Abstract
Purpose
Several adjuvant phase III trials are evaluating cyclin-dependent kinase 4/6 inhibitors (CDK4/6is) in combination with endocrine therapy (ET) in hormonal receptor positive (HR+)/human epidermal growth factor receptor 2 negative (HER2-) early-stage breast cancer (eBC). This study examines preferences for this combination regimen and ET alone among patients, oncologists, and payers in the United States.
Methods
A web-based questionnaire, including a discrete choice experiment (DCE), was administered to patients, practicing oncologists, and payers. In the DCE, respondents selected between hypothetical treatment profiles with attributes associated with ET monotherapy and CDK4/6i + ET regimens. Each treatment alternative was defined by the following attributes: 5-year invasive disease-free survival (iDFS), nausea, diarrhea, neutropenia, alopecia, dosing schedule, and electrocardiogram (ECG) monitoring. Payers had the additional attribute of annual per-patient treatment cost. Hierarchical Bayesian models were used to estimate relative preference weights for each attribute-level and relative attribute importance.
Results
For patients (n=300) and oncologists (n=200), iDFS was most important (2 to 3 times more important than the next most important attribute), followed by neutropenia and diarrhea risks for patients and oncologists, respectively. Patients and oncologists required an improvement in iDFS of 8.0 and 5.6 percentage-points, respectively, to accept an increase in diarrhea risk from 11% to 81%. Payers (n=60) viewed annual per-patient cost as most important for treatment access decision-making, closely followed by iDFS. Payers required an improvement in iDFS of 21.8 percentage-points to accept an increase in cost from $5,100 to $149,400. Across all stakeholder groups, dosing schedule, alopecia risk, and ECG monitoring were perceived as least important.
Conclusion
Patients, oncologists, and payers expect a large absolute risk reduction in efficacy to offset the potential risks and costs of adding a CDK4/6i to current standard of care. An open discussion between all stakeholders is necessary to ensure that decision-making, whether at patient- or system-level, is informed by preferences for novel treatments, like CDK4/6is.
Introduction
Breast cancer (BC) is the most common cancer in women and the leading cause of cancer deaths among women, globally. Despite advances in diagnosis and treatment, BC remains a significant global burden.Citation1 Hormone receptor positive (HR+)/human epidermal growth factor receptor 2 negative (HER2-) disease is the most common subtype of BC, comprising approximately two-thirds of all BCs.Citation2 Endocrine therapy (ET), typically tamoxifen or an aromatase inhibitor (AI), is the mainstay of treatment for patients with HR+/HER2- early-stage BC (eBC) to reduce the risk of recurrence and mortality.Citation3 However, a risk of recurrence remains despite treatment with standard ET, particularly among patients with high-risk features.Citation4 One strategy currently under investigation is the addition of a cyclin-dependent kinase inhibitor (CDK4/6i) to standard adjuvant ET.
Currently, three CDK4/6i, palbociclib, abemaciclib, and ribociclib, are approved for use in the advanced BC setting in the United States (US). The addition of a CDK4/6i to ET (hereafter referred to as CDK4/6i + ET) has demonstrated significantly improved progression-free survival among patients with advanced or metastatic BC.Citation5 On this basis, several phase III trials have recently been conducted or are presently underway to evaluate the efficacy of adjuvant CDK4/6i in combination with ET, with the aim of demonstrating a significant improvement in invasive disease-free survival (iDFS).Citation6–Citation8 However, these studies have thus far yielded mixed evidence.
The benefit of improving iDFS (a combination of BC and mortality events) achieved by combination therapy must be weighed against the additional safety risks and treatment costs. CDK4/6i + ET regimens are associated with toxicities distinct from tamoxifen or AIs alone.Citation9–Citation13 Hematological toxicities, primarily neutropenia, are the main adverse events (AEs) experienced by patients receiving CDK4/6i.Citation14 As with most cancer treatments, CDK4/6is can also induce gastrointestinal (GI) AEs, including nausea, vomiting, and diarrhea.Citation14 Alopecia is also an AE associated with all three CDK4/6is, with patients having at least a two-fold higher risk of experiencing grade 1 or grade 2 hair loss.Citation14 Additionally, an association between ribociclib and QTc prolongation has been reported and consequently, patients taking this medication are required to undergo electrocardiogram (ECG) monitoring.Citation15 Estimated rates of any grade 3/4 AEs reported in prior clinical trials in the metastatic setting for the CDK4/6i treatment arm were 71.2% to 83.1% (vs 22.1% to 25.0% for control arm), 55.0% (vs 21.7% for control arm), and 81.1% (vs 32.7% for control arm) for palbociclib (PALOMA-3),Citation16 abemaciclib (MONARCH 3),Citation11 and ribociclib (MONALEESA-2),Citation12 respectively. It should be noted that most AEs are manageable with dose modification and supportive care measures. In terms of costs, the monthly price of ET monotherapy is less than US $50, compared with over US $12,000 for a CDK4/6i.Citation17 Secondary costs may also be higher, due to the need for more frequent monitoring to detect and treat AEs.Citation18 It is possible that this may create a barrier to treatment, as both private insurance plans and single-payer national healthcare systems may potentially be less willing to accept higher treatment costs and therefore may be less likely to grant access to these drugs. For patients, the access to CDK4/6i and associated out-of-pocket costs largely depend on their insurance status and insurance plan and can vary greatly.
Prior studies have evaluated treatment preferences for surgery and/or chemotherapy in advanced/metastatic BC;Citation19–Citation21 more recently, preferences for CDK4/6i + ET regimens have also been examined in the advanced/metastatic setting.Citation22 However, such data are lacking in the adjuvant setting. This study aimed to understand patient, oncologist, and payer preferences for CDK4/6i + ET regimens for treatment of HR+/HER2- eBC. Specifically, this study sought to determine the trade-offs that these stakeholders are willing to make among key adjuvant treatment-related attributes (eg, efficacy vs safety), the relative importance of treatment attributes, as well as the degree to which these features may impact their decision to choose a CDK4/6i + ET regimen.
Participants and Methods
Study Design
This study was conducted in two phases: cognitive interviews to confirm questionnaire content and a cross-sectional online questionnaire that included sections designed to elicit preference for adjuvant treatment-related attributes. Questionnaires were administered to respondents representing three stakeholder groups: (1) patients with eBC, (2) oncologists who treat patients with eBC, and (3) payers. This research was conducted according to the recommendations of the International Society of Pharmacoeconomic and Outcomes Research (ISPOR) Good Research Practices for Conjoint Analysis Task Force.Citation23 All study participants endorsed an electronic informed consent form, and the study protocol received an exemption determination from Pearl Institutional Review Board (Indianapolis, IN, USA).
Participants
All patient respondents were adult women (≥18 years) with a diagnosis of stage II or III HR+/HER2- BC who had received adjuvant ET. Oncologists were board-certified and practicing for between 2 and 35 years with at least 50% of their time dedicated to direct patient care. They were required to have managed a minimum of 25 patients with BC and 10 patients with HR+/HER2- eBC, as well as treated at least one patient with HR+/HER2- eBC using adjuvant therapy, in the previous three months. Both patients and oncologists were identified through the Lightspeed Research general panel.Citation24
Payers consisted of current pharmacy and medical directors and other decision-makers (>2 years in role) who serve on a Pharmacy and Therapeutics (P&T) committee, are involved in coverage and reimbursement decisions for BC treatments, and are aware of oral oncology therapeutics for BC. Their organization was required to be a national managed care organization (MCO), regional/state MCO, pharmacy benefit manager (PBM), or Blues affiliate with plans of ≥450,000 covered lives for medical benefits and/or ≥350,000 covered lives for pharmacy benefits, or alternatively an integrated delivery network (IDN) or hospital. Payers were recruited via a payer panel affiliated with Kantar. All respondents were based in the US.
Questionnaire Design
Discrete choice experiments (DCEs) were used to assess stakeholder treatment preferences. These involved a series of choice tasks presenting hypothetical treatment profiles that varied with respect to their attributes, including efficacy and risk of toxicities, and respondents chose which they preferred.Citation25 By contrasting the attribute levels across choice tasks, DCEs force respondents to make trade-offs between the presented treatment profiles, which differs from methodologies that simply involve ranking or rating treatment attributes.Citation26
The DCE completed by participants involved the choice between two treatment profiles varying on seven attributes associated with ET monotherapy (AI or tamoxifen) and CDK4/6i + ET combination regimens ( shows an example DCE choice task). The wording for the choice tasks differed between stakeholder groups, as appropriate; for example, non-clinical terminology was used for the patient survey. The purpose of the DCE was to assess the trade-offs made across all adjuvant treatments, including CDK4/6i combinations and ET monotherapy.
Figure 1 Example DCE choice task.
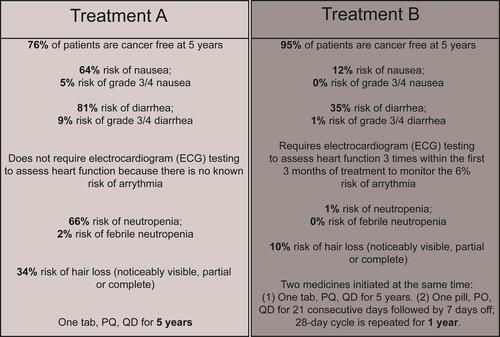
lists the attributes and levels shown in the DCE for patients, oncologists, and payers. Given that clinical trial data were not yet available for CDK4/6is in eBC at the time the study was conducted, the lowest and highest levels for each AE were based on registrational trials for CDK4/6i and ET combinations in the advanced HR+/HER2- BC setting,Citation10,Citation12,Citation13,Citation27–Citation29 as well as key trials for ET in the eBC setting.Citation30,Citation31 Efficacy was estimated based on results from clinical trials evaluating ET in eBC.Citation32,Citation33 For patients and oncologists, instructions for each choice task asked which profile was most preferred or most preferred to prescribe, respectively. For payers, instructions were to identify which profile they would most prefer to provide favorable access, ie,
Assuming everything else to be the same about the imaginary adjuvant treatment options below for early breast cancer, which would you most prefer to provide favorable access, with few hurdles or little management?
Table 1 Attributes and Levels Included in the DCE
In each survey, the DCE choice tasks were preceded by a section to help familiarize respondents with the DCE attributes and levels. Specifically, respondents were asked to rate the different treatment levels from “very bad” to “very good”. The survey also collected demographic data from all participants and clinical, practice, and plan characteristics data for patients, oncologists, and payers, respectively. The surveys were initially pilot tested in cognitive interviews with 10 patients with eBC, 10 practicing oncologists, and 6 payers to ensure that key treatment attributes were included, and item wording was clear, appropriate, and understood as intended.
Analysis
First, the consistency of responses to the very bad/very good rating scales was examined to identify those whose response patterns were indicative of inattention in completing the survey. Respondents were excluded from analysis if they failed validity checks including completing the survey much faster than expected (ie, completing the entire survey in <50% median completion time or completing the set of choice tasks in an average of <5 seconds per task) or providing two or more inconsistent ratings on the very bad/very good scales. Demographics and characteristics of the study sample were described using means and standard deviations (SDs) or medians and interquartile ranges (IQRs), as appropriate, for continuous variables, with frequencies and percentages reported for categorical variables.
Hierarchical Bayesian logistic regression models with effects coding were used to estimate relative preference weights for each attribute level in the DCEs. The outcome variable of this model was treatment choice, and the predictor variables were the levels within each attribute. The resulting parameter estimate for each attribute levels represents the preference weight. A higher preference weight for a specific attribute level indicates a greater likelihood that a treatment including that attribute level will be chosen. By directly comparing the relative importance of a change in the levels of one attribute to the change in levels of another attribute, the magnitude of the trade-offs that stakeholders are willing to make among the attribute levels was assessed.
To assess the importance of each attribute relative to the others in the study, relative importance estimates were calculated. The range of each attribute (the utility of the most favorable level minus the utility of the least favorable level) was divided by the sum of the ranges of all attributes and then multiplied by 100 and averaged for each stakeholder group. These estimates are ratio-scaled (eg, an attribute with a relative importance of 20% is twice as important as an attribute with a relative importance of 10%). The percentage point increase in iDFS required by participants to accept a change from the most to the least favorable attribute-levels for each safety and dosing attribute was computed. Using the range of both the attribute-levels and their associated preference weights for 5-year iDFS, a preference weight was calculated for each incremental unit-level change in the attribute. The unit-level preference weight was then multiplied by the range of the other attributes to determine the increase in 5-year iDFS over the base level of 76% to accept an increase of each safety and dosing attribute independently. To align these calculations with the most recent clinical trial safety data,Citation34 interpolation was used to estimate preference weights corresponding with a maximum level of 45% for risk of neutropenia.
The analyses were performed using the software packages SAS v9.3 (SAS Institute Inc., Cary, NC, USA), SPSS v25.0 (IBM, Armonk, NY, USA), and Sawtooth’s Lighthouse Studio, 2018 (Sawtooth Inc., Orem, UT, USA).
Results
A total of 310 patients with eBC, 216 oncologists, and 60 payers met eligibility criteria and completed the study survey. Of these, the data from 10 patients and 16 oncologists were flagged for failing validity and quality checks. Analyses were initially run with and without the flagged data. As the resulting preference weights diverged, the data from these respondents were excluded from further analyses, thus, the final sample included in the analyses consisted of 300 patients, 200 oncologists, and 60 payers.
Sample Characteristics
Sample characteristics are provided in and . The mean age of patients was 58.9±10.1 years. Most patients were diagnosed with stage II BC (73.3%) with a mean time of 7.3±7.1 years since diagnosis. Most patients were post-menopausal (natural: 46.7%, induced: 47.7%) and just over half of all patients were currently receiving treatment for their BC (56.3%). Oncologists reported a mean of 16.0±7.6 years in practice, and a majority (52.0%) were in a community-based solo or group practice. They had seen/treated a mean of 52.0±53.9 patients with HR+/HER2- eBC in the past three months, of which 40.4±46.0 were receiving adjuvant treatment. The majority of payers worked for a national/regional MCO (51.7%) with plans managing both pharmacy and medical benefits (60.0%). Payer plans represented a median of 1.2 million lives and 1.0 million lives with pharmacy and medical benefits, respectively.
Table 2 Sample Characteristics: Patients and Oncologists
Table 3 Sample Characteristics: Payers
Attribute-Level Preference Weights
Mean preference weights increased with improvements in attribute levels. With the exception of ECG requirements for payers, preference weights were statistically significant, indicating that each had an influence on treatment preferences. The preference weights and their 95% confidence intervals (CIs) are presented in for patients and oncologists and for payers. The magnitude of the difference across attribute-levels reflects the strength of preference for the change.
Figure 2 Preference weights for (A) patients, oncologists and (B) payers.
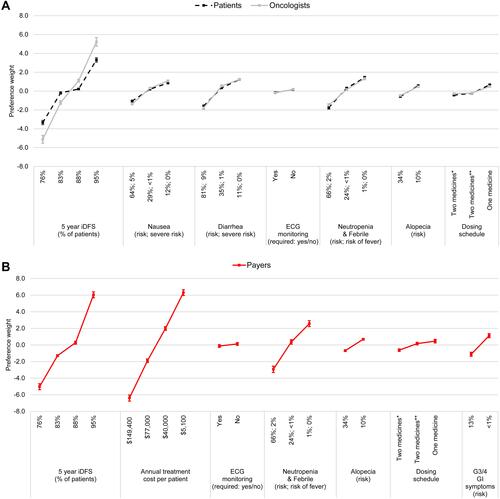
Attribute-level preference weights were similar between patients, oncologists, and payers. Improvements in 5-year iDFS from 76% to 95% were more important, relative to improvements in other attributes for both patients (difference in preference weights=6.6) and oncologists (10.3). While this attribute was also important to payers (11.1), it was less important than reducing annual treatment cost per patient from $149,400 to $5,100 (12.7). For patients, reducing the risk of neutropenia from 66% to 1% was more important than reducing the risk of diarrhea from 81% to 11% (3.2 and 2.8, respectively), while the opposite pattern was observed for oncologists (2.8 and 3.0, respectively).
The differences in preference weights were compared to those among other attributes to assess trade-offs that respondents were willing to make when choosing a treatment; ie, if a difference between two levels of one attribute was similar to or less than the difference between two levels of another attribute, this indicated a willingness to accept a worsening of one for an improvement in the other. Patients would be willing to accept an increased risk of neutropenia from 1% to 66% (difference in preference weights=3.2) in exchange for an increase in iDFS from 76% to 88% (3.6). Payers would accept an increased risk of neutropenia from 1% to 24% (2.2) or from 24% to 66% (3.3) for an improvement in iDFS from 76% to 83% (3.7). To compensate for an increased risk of neutropenia from 1% to 66%, oncologists were willing to accept an improvement in iDFS from 76% to 83% (2.8 vs 3.9). Regarding the remaining safety-related attributes, both patients and oncologists were willing to trade-off the largest increase possible in the risks of diarrhea, nausea, and alopecia for an improvement in iDFS from 76% to 83%. For improvements in iDFS from 76% to 88%, payers were willing to accept an increase in annual treatment cost per patient from $5,100 to $40,000 (5.3 vs 4.3, respectively); however, they were unwilling to accept an increase in annual per patient treatment cost from $5,100 to $149,400 in exchange for a larger improvement in iDFS from 76% to 95% (12.7 vs 11.1, respectively).
Efficacy Improvements Needed to Accept the Highest Toxicity Risks
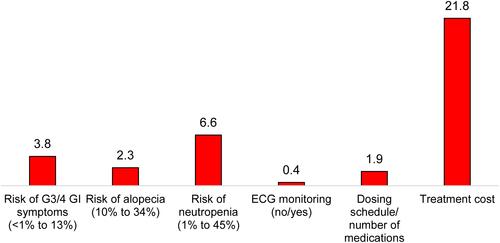
The percentage-point improvement in iDFS over the base level of 76% required to accept increases in each of the other attributes examined are shown in (patients and oncologists) and (payers). Patients required an improvement in iDFS of 8.0 and 6.1 percentage-points over the base level to offset an increase in risk of diarrhea from 11% to 81% and an increase in the risk of neutropenia from 1% to 45%, respectively. Oncologists required an improvement in iDFS of 5.6 percentage-points over the base level to offset an increase in risk of diarrhea from 11% to 81%, with 3.6 percentage-points over the base level iDFS needed to compensate for an increase in the risk of neutropenia from 1% to 45%. For payers, an improvement in iDFS of 3.8 and 6.6 percentage-points over the base level was required to offset an increase in risk of GI symptoms from <1% to 13% and an increase in the risk of neutropenia from 1% to 45%, respectively. The greatest improvement (21.8 percentage points over base level) in iDFS was required by payers in exchange for an increase in per-patient treatment cost from $5,100 to $149,400.
Figure 3 Improvement in iDFS required to accept a change from the most to the least favorable safety and dosing attribute-levels among patients and oncologists.
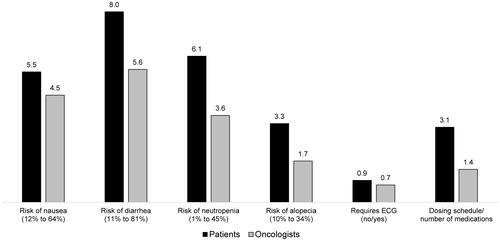
Figure 4 Improvement in iDFS required to accept a change from the most to the least favorable safety and dosing attribute-levels among payers.
Relative Attribute Importance
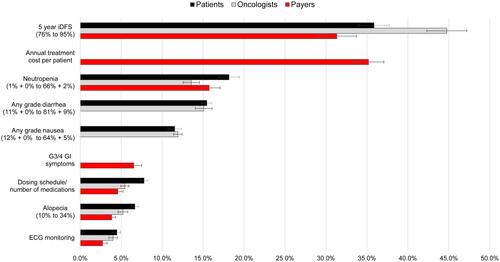
Attribute relative importance estimates are shown in . For patients, improvements in iDFS were around 2 times more important (35.9%), relative to reduction in risks of neutropenia (18.2%) and diarrhea (15.5%). Similarly, oncologists considered improvements in iDFS to be around 3 times more important (44.8%) than risk reductions in neutropenia (13.6%) and diarrhea (15.1%). Payers viewed both annual treatment costs (35.2%) and improvements in iDFS (31.3%) to be at least 2 times more important than increased neutropenia risk (15.8%). The requirement for ECG monitoring was the least important attribute for all three stakeholders.
Figure 5 Relative attribute importance for patients, oncologists and payers.
Discussion
This study examined and compared patient, oncologist, and payer preferences for CDK4/6i + ET regimens in the adjuvant setting. The results indicate that patient and oncologist preferences were mainly driven by improvements in iDFS and avoidance of neutropenia and diarrhea risks. Among payers, the most influential attribute was reducing annual per-patient treatment costs.
The finding that patients placed the greatest importance on improved efficacy is consistent with previous preference studies, which found that patients with BC are willing to accept the risk of AEs in exchange for efficacy gains.Citation19,Citation21,Citation35 Notably, the tolerance for increased risk as a trade-off for improved efficacy varied between patients and oncologists. For patients, the trade-off between AEs and iDFS was dependent on the magnitude of the benefit, which is consistent with the results of a recent study on the willingness of patients with eBC to add a CDK4/6i to their ET regimen.Citation36 Conversely, oncologists were willing to exchange a higher risk of all AEs examined for more modest improvements in efficacy.
In terms of the importance of safety-related attributes, patients considered the reduced risk of neutropenia more important than the reduced risk of diarrhea, whereas the opposite was observed for oncologists. Moreover, by using interpolation, when applicable, we were able to approximate the AE risks reported in the monarchE trial interim data;Citation34 this allowed us to determine the percentage-point improvement over base level of 5-year iDFS required to accept changing from the lowest to the highest level of risk for each safety-related attribute. Results showed that patients required greater improvements in iDFS than oncologists to accept increased risks of diarrhea and neutropenia. The different importance that patients and oncologists placed on diarrhea and neutropenia may represent divergent perspectives in the manageability of these AEs and their impact on patients’ quality of life. These findings emphasize the importance of open communication and shared decision-making among patients and oncologists in order to optimally balance efficacy outcomes with potential toxicity risks.
Little research has been performed regarding payer priorities for funding high-cost medicines in eBC. The current study’s findings provide important insights into the treatment preferences that may underlie payer decision-making. Results indicated that cost was a primary driver of treatment access decision-making among US payers. However, payers are willing to accept higher costs and additional safety considerations in exchange for the potential efficacy benefits associated with CDK4/6i + ET regimens for eBC. Nevertheless, payers appeared to be very sensitive to per-patient costs at the higher end of the spectrum. For instance, results indicated that payers would require an increase in 5-year iDFS of 97.8% (ie, 21.8 percentage points above the base level of 76%) to accept an increase in per-patient cost from $5,100 to $149,400. As interim analysis from the monarchE trial showed a 2-year iDFS of 92.2% for abemaciclib + ET,Citation34 payer expectations for efficacy improvements, relative to costs, may be unrealistic, although further data with longer-term follow-up is needed to verify this possibility. Overall, these findings can help to inform payers about the potential risk-benefit trade-offs entailed when making treatment access decisions about novel CDK4/6i regimens, relative to the current standard of care, in the adjuvant setting.
Disease recurrence in eBC is associated with high mortality rates and treatment costs.Citation33,Citation37 Pending the results of ongoing adjuvant phase III trials, CDK4/6i + ET regimens for HR+/HER2- eBC may hold promise for improving iDFS in patients, thereby filling this unmet need. However, the improvement in iDFS potentially offered by CDK4/6i + ET regimens is offset by high treatment costs and a greater risk of AEs. This study has shown that patients, oncologists, and payers were generally willing to accept this trade-off between increased risk of AEs for improvements in iDFS. Additionally, payers were willing to exchange improvements in iDFS for increased treatment costs, although the willingness to accept higher costs appears sensitive to the magnitude of improvement in iDFS. To date, the evidence available for the efficacy benefits associated with a CDK4/6i + ET regimen in the adjuvant setting is based on interim data from a single clinical trial.Citation34 Of importance, the iDFS improvements reported were based on a follow-up of roughly 15 months for a 2-year treatment duration of abemaciclib + ET, with only 12% of patients reaching 2 years of treatment duration.Citation34 Therefore, the 3.5 percentage point difference in iDFS in that analysis, which favored abemaciclib + ET over ET monotherapy, represents 2-year iDFS. It is thus unclear if this benefit will be sustained or change with longer follow-up, consequently making it challenging to apply results from the current study, which are based on 5-year iDFS, to those from monarchE.
Our results should be interpreted within the context of relevant study limitations. DCEs present hypothetical treatment scenarios that do not necessarily have the same clinical, financial, or emotional consequences as real-life decisions. DCE attributes were designed prior to the availability of clinical trial results for the adjuvant setting; however, the range of attribute-levels were selected to adequately reflect the possible ranges, particularly for safety-related attributes, which were informed by the literature on CDK4/6 + ET regimens in the advanced/metastatic BC setting. Potential hypothetical bias was minimized as much as possible by constructing choices that closely mimicked real-world trade-offs. Furthermore, the DCEs were developed by incorporating the feedback of patients, oncologists, and payers and by closely mirroring the clinical evidence and treatment costs available at the time the study was conducted. Furthermore, we relied on patient self-reporting of diagnosis, disease stage, and treatment, and this information could not be corroborated. However, a number of studies have found that this approach is reliable, with patient responses corresponding with their medical records.Citation38–Citation40 Given that costs are highly variable within the US healthcare system, especially for cancer treatments, the decision was made to narrow the scope of the study design to evaluate preferences independently of costs among patients and oncologists. Thus, we were unable to determine the extent to which costs may influence patient and oncologist preferences for CDK4/6i + ET regimens.
Conclusion
For patients and oncologists improving iDFS had the greatest influence on treatment choice, although oncologists placed greater emphasis on efficacy than patients. The different perspectives on neutropenia between patients and oncologists suggest that this side effect, in particular, deserves further discussion in treatment decision-making. For payers, cost was a primary driver when choosing therapies, with more favorable access and fewer barriers to access. Ultimately, by comparing treatment-related preferences among key stakeholder groups, the current study’s results provide a more comprehensive understanding of where priorities for CDK4/6i combination regimens in the adjuvant setting, relative to the current standard of care, are similar and where there is discordance.
Abbreviations
AI, aromatase inhibitor; AJCC, American Joint Committee on Cancer; BC, breast cancer; CDK4/6i, cyclin-dependent 4/6 kinase inhibitor; CI, confidence interval; DCE, discrete choice experiment; eBC, early-stage breast cancer; ECG, electrocardiogram; ET, endocrine therapy; G, grade; GI, gastrointestinal; HER2-, human epidermal growth factor receptor 2 negative; HR+, hormone receptor positive; iDFS, invasive disease-free survival; IDN, integrated delivery network; IQR, interquartile range; ISPOR, International Society of Pharmacoeconomic and Outcomes Research; MCO, managed care organization; NCCN, National Comprehensive Cancer Network; P&T, pharmacy and therapeutics; PBM, pharmacy benefits manager; PO, orally; QD, daily; SD, standard deviation; SVP, senior vice president; US, United States; VP, vice president.
Data Sharing Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Author Contributions
All authors contributed to data analysis or interpretation of the data, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Dr. Ann-Marie Waldron (Kantar Health GmbH, Munich) for medical writing assistance, which was funded by Pfizer. A portion of the results reported on in this manuscript was presented at the European Society for Medical Oncology (ESMO) Annual Congress, which was held virtually on September 19–21, 2020, and was presented at the Academy of Managed Care Pharmacy (AMCP) Nexus Conference, which was held virtually on October 20–23, 2020.
Disclosure
KB, BH, MMG, and MCM are employees of Kantar, which received funding from Pfizer Inc to conduct and report on the study; EHL is an employee and stockholder of Pfizer Inc; MLS is part of the advisory board for Pfizer and an advisor for Novartis. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Fitzmaurice C, Abate D, Barber RM, et al.; Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi:10.3322/caac.21583
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi:10.1016/S0140-6736(15)61074-1
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi:10.1146/annurev-med-070909-182917
- Zheng J, Wu J, Wang C, Zhuang S, Chen J, Ye F. Combination cyclin-dependent kinase 4/6 inhibitors and endocrine therapy versus endocrine monotherapy for hormonal receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: a systematic review and meta-analysis. PLoS One. 2020;15(6):e0233571. doi:10.1371/journal.pone.0233571
- Pfizer. A study of palbociclib in addition to standard endocrine treatment in hormone receptor positive Her2 normal patients with residual disease after neoadjuvant chemotherapy and surgery (PENELOPE-B). NLM identifier: NCT01864746. Available from: https://clinicaltrials.gov/ct2/show/NCT01864746. Accessed December 4, 2020.
- Eli Lilly and Company. Endocrine therapy with or without abemaciclib (LY2835219) following surgery in participants with breast cancer (monarchE). NLM identifier: NCT03155997. Available from: https://ClinicalTrials.gov/show/NCT03155997. Accessed December 4, 2020.
- Novartis Pharmaceuticals. A trial to evaluate efficacy and safety of ribociclib with endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer (NATALEE). NLM identifier: NCT03701334. Available from: https://ClinicalTrials.gov/show/NCT03701334. Accessed December 4, 2020.
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, Phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi:10.1016/S1470-2045(15)00613-0
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi:10.1056/NEJMoa1607303
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. doi:10.1200/JCO.2017.75.6155
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi:10.1056/NEJMoa1609709
- Sledge W, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. doi:10.1200/JCO.2017.73.7585
- Thill M, Schmidt M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol. 2018;10:1758835918793326. doi:10.1177/1758835918793326
- Ball S, Swarup S, Sultan A, Thein KZ. Increased risk of cardiac conduction abnormalities with ribociclib in patients with metastatic breast cancer: a combined analysis of phase III randomized controlled trials. Hematol Oncol Stem Cell Ther. 2020:S1658-3876(20)30047-30049. doi:10.1016/j.hemonc.2020.03.001
- Loibl S, Turner NC, Ro J, et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist. 2017;22(9):1028–1038. doi:10.1634/theoncologist.2017-0072
- GoodRx.com [homepage on the internet]. Breast Cancer Medications; 2020. Available from: https://www.goodrx.com/breast-cancer/drugs. Accessed September 20, 2020.
- Schaecher KL. A payer’s perspective on CDK4/6 inhibitors. J Hematol Oncol Pharm. 2018. Available from http://jhoponline.com/jhop-supplements?view=article&secid=15250:march-2018-abemaciclib-fda-approval&artid=17419:a-payer-s-perspective-on-cdk4-6-inhibitors. Accessed January 21, 2021.
- Guerra RL, Castaneda L, de Albuquerque R, et al. Patient preferences for breast cancer treatment interventions: a systematic review of discrete choice experiments. Patient. 2019;12(6):559–569. doi:10.1007/s40271-019-00375-w
- daCosta DiBonaventura M, Copher R, Basurto E, Faria C, Lorenzo R. Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am Health Drug Benefits. 2014;7(7):386–396.
- Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279–287. doi:10.2147/IJWH.S31331
- Maculaitis MC, Liu X, Will O, et al. Oncologist and patient preferences for attributes of CDK4/6 inhibitor regimens for the treatment of advanced/metastatic HR positive/HER2 negative breast cancer: discrete choice experiment and best-worst scaling. Patient Prefer Adherence. 2020;14:2201–2214. doi:10.2147/PPA.S254934
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
- Crivera C, Nelson WW, Schein JR, Witt EA. Attitudes toward anticoagulant treatment among nonvalvular atrial fibrillation patients at high risk of stroke and low risk of bleed. Patient Prefer Adherence. 2016;10:795–805.
- Hauber BA, Fairchild AO, Johnson FR. Quantifying benefit-risk preferences for medical interventions: an overview of a growing empirical literature. Appl Health Econ Health Policy. 2013;11(4):319–329. doi:10.1007/s40258-013-0028-y
- Louviere J, Hensher D, Swait J. Stated Choice Methods: Analysis and Application. Cambridge: Cambridge University Press; 2000.
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5(1):5. doi:10.1038/s41523-018-0097-z
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a Phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–5224. doi:10.1158/1078-0432.CCR-17-0754
- Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi:10.1056/NEJMoa1505270
- Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366(9484):455–462. doi:10.1016/S0140-6736(05)67059-6
- Fisher B, Dignam J, Bryant J, Wolmark N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 2001;93(9):684–690. doi:10.1093/jnci/93.9.684
- Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34(21):2452–2459. doi:10.1200/JCO.2015.63.8619
- Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group trials I to V. J Clin Oncol. 2016;34(9):927–935. doi:10.1200/JCO.2015.62.3504
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–3998. doi:10.1200/JCO.20.02514
- Wouters H, Maatman GA, Van Dijk L, et al. Trade-off preferences regarding adjuvant endocrine therapy among women with estrogen receptor-positive breast cancer. Ann Oncol. 2013;24(9):2324–2329.
- Lipton NJ, Jesin J, Warner E, et al. Willingness of women with early estrogen receptor-positive breast cancer to take adjuvant CDK4/6 inhibitors. Curr Oncol. 2020;27(3):127–134. doi:10.3747/co.27.6131
- Ritzwoller DP, Fishman PA, Banegas MP, et al. Medical care costs for recurrent versus de novo stage IV cancer by age at diagnosis. Health Serv Res. 2018;53(6):5106–5128. doi:10.1111/1475-6773.13014
- Gast KC, Cathcart-Rake EJ, Norman A, et al. Accuracy of self-reported cancer treatment data in young breast cancer survivors. J Patient Rep Outcomes. 2019;3(1):24. doi:10.1186/s41687-019-0114-5
- Kool M, Bastiaannet E, Van de Velde CJH, Marang-van de Mheen PJ. Reliability of self-reported treatment data by patients with breast cancer compared with medical record data. Clin Breast Cancer. 2018;18(3):234–238. doi:10.1016/j.clbc.2017.08.005
- Barisic A, Glendon G, Weerasooriya N, Andrulis IL, Knight JA. Accuracy of self-reported breast cancer information among women from the Ontario site of the breast cancer family Registry. J Cancer Epidemiol. 2012;2012:310804. doi:10.1155/2012/310804
