria: AbbVie, Agios, BMS/Celgene; Grants/research contracts: American Cancer Society, AstraZeneca, CareVive, Duke University, Flatiron, Helsinn, Heron, Jazz Pharmaceuticals, NINR/NIH, Seattle Genetics. The authors report no other conflicts of interest in this work.