Abstract
Moderate-to-severe asthma represents about a quarter of the nearly 10% of Americans diagnosed with asthma. Many patients with moderate-to-severe asthma have uncontrolled symptoms that lead to exacerbations requiring oral corticosteroids. There are many factors contributing to poor asthma control, including poor adherence to prescribed therapies, the under-prescribing of biologics and therapeutic inertia. We convened an eight-member panel from fields of primary care, pulmonology, immunology, health services and clinical research, behavioral science and pharmaceutical medical affairs, with the goal of identifying contributing factors and solutions to therapeutic inertia with asthma biologics. We used the Capability, Opportunity, and Motivation (COM-B) model to classify patient and provider behavior towards therapeutic inertia. The model incorporates existing behavior theories and is driven by the interaction of capability, opportunity, and motivation. We used a Delphi method to identify and develop six primary solutions: 1) integration of patient-centered outcomes into asthma management practice; 2) provider education about asthma treatment; 3) moderate-to-severe asthma care delivery redesign; 4) harmonized, evidence-based protocol for the management of moderate-to-severe asthma; 5) designated coordinator approach for optimal asthma management; and 6) a case coordination digital support tool. Integration of patient-centered outcomes into asthma management practice and provider education were identified as having the highest potential to impact therapeutic and clinical inertia. The COM-B model is effective in identifying improvement within therapeutic inertia targeting the capabilities, opportunities, and motivations of patients, providers, and payer systems.
Plain Language Summary
What is already known about this subject:
For asthma patients with uncontrolled disease, the majority of moderate-to-severe patients are not prescribed biologic therapies as indicated by current guidelines.
Therapeutic inertia refers to the lack of treatment intensification according to evidence-based guidelines for those patients who are considered eligible but not receiving therapy and is often driven by complex factors involving patients, providers, and practice systems.
What this study adds:
An expert panel identified 6 solutions to address therapeutic inertia for moderate-to-severe asthma patients across patient, provider, and practice system levels by using a behavior change framework.
The integration of patient-centered outcomes and provider-focused educational solutions were identified as most significant to improve asthma management.
Asthma Prevalence and Therapeutic Inertia
Of the nearly 10% of American adolescents and adults diagnosed with asthma, about 5–10% of those with asthma have severe persistent disease.Citation1 Severe asthma is associated with asthma attacks and symptoms that limit patients’ daily living and activities like sleep, exercise, and work and school attendance. These limitations further exacerbate asthma as well as comorbidities like diabetes, obesity, anxiety and depression. Exacerbation of asthma also increases individuals’ risk of hospitalization and death, and impair quality of life.Citation2,Citation3 Patients with moderate-to-severe asthma may have Type 2 inflammation driven by inflammatory cytokines IL-4, IL-13, and IL-5.Citation4 These patients are often identified by their treatment responsiveness to inhaled or oral corticosteroids (OCS) with or without atopic comorbidities. Despite appropriate escalations in inhaled therapies, there is a significant portion of patients whose symptoms remain poorly controlled despite medium to high dose inhaled corticosteroids and/or experience adverse effects from frequent OCS requirements who would be ideal candidates for biologic initiation. With an estimated 20% of severe asthma patients having uncontrolled disease,Citation3,Citation5 the majority of moderate-to-severe patients are not prescribed biologic therapies as indicated by current guidelines.Citation6,Citation7 This lack of appropriate evidence-based treatment represents a complex issue and may be partially attributed to factors such as the need to address poor inhaler adherence, diagnostic uncertainty, an overabundance of medication choices, managing comorbidities, patient preferences and healthcare system barriers.Citation8 This lack of guideline treatment is an example of therapeutic inertia, which refers to the lack of treatment intensification according to evidence-based guidelines for those patients who are considered eligible but not receiving therapy and is often driven by complex factors involving patients, providers and practice systems.
The COM-B Framework
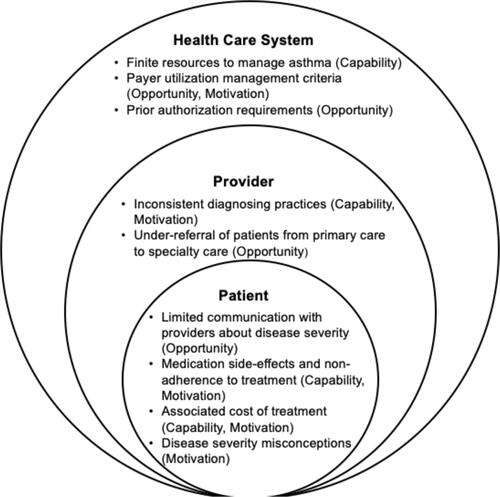
The multifaceted management of chronic disease and the practice of therapeutic inertia is intrinsically related to the behaviors of patients and providers within the context of the practice system. We used the Capability, Opportunity, and Motivation (COM-B) model to qualitatively describe the behaviors that influence the drivers of therapeutic inertia for the management of moderate-to-severe asthma.Citation9 The COM-B model was selected prior to the workshop to help facilitate framing and categorizing factors that may contribute to challenges using biologics for individuals with moderate-to-severe asthma. The COM-B model was selected because it is a comprehensive framework used to facilitate, understand, and affect behavior change. Capability (C) refers to the physical and physiological capacity to engage in a target activity; Opportunity (O) refers to the external influences that impact a behavior; and Motivation (M) refers to the reasons underlining a behavior. Each of the three COM-B parts can operate independently to impact behavior, though Capability and Opportunity can impact Motivation and thus effect Behavior (B).Citation10 Provider, patient, and practice system drivers of therapeutic inertia in moderate-to-severe asthma management are associated with the domains of the COM-B model ().
At the provider level, therapeutic inertia can be attributed to inconsistent diagnosing practices among providers (Capability, Motivation) and the under-referral of patients from primary to specialty care (Opportunity). Therapeutic inertia is driven by an inconsistency in diagnosis between primary care physicians, allergists, and pulmonologists– health providers most familiar with the diagnosis and treatment of asthma. This inconsistency in diagnosis can lead to differences in treatment practice. While primary care physicians may provide an initial asthma diagnosis, allergists primarily diagnose allergic asthma and report prescribing biologics to 5% more moderate-to-severe asthma patients than controlled patients, while pulmonologists diagnose a greater variety of asthma types.Citation6,Citation11 This discrepancy in diagnosing asthma types and subsequently prescribing appropriate treatment modalities related to such diagnoses partially explains the therapeutic inertia towards asthma biologics. However, this inconsistency is further underscored by the under-referral of patients from primary care to asthma-focused specialists, partially by restrictions put in place by healthcare systems and limited access to specialists. Referrals to specialty care have been identified to occur primarily following significant exacerbations and emergency room visits by moderate-to-severe asthma patients; however, a referral to an asthma specialist is recommended by Global Initiative for Asthma (GINA) at step 5, or when the patient requires high dose ICS-LABA to control disease. The lack of prompt recognition and specialist referral for patients requiring high dose ICS-LABA or other combinations of therapy may limit the appropriate prescribing of biologics to eligible patients.Citation12–Citation14
There are several patient level factors that may contribute to provider therapeutic inertia. These patient-level factors can reflect poor patient-provider communication (Opportunity) which may result in non-adherence to both inhaled and biologic treatments (Capability, Motivation). Patient behavior towards non-adherence of asthma treatment is driven by the potential of medication side-effects and improper administration technique (Capability, Motivation), associated cost of treatment (Opportunity), and the misperceptions of the severity of their asthma (Motivation).Citation15–Citation17 Discrepancies in the patients’ perception of condition severity can stem from a limited understanding of chronic asthma management, including the risk and benefits of asthma treatment and low-awareness of biologic treatment options. Similarly, logistical and systemic barriers like high out-of-pocket costs, insurance approval, time, and transportation to and from treatment sites impact patients’ engagement in and adherence to biologic treatment, contributing to therapeutic inertia.Citation12,Citation18 For some medications, there are now home injections, COVID notwithstanding, which may reduce some of these logistical barriers like access to care.
At the health care system level, therapeutic inertia is emphasized by finite resources to manage asthma (Capability) and payer utilization management criteria for the use of biologics in asthma treatment (Opportunity, Motivation). Payers report medical utilization to account for the majority of expenditure in all treated asthma patients, while quality measures like hospital readmissions and length of stay are identified as management priorities for payers in regards to moderate-to-severe asthma patients. These payer-level drivers are directly related to the low prescribing rates of biologics—as payers’ influence on prescription trends keep biologics costs up, low prescribing of these medications is perpetuated to counter this influence.Citation12 In addition, within the United States, payers use strict management criteria for the approval of biologics in asthma management with inconsistent criteria across insurance plans. These criteria are underscored by the process of securing prior authorization support, in which a healthcare provider must appeal to an insurance company and provide supporting information for approval before a patient can fill a prescription. Prior authorization can delay medication access and impact both provider prescription practices and patient adherence and expectations of moderate-to-severe asthma self-management.Citation19
The interaction between the practice system, provider, and moderate-to-severe asthma patient further attributes to therapeutic inertia. Providers may not adequately communicate to patients the importance of adherence for treatment plans, the susceptibility of moderate-to-severe asthma patients to frequent exacerbations, and the perception of diagnosis severity. Patients also may not engage with providers about concerns with their diagnosis, symptoms, or management plans. Furthermore, primary care providers may not be fully aware or have the opportunity to engage in discussions with patients about the appropriate range of eosinophil counts – as a higher count signals an increased likelihood for exacerbations and uncontrolled asthma – or other factors that may impact their diagnosis and treatment plans, leading to misguided expectations of asthma medications.Citation20 Lastly, within the context of the practice system, both provider and patient behavior can be affected by high-costs of prescriptions, payer formalities, and restrictions to specific therapies.
Panel Solutions to Therapeutic Inertia in the Context of Moderate-to-Severe Asthma ManagementCitation6,Citation21–Citation23
Experts from the fields of primary care, pulmonology, immunology, health services and clinical research, behavioral science, and pharmaceutical medical affairs gathered to engage in a structured process to identify solutions to address therapeutic inertia in moderate-to-severe asthma management. Both Sanofi (the sponsor) and Dr. Bosworth who was a moderator of the panel, identified a diverse group of stakeholders to serve as panel members. The sponsor (Sanofi) confirmed these eight individuals as having significant experience in treating individuals with severe asthma and individually representing a diverse set of large health care systems by affiliation and experience. There was no discussion of any specific medications and panel members were reimbursed by the sponsor for their participation. This panel utilized comparable methodology to discuss therapeutic inertia as other similar panels funded by the sponsor.Citation24 The panel represented academia and industry and provided perspectives on the patient, provider, and practice system-level challenges impacting therapeutic inertia. Panel members also utilized behavioral science to develop solutions that address these drivers of therapeutic inertia at each level and discussed implementation strategies and recommendations to offer behavior-driven therapeutic inertia solutions.
The panel was asked to list all potential barriers to therapy intensification for asthma; they generated over 50 potential solutions for addressing barriers to therapy intensification in moderate-to-severe asthma in a daylong, in-person session. Using a Delphi process, the panel engaged in a structured multi-round voting process to reach consensus and identify the most promising 18 solutions through iterative rounds of discussion. The Delphi process is a structured approach to gather input from a panel of experts.Citation25–Citation27 Through voting, a list of six action-oriented solutions that received the highest votes were identified for their potential to significantly impact therapeutic inertia. These solutions were developed into descriptive prototypes and mapped onto a primary domain of the COM-B model (). The solutions identified included 1) integration of patient-centered outcomes into asthma management practice (motivation); 2) provider education (motivation); 3) moderate-to-severe asthma care delivery redesign (capability); 4) harmonized, evidence-based protocol for the management of moderate-to-severe asthma (opportunity); 5) designated coordinator approach for optimal asthma management (capability); 6) case coordination digital support tool (capability). We describe each potential solution and how the solution relates to the COM-B model below ().
Table 1 Expert Solutions to Reduce Therapeutic Inertia in the Treatment of Moderate-to-Severe Asthma
Potential Solution 1: Integration of Patient-Centered Outcomes into Asthma Management Practice – Motivation
One strategy discussed to reduce therapeutic inertia for moderate-to-severe asthma patients is to integrate patient-centered outcomes and patient-defined milestones into asthma management practice. Patient-focused care enhances the patient–provider relationship by combining elements of education and evidence-based medical practice to focus on patients’ knowledge and understanding of their disease.Citation28,Citation29 Incorporating patient-focused care is of high importance in managing chronic conditions like asthma and results in increased patient adherence to treatment management guidelines, and subsequent improved patient outcomes and quality of life.Citation29 In addition, integrating patient-centered outcomes in chronic disease management mitigates discordance between physicians and patients’ beliefs and expectations about asthma management by addressing patients’ knowledge of asthma, individual treatment plans, and simplified treatment regimens.Citation29,Citation30 For example, in the context of severe asthma, the ability to reduce the oral corticosteroids (OCS) maintenance dose or OCS bursts because of the negative side effects (weight gain, osteoporosis, infection risk, among others) is viewed as a high priority patient-centered outcome. Most (not all) biologics have been proven to reduce OCS needs in the appropriately selected patient.
The COM-B framework can be utilized to best define and integrate patient-centered outcomes into asthma management evidence-based guidelines and practice. Patient-level barriers include challenges with medication adherence and a limited understanding and expectation of asthma management, patients’ capabilities in their care management (eg, having adequate knowledge regarding biologic intensification and treatment management), motivation (eg, understanding the value of their medication in asthma management), and opportunity (eg, designated discussions between patients and providers to design treatment plan). In addition, integrating patient-centered outcomes yields improved treatment management and positively impact payer-level benchmarks like hospitalizations and emergency room visits.
Potential Solution 2: Provider Education – Motivation
A lack of provider education serves as the root cause for several of the problems and solutions towards targeting therapeutic inertia for moderate-to-severe asthma patients. Educational programs and interactive, web-based trainings can target primary care providers, allergists, and pulmonologists with background and treatment guidelines to enhance the understanding of the appropriate use of biologics. Trainings should be tailored to specific providers addressing diagnosis and treatment patterns and patient populations to ensure uptake of the latest evidence-based treatment guidelines in clinical practice. Consideration of training focused on clarifying the approval process for initiating these medications should also be considered. The goal of provider-specific training would be to link theoretical and case-based education to issues facing providers’ hesitation towards intensifying biologic treatments to standardize the diagnoses and treatment of patients with moderate-to-severe asthma. Provider education addresses motivation and capability-related barriers (eg modifying treatment outside of symptom triggers; ensuring the appropriate diagnosis of moderate-to-severe asthma; warranting appropriate prescription and management of biologic treatment) across varying physician types with varied background in treating individuals with moderate-to-severe asthma.
When applied to the COM-B framework, incentivizing specific and tailored training and education programs through continuing medical education credit can increase provider motivation to complete such training. Such training can also increase a providers’ confidence in prescribing and communicating appropriate treatment and management strategies. This provider education solution can also impact patients’ understanding of the severity of their moderate-to-severe asthma diagnosis and adherence to treatment through downstream effects of provider education.
Potential Solution 3: Moderate-to-Severe Asthma Care Delivery Redesign – Capability
Redesigning the care delivery process for moderate-to-severe asthma patients can significantly improve the process for both payers and providers. Panelists agreed that redesigning care delivery by increasing payer motivation can significantly impact insurance costs, prescribing practices, and medication adherence, thereby targeting therapeutic inertia from the capability and motivation domains in regards to payers, providers, and patients.
When aligned with the COM-B model, redesigning the care continuum for moderate-to-severe asthma patients can impact intersecting challenges at each level and domain that can worsen therapeutic inertia. Identifying patients who may benefit most from particular biologics may mitigate practice-provider barriers to prescribing biologics (eg, payer-delayed authorizations of biologic prescriptions). In addition, redesigning asthma care delivery to improve access to appropriate biologic prescriptions for patients would increase patient motivation towards asthma management through adherence. Increased adherence may result in positive downstream effects for moderate-to-severe asthma patients, which could lead to reduced asthmatic exacerbations and readmission rates and an increase in payer motivation. The panel emphasized that the value of the appropriate use of medications in moderate-to-severe asthma patients must underscore discussions of bottom-line cost at the payer-level.
Potential Solution 4: Harmonized, Evidence-Based Protocol for the Management of Moderate-to-Severe Asthma – Opportunities
Significant differences in the rates of prescription of biologics and a lack of biologic intensification lend to the need for harmonization amongst treatment protocol management of moderate-to-severe asthma. Though published provider-focused diagnosis and treatment resources aid in decision-making,Citation31 the emergence of therapeutic inertia in biologic intensification suggests that these resources and guidelines have not been systematically implemented.Citation8 The panel identified the need for an implementation plan following the creation of a harmonized management protocol for treating moderate-to-severe asthma. The proposed plan would feature dissemination via multiple messaging channels with the visual support of stakeholders and clinical and administrative leadership to guide implementation within healthcare systems and at the individual-provider level. This plan could also include integration of guidelines and protocols into the electronic health record through check-point reminders targeted towards specific patients and prescriptions.
Using the COM-B framework to guide implementation of a harmonized protocol can address the barriers surrounding provider capability and opportunity. Provider access to protocol measures through the electronic health record and informed messaging decreases barriers to knowledge of current asthma management guidelines. Increasing the presence of protocols and guidelines within the clinical environment also creates the opportunity for providers to engage with current protocols and training through reminders, info-graphics, and alerts, which could in turn encourage the appropriate prescriptions and intensifications suited for a moderate-to-severe asthma patient.
Potential Solution 5: Designated Coordinator Approach for Optimal Asthma Management – Capability
To address discrepancies in primary care providers, allergists, and pulmonologists diagnosis and treatment for moderate-to-severe asthma patients, the expert panel acknowledged the benefit of designating a singular care-coordinator to manage these asthma patients. An identified provider designated as the sole coordinator of asthma management would address differences in therapeutic inertia and treatment patterns that can arise across patient care teams. Similarly, this solution can lead to the creation or expansion of a mid-level health care provider’s role to include treatment management of moderate-to-severe asthma patients. This expanded role includes establishing specific appointments or messaging streams between moderate-to-severe asthma patients and the care coordinator to communicate symptom and prescription changes, as well as monitoring referrals from primary care providers to asthma-focused specialty providers. Establishing a designated care team member to monitor and alter moderate-to-severe asthma patients’ biologic intensification can reduce therapeutic inertia.Citation32–Citation35
This solution’s integration with the COM-B model directly addresses provider capability to manage and address therapeutic inertia. By establishing a care coordinator to manage the treatment and biologic intensification of moderate-to-severe patients, this solution acts to address under referral practices by primary care providers to asthma specialists by serving as a communication bridge between all providers and patients to manage the care team’s role in a moderate-to-severe patients’ treatment plan.
Potential Solution 6: Case Coordination Digital Support Tool – Capability
Advancements in digital medicine can be utilized to improve and streamline care coordination for moderate-to-severe asthma patients. The panel discussed digital medicine tools focused on medication adherence and tracking as a means to address patients’ capability and opportunity to engage in the management of their asthma diagnosis. Mobile and digital medicine platforms with messaging capabilities can remind patients to engage in their care and monitor their symptoms, while integration with patient education materials about moderate-to-severe asthma can alleviate discrepancies in patients’ perceptions of diagnosis severity and management of chronic conditions. Developing and implementing an electronic health record digital support tool to streamline the biologic prescription process and ensure consistent therapeutic changes for individual cases targets the fragmented case coordination that can exist between prescribing providers and across a multifaceted asthma care team, as seen in relation to non-asthma diagnosesCitation36,Citation37 care management.
When applied to the COM-B model, digital support tools increase the capability for patients and providers to engage in treatment management, which can have downstream effects on treatment intensification from an awareness of symptom patterns and case progression. Patient use of a digital support tool to manage asthma symptoms can reduce the barriers that may exist in communicating such symptoms to providers at routine visits and can be used to streamline decision-making when considering treatment intensification.
The strategies discussed highlight a number of barriers that exist within the treatment of moderate-to-server asthma patients. These strategies also identified and prioritized solutions to help improve the uptake and use of appropriate biologic treatments for this patient population. Research regarding the appropriate biologic therapies for moderate-to-server asthma patients should continue to consider limitations that may exist at the intersection of provider, patient, and health system preferences and needs.
Conclusions
Moderate-to-severe asthma affects a significant fraction of diagnosed asthma patients. Drivers of therapeutic inertia, the sub-optimal use of biologics, in the severe asthma setting were identified and grouped into provider-related, patient-related or payer-related factors. Multi-level solutions to the issues were identified involving patients, providers, payers, and health systems with integration of patient-centered outcomes into asthma management practice and provider education being ranked the highest in terms of their potential impact on therapeutic inertia. The COM-B model is both an effective and useful framework to identify areas of improvement within therapeutic inertia targeting the capabilities, opportunities, and motivations of patients, providers, and payer systems.
Ethics Approval
According to the policy activities that constitute research at Duke University, this work met the criteria for quality improvement activities and was considered exempt from review by the Duke University Medical Center Institutional Review Board.
Acknowledgments
We thank Alvin Ong, PharmD, former Sanofi fellow, for his contributions to the workshop content development. The authors also acknowledge support from the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) [CIN 13-410] within the Durham VA Health Care System.
Disclosure
Sheila M. Thomas, Thomas Barsanti, and Daniel T. Kennedy are employees of Sanofi. Lisa Egbuonu-Davis is a former employee of Sanofi and is a current employee of Danaher Diagnostics. Hayden B. Bosworth reports research grants from Otsuka, Novo Nordisk, Sanofi, Improved Patient Outcomes, and Boehringer Ingelheim; consulting for Preventric Diagnostic, outside the submitted work. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Hekking P-PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042
- Stubbs MA, Clark VL, McDonald VM. Living well with severe asthma. Breathe. 2019;15(2):e40–e9. doi:10.1183/20734735.0165-2019
- Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139–1151. doi:10.1016/j.rmed.2006.03.031
- Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2015;15:35. doi:10.1038/nrd4624
- Rabe KF, Adachi M, Lai CKW, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114(1):40–47. doi:10.1016/j.jaci.2004.04.042
- Genzyme S. Addressing Therapeutic Inertia in Moderate-to-Severe Asthma: behavioral Science Co-Creation Workshop. 2018; Dalls. 2019.
- Wechsler ME. Current and Emerging Biologic Therapies for Asthma and COPD. Respir Care. 2018;63(6):699–707. doi:10.4187/respcare.06322
- Glonal Initiative of Asthma. Diagnosis and Management of Difficult-to-Treat and Severe Asthma Pocket Guide. Global Initiative for Asthma; 2019:V2. Available from: https://ginasthma.org/wp-content/uploads/2018/11/GINA-SA-FINAL-wms.pdf. Accessed April 01, 2021.
- McDonagh LK, Saunders JM, Cassell J, et al. Application of the COM-B model to barriers and facilitators to chlamydia testing in general practice for young people and primary care practitioners: a systematic review. Implement Sci. 2018;13(1):130. doi:10.1186/s13012-018-0821-y
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Sci. 2011;6(1):42. doi:10.1186/1748-5908-6-42
- Menzies-Gow A, Canonica GW, Winders TA, Correia de Sousa J, Upham JW, Fink-Wagner A-H. A charter to improve patient care in severe asthma. Adv Ther. 2018;35(10):1485–1496. doi:10.1007/s12325-018-0777-y
- Genentech. The 2017 Genentech Respiratory Trend Report: perspectives from Payers, Specialty Pharmacies, Practicing Physicians, and Provider Administrators. 1st ed. Genentech; 2017.
- Price D, Bjermer L, Bergin DA, Martinez R. Asthma referrals: a key component of asthma management that needs to be addressed. J Asthma Allergy. 2017;10:209–223. doi:10.2147/JAA.S134300
- Aragona E, Wang J, Scheckelhoff T, et al. Asthma specialty clinics decrease emergency department visits in inner-city children hospitalized for asthma exacerbation. B107 Understanding Disease Complexity in Asthma. Am Thoracic Soc. 2014;A3838–A.
- Lee J, Tay TR, Radhakrishna N, et al. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J. 2018;51(4):1701836. doi:10.1183/13993003.01836-2017
- Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med. 2009;180(9):817–822. doi:10.1164/rccm.200902-0166OC
- Asthma and Allergy Foundation of America. My Life with Asthma Survey Report. 2017. Available from: https://www.aafa.org/media/1684/my-life-with-asthma-in-2017-survey-findings-report.pdf. Accessed April 01, 2021.
- Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B. Patient and Physician Preferences for Attributes of Biologic Medications for Severe Asthma. Patient Preference Adherence. 2018;13:1253.
- Goldstein EJC, Raper JL, Willig JH, et al. Uncompensated Medical Provider Costs Associated with Prior Authorization for Prescription Medications in an HIV Clinic. Clin Infect Dis. 2010;51(6):718–724. doi:10.1086/655890
- Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3(11):849–858. doi:10.1016/S2213-2600(15)00367-7
- Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Managed Care Pharm. 2009;15(8):690–695. doi:10.18553/jmcp.2009.15.8.690
- Byrnes P. Why haven’t I changed that? Therapeutic inertia in general practice. Aust Fam Physician. 2011;40:24–28.
- O’Connor PJ, Sperl-Hillen JAM, Johnson PE, Rush WA, Biltz G. Advances in Patient Safety Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, Lewin DI, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005.
- Zullig LL, Egbuonu-Davis L, Trasy A, Oshotse C, Goldstein KM, Bosworth HB. Countering clinical inertia in lipid management: expert workshop summary. Am Heart J. 2018;206:24–29. doi:10.1016/j.ahj.2018.09.003
- Humphrey-Murto S, Varpio L, Wood TJ, et al. The use of the delphi and other consensus group methods in medical education research: a review. Acad Med. 2017;92(10):1491–1498. doi:10.1097/ACM.0000000000001812
- Jairath N, Weinstein J. The Delphi methodology (Part one): a useful administrative approach. Can J Nurs Adm. 1994;7(3):29–42.
- Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and Reporting the Delphi Method for Selecting Healthcare Quality Indicators: a Systematic Review. PLoS One. 2011;6(6):e20476. doi:10.1371/journal.pone.0020476
- Irwin RS, Richardson ND. Patient-Focused Care: using the Right Tools. Chest. 2006;130(1, Supplement):73S–82S. doi:10.1378/chest.130.1_suppl.73S
- Qamar N, Pappalardo AA, Arora VM, Press VG. Patient-centered care and its effect on outcomes in the treatment of asthma. Patient Relat Outcome Meas. 2011;2:81–109. doi:10.2147/PROM.S12634
- Goeman DP, Douglass JA. Optimal management of asthma in elderly patients. Drugs Aging. 2007;24(5):381–394. doi:10.2165/00002512-200724050-00003
- Becker AB, Abrams EM. Asthma guidelines: the Global Initiative for Asthma in relation to national guidelines. Curr Opin Allergy Clin Immunol. 2017;17(2):99–103. doi:10.1097/ACI.0000000000000346
- Johnson SA, Giesie PD, Ireland AM, Rice RD, Thomson BK. On the scene: developing a nurse care coordinator role at city of hope. Nurs Adm Q. 2016;40(1):39–50. doi:10.1097/NAQ.0000000000000139
- Isik E, Isik IS. Asthma care coordination in schools by school nurses: an integrative literature review. Public Health Nurs. 2019;36(4):498–506. doi:10.1111/phn.12610
- Janevic MR, Baptist AP, Bryant-Stephens T, et al. Effects of pediatric asthma care coordination in underserved communities on parent perceptions of care and asthma-management confidence. J Asthma. 2017;54(5):514–519. doi:10.1080/02770903.2016.1242136
- Pike KC, Levy ML, Moreiras J, Fleming L. Managing problematic severe asthma: beyond the guidelines. Arch Dis Child. 2018;103(4):392–397. doi:10.1136/archdischild-2016-311368
- Bangash H, Pencille L, Gundelach JH, et al. An implementation science framework to develop a clinical decision support tool for familial hypercholesterolemia. J Pers Med. 2020;10(3):67. doi:10.3390/jpm10030067
- Chang TS, Buchipudi A, Fonarow GC, Pfeffer MA, Singer JS, Cheng EM. Physicians voluntarily using an EHR-based CDS tool improved patients’ guideline-related statin prescription rates: a retrospective cohort study. Appl Clin Inform. 2019;10(3):421–445. doi:10.1055/s-0039-1692186