Abstract
Background
Non-adherence to medication (range 30–107%) is a major issue in patients with rheumatoid arthritis (RA). Previous research has shown that electronic monitoring feedback (EMF) might be an effective strategy to improve medication adherence in chronic conditions. Therefore, this study investigated the effectiveness of electronic monitoring feedback in patients with early RA to improve medication adherence and clinical outcomes compared to usual care.
Methods
An open-label randomized clinical trial was performed to compare EMF with standard care during a 12-month follow-up period on two sites of the Sint Maartenskliniek (Nijmegen and Boxmeer) in the Netherlands. Patients were eligible if they: (1) had a (working) diagnosis of early RA, (2) were currently using methotrexate, (3) were aged ≥18 years, and (4) had a life expectancy of ≥12 months. Primary outcome was the difference in proportion of non-adherent patients measured with the Compliance Questionnaire on Rheumatology after 12 months. Secondary outcomes were beliefs about medicines, medication adherence measured with the MMAS-8®, patients' health status, prescription of biologic DMARDs, and disease activity after 12 months.
Results
Of the 367 initially-invited patients, 93 patients with early RA agreed to participate in this study. No significant difference was found in the proportion of non-adherent patients between the intervention arm and the usual care arm after 12 months follow-up (60.0% and 61.3%, p=0.93, respectively). Patients in the intervention arm tended to discontinue methotrexate earlier than patients in the usual care arm (median time in weeks: 15.7 (9.1–33.6) and 21.9 (19–28.4), respectively, p=0.31), whereas patients in the usual care arm tended to initiate biologic DMARDs earlier than those in the intervention arm (median time in weeks: 11.9 (5.7–22) and 17 (9.9–40.9), respectively, p=0.55).
Conclusion
This study illustrates the challenge of targeting non-adherence with EMF in patients with early RA and shares important lessons learned about designing adherence intervention trials with respect to study attrition, accounting for drug survival, intervention fidelity, intervention uptake, and technical aspects.
Introduction
In rheumatoid arthritis (RA), maximum treatment benefits can only be achieved when patients take their disease-modifying antirheumatic drugs (DMARDs).Citation1,Citation2 However, previous research revealed that medication adherence to DMARDs ranged from 30–107%, depending on the measurement methods used to assess medication-taking behavior.Citation3–Citation5 Non-adherence to DMARDs is associated with higher disease activity scores and an increased risk of radiographic damage.Citation2,Citation6–Citation8 Since irreversible articular damage mainly occurs early in the course of RA, with most rapid radiologic progression during the first two years after diagnosis, it is likely that patients with early RA will benefit most from adherence-improving interventions.Citation9,Citation10 Additionally, intervening in non-adherence to medication in the early course of RA might prevent or delay the initiation of the more expensive biologic DMARDs (bDMARDs), which has the potential to reduce healthcare expenditures and might possibly be cost-effective.Citation11 Therefore, effective interventions to improve medication adherence in the early course of RA are warranted.
So far, studies on adherence-improving interventions in patients with rheumatic diseases are limited, with studies often reporting inconsistent results on adherence and clinical outcomes.Citation12,Citation13 However, electronic monitoring feedback (EMF) is considered to be one of the most promising interventions in improving adherence outcomes in adult patients.Citation13–Citation15 EMF is defined as tailored feedback (instant or non-instant) on electronically obtained adherence data to improve or sustain medication-taking behavior.Citation13,Citation14 Electronic devices, such as Medication Event Monitoring Systems (MEMS), register time intervals between consecutive openings of MEMS bottles and, therefore, provide insight in medication adherence patterns of patients based on device usage.Citation5,Citation14,Citation16,Citation17 These data can be used to (1) confront patients with their own medication-taking behavior, and (2) support healthcare professionals in providing tailored and non-judgmental feedback.Citation14 Such feedback includes the elicitation and strengthening of patients' intrinsic motivation to overcome practical and perceptual barriers to adequate medication intake in daily life (eg concerns about the prescribed medication and beliefs related to medication necessity), and to overcome knowledge gaps regarding DMARD treatment.Citation14 Several barriers to adequate medication intake might exist in patients with chronic conditions, such as therapy-related factors, condition-related factors, patient-related factors, socio-economic factors, and health system-related factors.Citation18–Citation21 EMF provides the opportunity to identify such barriers to adequate medication intake, thereby anticipating the large (intra- and interpatient) variety over time. The barriers identified during these feedback conversations can subsequently act as targets for intervening for individual patients.Citation14 In the early course of RA, methotrexate is considered to be an excellent candidate to improve medication adherence and clinical outcomes in the long term.Citation8 Randomized controlled trials on the effectiveness of EMF on medication adherence and clinical outcomes in patients with early RA are, however, lacking.Citation12,Citation14
Therefore, the primary objective of this study was to examine the effectiveness of providing EMF in standard care for patients with early RA, using methotrexate, in improving medication adherence compared to standard care without EMF. The secondary objective was to examine the effectiveness of the intervention on patients’ disease activity, health status, beliefs about medicines, and time to first anti-TNFα prescription.
Methods
The CONSORT (Consolidated Standards of Reporting Trials) statement and EMERGE (ESPACOMP Medication Adherence Reporting Guideline) were used as guidance for adequate reporting in this study (see Appendix A1).Citation22,Citation23
Trial Design
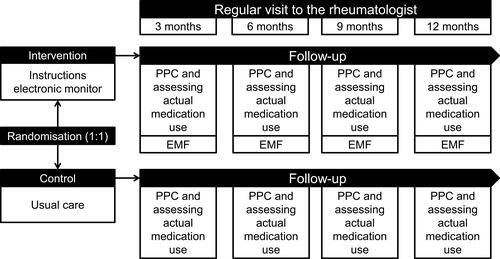
This open-label, randomized clinical trial assessed the effectiveness of providing EMF embedded in standard care for patients with early RA (Netherlands Trial Register: Trial NL4532 (NTR4667)). This study was conducted according to the ethical principles for medical research as stated in the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, 2013). The research protocol was submitted for consideration, comment, guidance and approval to the Medical Research Ethics Committee (MREC) of Arnhem-Nijmegen before the study began. The MREC of Arnhem-Nijmegen waived official ethical approval (file number: 2014–137) and assessed the trial as not being subject to the Medical Research Involving Human Subjects Act (WMO). Providing electronic monitoring feedback in addition to standard care was not considered an infringement of the physical and/or psychological integrity of the subject.
Participants and Selection Procedures
Patients were recruited on two sites of the Sint Maartenskliniek (Nijmegen and Boxmeer) in the Netherlands. All consecutive patients who fulfilled the following inclusion criteria were eligible to participate in this study: (1) a (working) diagnosis of early RA (≤ one year) by a rheumatologist, (2) currently using oral or subcutaneous methotrexate, (3) aged ≥18 years, (4) sufficient proficiency in the Dutch language, (5) no large cognitive limitations, (6) no assistance in taking drugs (ie home care or pill boxes), and (7) a life expectancy of at least 12 months given the one-year follow-up. A first selection was made by a local researcher who screened for eligibility based on the dispensing data of the outpatient pharmacy of the Sint Maartenskliniek. For each patient using methotrexate, the (working) diagnosis was checked in their medical file or verified by their rheumatologist. Subsequently, after receiving permission from the treating healthcare professional, written information with an informed consent form and return envelope were sent to all eligible patients on behalf of the research team. An independent rheumatologist (ie not a member of the research team) was available to discuss this information. After one week, the researcher contacted these patients by telephone to explore their willingness to participate in this study, to check for additional inclusion criteria, and to answer questions regarding this study. After a patient agreed to participate in this study, a research appointment during their next visit to the Sint Maartenskliniek was planned to facilitate their signing of the informed consent form and perform baseline measurements.
Study Arms
Intervention Arm
During the 12-month study period, patients allocated to the intervention arm received their methotrexate packaged in an electronic device to provide them with electronic monitoring feedback. In the case of discontinuation of methotrexate, patients left the study. After inclusion of 62 patients, medication blisters (MediccineTM system, Confrérie Clinique) were replaced by Medication Event Monitoring Systems (MEMS, Aardex®) due to quality issues related to the usability of the blisters.
Patients in the intervention arm were provided with EMF before each regular consultation (on average, two times during a 12-month period) with the rheumatologist. The intervention providers (N=6, all pharmacists, 66.7% female) completed training in motivational interviewing (ie at least one full day's training) to master the basic motivational interviewing techniques and to practice the communication strategy based on a semi-structured conversation model (see Appendix A2 and Figure A2).Citation24,Citation25 This model was used as basis for an open, patient-tailored and non-judgmental conversation between patients and trained pharmacists in which the following topics were discussed: (recognition of their own) medication-taking behavior based on electronic device usage, practical barriers, perceptual barriers (eg concern beliefs about the prescribed medication and beliefs related to medication necessity), information needs, implementation in daily life, and goal setting.
Control Arm
Patients in the control arm received standard care (ie a consultation with the pharmacy consultant without the use of electronic monitors and EMF before each visit to the rheumatologist). During this consultation the following topics were discussed: actual medication use, possible side-effects, and medication-related issues.
Study Outcomes
The primary study outcome was to identify the difference in proportion of non-adherent (taking compliance ≤80%) patients with RA after one year between the intervention arm and the usual care arm, assessed with the validated Compliance Questionnaire on Rheumatology (CQR).Citation26,Citation27 Secondary outcomes were to record time to first anti-TNFα (bDMARD) prescription, proportion of patients on anti-TNFα therapy after one year, patients' health status after one year, beliefs about medicines after one year, and mean disease activity (ie DAS28-CRP) after one year.Citation28–Citation30
Sample Size
Previous research revealed that 35% of patients with RA using a DMARD did not adhere to their treatment (taking compliance ≤80%).Citation31 Assuming that half of these non-adherent patients became adherent after receiving EMF, we expected that 65% of the patients in the usual care arm and 83% of the patients in the intervention arm would be adherent. A sample of 84 patients per study arm (ie study sample size = 168) was required to detect this difference of 18% between the intervention and control arm with a power of 80% and alpha=0.05, allowing for a 10% loss to follow-up.
Randomization and Blinding
Randomization was conducted by an independent research assistant using a computer-generated randomization list (ie generated with a random allocation procedure with a 1:1 allocation ratio). This list was transferred to sheets of paper and these sheets of paper were sealed in opaque envelopes. With every new patient enrolled in this study, a new envelope was opened by the researcher to allocate patients to the different study groups. Blinding of patients and researchers was not feasible since electronic drug monitors were only used in the intervention arm.
Measurement Instruments
In both study arms, self-reported medication adherence was measured with the validated Compliance Questionnaire on Rheumatology (CQR, 19 Likert-scaled items, item scores ranging from 1 to 4). Self-reported medication adherence was operationalized as “taking compliance” calculated with the discriminant function for CQR items as described by de Klerk et al.Citation27 The critical cut-off score of −0.5849 for taking compliance ≤80% was used to identify adherent and non-adherent patients.Citation27 The Morisky Medication Adherence Scale® (MMAS, 8 items) was used to classify patients as low (total score of <6), medium (total score of 6 or 7) and high (total score of 8) adherent.Citation26,Citation27,Citation32 Permission to use the MMAS-8 was granted by the copyright holder of this questionnaire, Donald Morisky. Necessity and concern beliefs about the prescribed medication were assessed with the Beliefs about Medicines Questionnaire Specific (BMQ-Specific, 10 Likert-scale items, item scores ranging from 1 to 5).Citation28,Citation29 These beliefs about medicines were operationalized as sum scale scores for necessity beliefs (range: 5–25), as sum scale scores for concern beliefs (range: 5–25), and as necessity–concerns differential (NCD) scores.Citation28,Citation29 NCD scores were calculated by subtracting the sum of item scores for concern beliefs from the sum of item scores for necessity beliefs.Citation24,Citation25 A positive NCD indicates that necessity beliefs dominate concern beliefs.Citation24,Citation25 Patients' health status was routinely assessed with the Health Assessment Questionnaire (HAQ, 20 items with 5 dimensions) to provide a single index value for health status. For the Health Assessment Questionnaire Disability Index (HAQ-DI), the highest subcategory score determined the value for each category, unless aids or devices were used.Citation30 Patients were included in this calculation only if at least six of the eight categories were completed. Averaging these eight category scores resulted in the HAQ-DI (range: 0–3), with higher scores indicating more disability (category 0–1: mild to moderate disability, 1–2: moderate to severe disability, 2–3: severe to very severe disability).Citation30 Disease activity was routinely assessed with the DAS28-CRP.Citation1,Citation30 The behavior change counselling index (BECCI, 11 Likert-scale items ranging from 0 “not at all” to 4 ”to a great extent”) and a quality assessment form (see Appendix A3) based on our semi-structured conversation model were used to test for intervention fidelity and to measure a practitioner’s competence in behavior change counselling (ie more comprehensive than brief advice but less extensive than applying motivational interviewing during a brief consultation).Citation33 For the BECCI data, an overall mean “practitioner BECCI score” was calculated by adding up the individual scores of the applicable items divided by that number of items. The overall mean practitioner BECCI score corresponds with the points on the Likert scales: 0 (not at all), 1 (minimally), 2 (to some extent), 3 (a good deal), and 4 (to a great extent).Citation33
Data Collection Procedures
At baseline, the following patient and clinical characteristics were extracted from patients' medical files by the local researcher: age, sex, disease duration, medication use, comorbidities, and serology (ie anti-CCP). Disease activity was routinely assessed with the DAS28-CRP every three months in the first year after diagnosis as part of standard care in the Sint Maartenskliniek. Patients were asked to complete hardcopy questionnaires concerning beliefs about medicines and self-reported medication adherence at baseline and after 12 months. The first questionnaire was completed during the first research appointment, whereas the hardcopy questionnaire after 12 months was sent to all participants with an instruction to return this questionnaire in the return envelope. Non-response to questionnaires was reduced by using one written reminder and subsequently an additional telephone call. Persistence with methotrexate and reasons for discontinuation with methotrexate were registered during the follow-up period of the study. See for an overview of the study design. Intervention fidelity was assessed by audio-recording a random set of 10% of the consultations with the intervention providers. These audio-recordings were independently assessed by a researcher and a research assistant (see Appendix A3) on topics that had to be discussed according to the intervention protocol (ie methotrexate use over the past months, necessity beliefs, concern beliefs, implementation of methotrexate’s dosing regime in daily life, and needs for additional information), and on the application of the motivational interviewing techniques (ie BECCI practitioner’s score).Citation24 On whose initiative these topics were discussed fell outside the remit of this study.
Statistical Methods
Statistical analyses were performed using STATA version 13.1. Descriptive statistics were used to describe patient and disease characteristics. P-values of ≤0.05 were considered statistically significant.
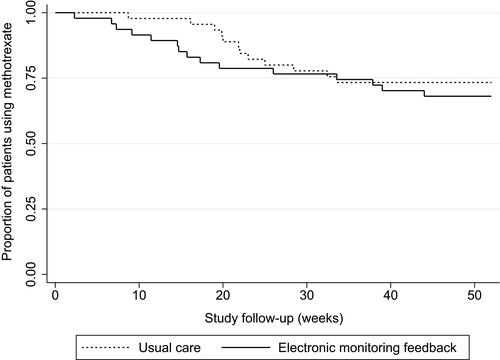
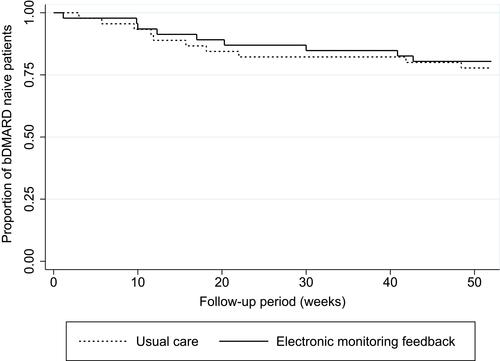
Two sample t-tests and two sample tests of proportions were performed to test for equality of means or proportions between study arms after one-year follow-up, respectively. Non-normally distributed data were presented as medians with interquartile ranges (p=25 to p=75). Two-sample Wilcoxon rank-sum tests were performed to test for differences in medians between study arms. Mixed-model procedures with intention-to-treat approach were planned for the main outcomes in this study. Between-group differences on methotrexate discontinuation and time to initiating a bDMARD were visualized with Kaplan–Meier time-to-event curves and tested with log rank testing.
Patient and Public Involvement
Two patient research partners were involved in the design phase of the intervention and the study. These patient research partners were updated annually about the progress of the study.
Results
Study Sample Characteristics
Patients were recruited between August 6, 2014 and June 8, 2018 on two sites of the Sint Maartenskliniek (Nijmegen and Boxmeer) in the Netherlands. Of the 367 initially invited eligible patients, 93 were willing to participate in this study (response rate: 25.3%; see ). Due to exceeding the scheduled period for patient recruitment (ie 47 months instead of 18 months) and lack of financial resources, this RCT ended before achieving the calculated sample size.
Figure 2 Flowchart of study participants.
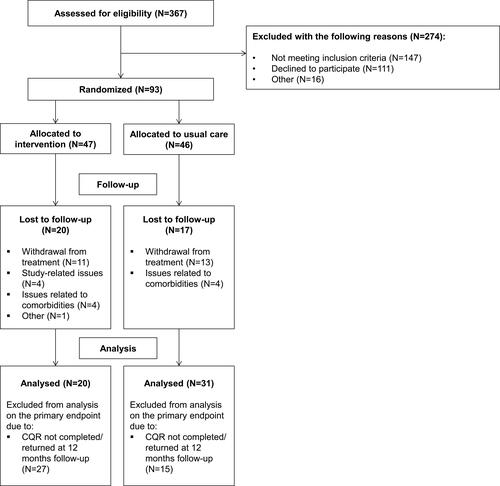
Among the 93 participating patients with RA, 65.6% was female. Their mean age was 58.9 years (SD=13.7), and 69.9% met the ACR/EULAR 2010 RA classification criteria at the time of inclusion (see for all patient characteristics at baseline). The majority of the study participants (71.0%) administered methotrexate as subcutaneous injection and two study participants allocated to the intervention arm used biologic DMARDs at time of inclusion. Overall, no relevant differences were found in baseline characteristics between the intervention arm and the control arm. Regarding attrition rates, 34 patients (36.6%) did not complete the 12-month follow-up period of the study. Main reasons for not completing the 12-month follow-up period were discontinuation of methotrexate (ie due to side-effects and/or inefficacy of methotrexate) (N=24), issues related to comorbidities (N=5), and study-related issues (N=4). This resulted in a large proportion of non-random missing data at 12-month follow-up.
Table 1 Baseline Characteristics of Study Participants for Both Study Arms
Primary and Secondary Study Outcomes
The intervention protocol could only be followed when patients were receiving active treatment with methotrexate. Due to the large proportion of patients who discontinued methotrexate (ie according to the treatment protocol), we were confronted with a large proportion of missing values (not at random) on the primary endpoint at 12 months. Therefore, we decided to deviate from the planned mixed-model procedures. Instead, all available data were presented as descriptive statistics on the primary and secondary study outcomes after 12 months (see ).
Table 2 Study Results of the RCT: Effectiveness of Electronic Monitoring Feedback (EMF) in Standard Care
No significant differences were found between the intervention arm and the usual care arm after 12-month follow-up for the following variables: proportion of adherent patients measured with the CQR (mean difference: 1.3%, 95% CI −30.0; 30.0), medication adherence measured with the MMAS-8® (median sum score: 7 (6–7) and 7 (6–7), respectively; p=0.27), sum scale scores for necessity beliefs (median: 18 (17–21) and 19 (16–20), respectively; p=0.76), sum scale scores for concern beliefs (median: 14 (9–17) and 14 (12–17), respectively; p=0.79), and necessity concern differential score (median: 4 (2–7) and 3 (2–6), respectively; p=0.56). Patients in the intervention arm tended to discontinue methotrexate earlier than patients in the usual care arm (median time in weeks: 15.7 (9.1–33.6) and 21.9 (19–28.4), respectively; p=0.31), whereas the median time to initiate a bDMARD tended to be shorter in the usual care arm than in the intervention arm (11.9 (5.7–22) and 17 (9.9–40.9), respectively; p=0.55). and visualize time to methotrexate discontinuation and time to start with a bDMARD between study arms over time. Log rank tests for equality of survivor functions showed no significant differences between study arms on methotrexate discontinuation and initiating bDMARDs (data not shown). Sensitivity analyses were avoided due to the small number of patients available for subgroup analyses.
Intervention Fidelity
Of all patients allocated to the intervention arm (N=47), 11 patients (23.4%) received no electronic monitoring feedback during the study. This was a result of the high attrition rate of study participants due to methotrexate discontinuation, together with technical issues related to the electronic circuits of the MediccineTM medication blisters during the first part of the study (ie not able to register blister openings and print read-outs). In total, 28 patients (59.6% of the intervention arm) received EMF based on MEMS devices, whereas 19 patients received at least one MediccineTM medication blister during the study. Audio-recordings (N=27) of the electronic monitoring feedback sessions were assessed by a researcher and research assistant. The mean duration of providing EMF was 8.3 minutes (SD=6.3) and electronically monitored adherence data were available for 25 patients. Two patients received no EMF due to the absence of electronically monitored adherence data. The proportion of topics discussed during the EMF consultations was as follows: concern beliefs (N=13, 48.1%), necessity beliefs (N=5, 18.5%), implementation of medication regimen in daily life (N=21, 77.8%), and patient’s need for additional information (N=15, 55.6%). Furthermore, more than half of the patients on the audio-recordings (N=15, 55.6%) showed irregular medication-intake patterns. The mean practitioner BECCI score was 1.6 (SD=0.70), which indicates that the trained pharmacists practiced behavior change counselling ranged from “minimally” to “to some extent”.
Discussion
Summary of Evidence
This study failed to show the effectiveness of EMF on self-reported medication adherence in patients with early RA. Negative findings were also found on beliefs about medicines, patients' health status, biologic DMARD use and disease activity scores after one-year follow-up. It cannot be excluded that these findings were impacted by the limitations of this study together with the encountered barriers for applying EMF in standard care. Therefore, this article shares lessons learned regarding feedback on electronically obtained adherence data as an intervention to improve medication adherence and clinical outcomes in patients with early RA in standard care. The lessons learned from this study relate to the following themes: (1) attrition, (2) drug survival, (3) intervention fidelity, (4) intervention uptake, and (5) technical issues.
Review of Findings
Several issues related to the intervention prevented drawing valid conclusions on the effectiveness of EMF in this patient population. Therefore, we conclude it is not justified to compare the findings of this study on the effectiveness of EMF with findings from previous research.Citation12–Citation14 Nevertheless, previous work indicated that EMF might be a promising intervention to enhance medication adherence in adult patients.Citation13–Citation15 It is, therefore, possible that EMF might be effective in improving medication adherence and clinical outcomes in patients with early RA, but this was not detected due to methodological issues. Statistical power issues (ie due to the small sample size of this study together with the high attrition rates after one-year follow-up) have complicated the possibility of detecting an intervention effect in this study. Similar difficulties were, however, encountered in previous adherence research (eg small sample sizes, high attrition rates, limited protocol adherence), thereby also recognizing the challenge of recruiting a representative study sample.Citation14,Citation34 Nevertheless, several methodological considerations are extensively discussed below to share the lessons learned from this study rather than concluding that this study does not contribute to the body of knowledge.
Strengths, Limitations and Lessons Learned
The key strengths of this study included a randomized controlled study design, the incorporation of disease outcomes besides adherence outcomes, the use of validated questionnaires (ie CQR and MMAS-8®) to assess medication adherence, and the long-term follow-up period since intervention effects on clinical outcomes often manifest over time. Previous work indicated that adherence rates of patients in the usual care arm were influenced by (regular) assessment of adherence to medication (ie due to awareness of being monitored).Citation35 Therefore, the authors decided to measure adherence to medication at baseline and 12-month follow-up only. Nevertheless, there is an ongoing debate on what measurement instruments and definitions for adherence outcomes should be used to accurately capture patients’ medication-taking behavior.Citation4,Citation17,Citation36–Citation39
The first lesson learned relates to attrition rate after randomization in this RCT together with drug survival. Although attrition rates did not significantly differ between study arms, the overall attrition rate contributed to statistical power issues for detecting an intervention effect. Low drug survival (ie due to methotrexate discontinuation) together with the relatively long follow-up period were the main reasons for the high attrition rate. The authors, however, did not take low drug survival into account in the power calculation.Citation40 Therefore, one of the lessons learned is that data on drug survival might be more relevant to include in power calculations than data on loss to follow-up. Furthermore, critically reviewing the planned study duration on feasibility and relevance for the study outcomes is recommended.
The next lessons learned relate to barriers/difficulties for applying EMF in standard care in patients with early RA. Regarding intervention fidelity, we found that the degree to which the trained pharmacists practiced behavior change counselling ranged from “minimally” to “to some extent” and the extent to which topics were discussed varied considerably. However, EMF is a tailored intervention and scoring the audio-recordings might, therefore, be too strict an approach upon which to decide whether the intervention protocol was (in)sufficiently followed. For future research, providing personal feedback on a regular basis to intervention providers, thereby using their own audio-recordings, is recommended. This makes it possible to intervene in case intervention providers deviate from the intervention protocol. Contamination of the intervention effect between study arms might also have occurred due to randomization on the level of patients instead of healthcare professionals. Most patients assigned to the usual care arm, however, received standard care from a pharmacy consultant instead of trained pharmacists.
Regarding intervention uptake in clinical practice, it is unknown whether this intervention fits patients’ needs and preferences in the early stage of the disease.Citation41 Patients included in this study were dealing with the ongoing process of accepting their new diagnosis and implementing chronic medication use in daily life. Several factors might have contributed to the low degree of patients’ willingness to participate in this study.Citation41 Therefore, we recommend conducting a pilot study before starting an RCT to provide insight into: (1) reasons for unwillingness to participate in a study, (2) inclusion rate, (3) practical issues, and (4) technical issues. Conducting a qualitative interview study on patients who refused to participate in this study might also provide in-depth information regarding reasons for unwillingness to participate in adherence trials. This information can be relevant for designing future adherence trials and to assess whether the study sample will be representative of the general population. Furthermore, it cannot be excluded that the technical issues encountered with the medication blisters might have negatively influenced patients’ engagement with treatment adherence. This might have strengthened the absence of an intervention effect. Therefore, we endorse performing pre-tests with innovative electronic devices to avoid technical issues during the conduct of the study.
Generalizability
The proportion of patients with “low adherence” based on the MMAS-8 at baseline was significantly lower than proportions of patients with “low adherence” reported in previous work.Citation42 Theoretically, this might indicate that patients enrolled in this study may be less able to show improvements on adherence outcomes compared with less adherent patients with RA. Considering the low mean disease activity scores at baseline for both study arms, the low response rate, and the large number of patients who discontinued methotrexate before inclusion, we conclude that selection bias prior to trial entry is likely. In addition, the high quality of standard care in the Sint Maartenskliniek might have decreased the contrast in interventions between study arms (ie standard care might not be standard care per definition). Also, the large number of study participants who were administered methotrexate subcutaneously compared to oral administration due to local treatment protocols might have limited the generalizability of the results. However, no differences were found on gender and mean age between patients who participated in this trial and patients who refused to participate in this study (female: 65.6% versus 63.5%; mean age: 58.9 years versus 57.9 years, respectively).
Conclusion
In conclusion, no evidence for the effectiveness of EMF on improving medication adherence and clinical outcomes was found in patients with early RA. Although several lessons learned can be drawn from this RCT, this study underlines the challenges that have to be overcome when conducting a behavioral intervention in a study setting. We therefore recommend learning from the lessons described in this article when designing an adherence-improving intervention, such as EMF, for patients with RA. Implementing EMF in clinical practice is not yet recommended in patients with (early) RA.
Abbreviations
ACR, American College of Rheumatology; Anti-CCP, Anti-cyclic citrullinated peptide; BECCI, Behavior Change Counselling Index; bDMARD(s), Biologic disease-modifying antirheumatic drug(s); BMQ, Beliefs about Medicines Questionnaire; CONSORT, Consolidated Standards of Reporting Trials; CQR, Compliance Questionnaire on Rheumatology; DAS28-CRP, Disease Activity Score based on 28 joints and using C-reactive protein; DMARD(s), Disease-modifying antirheumatic drug(s); EMERGE, ESPACOMP Medication Adherence Reporting Guideline; EMF, Electronic monitoring feedback; EULAR, European League Against Rheumatism; HAQ, Health Assessment Questionnaire; HAQ-DI, Health Assessment Questionnaire Disability Index; MEMS®, Medication Event Monitoring System®; MMAS-8®, Morisky Medication Adherence Scale®; NCD, Necessity–concerns differential; RA, Rheumatoid arthritis.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics Approval and Consent to Participate
This study was conducted according to the ethical principles for medical research as stated in the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, 2013). The research protocol was submitted for consideration, comment, guidance and approval to the Medical Research Ethics Committee (MREC) of Arnhem-Nijmegen before the study began. The MREC of Arnhem-Nijmegen waived official ethical approval (file number: 2014-137) and assessed the trial as not being subject to the Medical Research Involving Human Subjects Act (WMO). Providing electronic monitoring feedback in addition to standard care was not considered an infringement of the physical and/or psychological integrity of the subject. All study participants provided written informed consent before inclusion in this study. Patient data were handled according to the applicable laws and regulations. A document that linked the study codes to the patients’ identifying information was digitally stored and protected.
Principal Investigator Statement
The authors confirm that the PI for this paper is BJF van den Bemt and that he had direct clinical responsibility for patients.
Trial Registration
Netherlands Trial Register: Trial NL4532 (old NTR ID 4667, date registered July 3, 2014), https://www.trialregister.nl/trial/4532.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank all patients who participated in this study, as well as the healthcare professionals involved in this study. Special thanks are extended to the patient research partners for their assistance in the design phase of the study.
Disclosure
Dr Milou van Heuckelum reports receipt of grants from the Dutch Arthritis Foundation during the conduct of the study and grants from the Dutch Arthritis Foundation outside the submitted work. The authors reported no other potential conflicts of interest in this work.
Additional information
Funding
References
- Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–977. doi:10.1136/annrheumdis-2016-210715
- Waimann C, Marengo M, Achaval de S, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis. Consequences of low adherence. Arthritis Rheum. 2013;65:1421–1429. doi:10.1002/art.37917
- Salt E, Frazier S. Adherence to disease modifying anti-rheumatic drugs in rheumatoid arthritis patients: a narrative review of the literature. Orthop Nurs. 2010;29:260–275. doi:10.1097/NOR.0b013e3181e5c2c9
- Pasma A, den Boer E, van ’T Spijker A, et al. Nonadherence to disease modifying antirheumatic drugs in the first year after diagnosis: comparing three adherence measures in early arthritis patients. Rheumatology. 2016;55(10):1812–1819. doi:10.1093/rheumatology/kew247
- Michaud K, Vrijens B, Tousset E, et al. Real‐world adherence to oral methotrexate measured electronically in patients with established rheumatoid arthritis. ACR Open Rheumatol. 2019;1:560–570. doi:10.1002/acr2.11079
- Pasma A, Schenk CV, Timman R, et al. Non-adherence to disease-modifying antirheumatic drugs is associated with higher disease activity in early arthritis patients in the first year of the disease. Arthritis Res Ther. 2015:1–10. doi:10.1186/s13075-014-0514-0
- Hope HF, Bluett J, Barton A, Hyrich KL, Cordingley L, Verstappen SMM. Psychological factors predict adherence to methotrexate in rheumatoid arthritis; findings from a systematic review of rates, predictors and associations with patient-reported and clinical outcomes. RMD Open. 2016;2:e000171. doi:10.1136/rmdopen-2015-000171
- Wabe N, Lee A, Wechalekar M, McWilliams L, Proudman S, Wiese M. Adherence to combination DMARD therapy and treatment outcomes in rheumatoid arthritis: a longitudinal study of new and existing DMARD users. Rheumatol Int. 2017;37:897–904. doi:10.1007/s00296-017-3655-z
- Lindqvist E, Jonsson K, Saxne T, Eberhardt K. Course of radiographic damage over 10 years in a cohort with early rheumatoid arthritis. Ann Rheum Dis. 2003;62:611–616. doi:10.1136/ard.62.7.611
- Burgers LE, Raza K, Van der Helm-van Mil AH. Window of opportunity in rheumatoid arthritis – definitions and supporting evidence: from old to new perspectives. RMD Open. 2019;5(1):e000870. doi:10.1136/rmdopen-2018-000870
- Pasma A, Schenk C, Timman R, et al. Does non-adherence to DMARDs influence hospital-related healthcare costs for early arthritis in the first year of treatment? PLoS One. 2017;12(2):1–14. doi:10.1371/journal.pone.0171070
- Galo JS, Mehat P, Rai SK, Avina-zubieta A, De Vera MA. What are the effects of medication adherence interventions in rheumatic diseases: a systematic review. Ann Rheum Dis. 2016;75(4):667–673. doi:10.1136/annrheumdis-2014-206593
- Demonceau J, Ruppar T, Kristanto P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73(6):545–562. doi:10.1007/s40265-013-0041-3
- Van Heuckelum M, Van den Ende CHM, Houterman AEJ, Heemskerk CPM, Van Dulmen S, Van den Bemt BJF. The effect of electronic monitoring feedback on medication adherence and clinical outcomes: a systematic review. PLoS One. 2017;12:e0185453. doi:10.1371/journal.pone.0185453
- Herzer M, Ramey C, Rohan J, Cortina S. Incorporating electronic monitoring feedback into clinical care: a novel and promising adherence promotion approach. Clin Child Psychol Psychiatry. 2011;17:505–518. doi:10.1177/1359104511421103
- St Quinton T, Brunton JA. Implicit processes, self-regulation, and interventions for behavior change. Front Psychol. 2017;8:1–7. doi:10.3389/fpsyg.2017.00346
- Eliasson L, Vrijens B, Clifford S, Mulick A, Jackson C. How the EMERGE guideline on medication adherence can improve the quality of clinical trials. Br J Clin Pharmacol. 2020;86(4):687–697.
- Yeam CT, Chia S, Tan HCC, Kwan YH, Fong W, Seng JJB. A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos Int. 2018;29(12):2623–2637. doi:10.1007/s00198-018-4759-3
- Seng JJB, Tan JY, Yeam CT, Htay H, Foo WYM. Factors affecting medication adherence among pre-dialysis chronic kidney disease patients: a systematic review and meta-analysis of literature. Int Urol Nephrol. 2020;52:903–916. doi:10.1007/s11255-020-02452-8
- Goh H, Kwan YH, Seah Y, Low LL, Fong W, Thumboo J. A systematic review of the barriers affecting medication adherence in patients with rheumatic diseases. Rheumatol Int. 2017;37:1619–1628. doi:10.1007/s00296-017-3763-9
- Sabaté E. Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; 2003:1–175.
- Schulz K, Altman D, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c332. doi:10.1136/bmj.c332
- De geest S, Zullig LL, Dunbar-jacob J, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med. 2018;169(1):30–36. doi:10.7326/M18-0543
- Noordman J, van der Weijden T, van Dulmen S. Communication-related behavior change techniques used in face-to-face lifestyle interventions in primary care: a systematic review of the literature. Patient Educ Couns. 2012;89(2):227–244. doi:10.1016/j.pec.2012.07.006
- Linn A, van Weert J, Schouten B, Smit E, van Bodegraven A, van Dijk L. Words that make pills easier to swallow: a communication typology to address practical and perceptual barriers to medication intake behavior. Patient Prefer Adherence. 2012;6:871–885. doi:10.2147/PPA.S36195
- de Klerk E, van der Heijde D, van der Tempel H, van der Linden S. Development of a questionnaire to investigate patient compliance with antirheumatic drug therapy. J Rheumatol. 1999;26:2635–2641.
- de Klerk E, van der Heijde D, Landewé R, van der Tempel H, van der Linden S. The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: a validation study. J Rheumatol. 2003;30:2469–2475.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1–24. doi:10.1080/08870449908407311
- Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One. 2013;8:e80633. doi:10.1371/journal.pone.0080633
- Boers M, Jacobs JWG, Van Vliet Vlieland TPM, Van Riel PLCM. Consensus dutch health assessment questionnaire. Ann Rheum Dis. 2007;132–133. doi:10.1136/ard.2006.059451
- van den Bemt BJF, van den Hoogen FH, Benraad B, Hekster Y, van Riel PL, van Lankveld W. Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol. 2009;36(10):2164–2170. doi:10.3899/jrheum.081204
- Morisky DE, Green L, Levine D. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
- Lane C, Huws-thomas M, Hood K, Rollnick S, Edwards K, Robling M. Measuring adaptations of motivational interviewing: the development and validation of the behavior change counseling index (BECCI). Patient Educ Couns. 2005;56(2):166–173. doi:10.1016/j.pec.2004.01.003
- Jeffery RA, Navarro T, Wilczynski NL, et al. Adherence measurement and patient recruitment methods are poor in intervention trials to improve patient adherence. J Clin Epidemiol. 2012;67(10):1076–1082. doi:10.1016/j.jclinepi.2014.06.008
- Zwikker HE, van den Ende CH, van Lankveld WG, et al. Effectiveness of a group-based intervention to change medication beliefs and improve medication adherence in patients with rheumatoid arthritis: a randomized controlled trial. Patient Educ Couns. 2014;94(3):356–361. doi:10.1016/j.pec.2013.12.002
- Alili M, Vrijens B, Demonceau J, Evers S, Hiligsmann M. A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol. 2016;82(1):268–279. doi:10.1111/bcp.12942
- Vrijens B, de Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi:10.1111/j.1365-2125.2012.04167.x
- van den Bemt B, den Broeder A, van den Hoogen F, et al. Making the rheumatologist aware of patients’ non-adherence does not improve medication adherence in patients with rheumatoid arthritis. Scan J Rheumatol. 2011;40:192–196. doi:10.3109/03009742.2010.517214
- Van den Bemt BJF, Zwikker HE, Van den Ende CHM. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337–351. doi:10.1586/eci.12.23
- Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol. 2016;43(11):1997–2009. doi:10.3899/jrheum.151212
- Allemann SS, Nieuwlaat R, Van den Bemt BJF, Hersberger KE, Arnet I. Matching adherence interventions to patient determinants using the theoretical domains framework. Front Pharmacol. 2016;7:1–11. doi:10.3389/fphar.2016.00429
- Gadallah MA, Boulos DNK, Gebrel A, Dewedar S, Morisky DE. Assessment of rheumatoid arthritis patients’ adherence to treatment. Am J Med Sci. 2015;349:151–156. doi:10.1097/MAJ.0000000000000376