Abstract
Purpose
To determine the usability of the ergonomically designed certolizumab pegol pre-filled pen (CZP PFP) in Australian patients with active ankylosing spondylitis, active psoriatic arthritis or moderate-to-severe rheumatoid arthritis.
Patients and Methods
CZP-naive patients were recruited from six clinical centers in Australia between November 2018 and May 2019. Patients and healthcare professionals (HCPs) reviewed training materials and completed pre-injection surveys; patients then self-administered ≥1 injection with the CZP PFP during each of three visits. Patients and HCPs then completed post-injection surveys. Some survey questions were adapted from the self-injection assessment questionnaire (SIAQv2.0).
Results
Seventy patients participated (65 completed 3 visits); 33 were biologic-experienced. All patients agreed that training materials were informative; 94% found them easy to understand. Pre-injection, 89% of patients reported little or no anxiety about having injections; 67% (79% in biologic-experienced) were very confident about self-injecting the correct dose with the PFP. Ninety percent of patients were satisfied/very satisfied with their first experience with the CZP PFP; those with pre-injection anxiety reported lower satisfaction (43% vs 79% “very satisfied”). Confidence and satisfaction increased as visits progressed (for Visit 3 vs Visit 2: 69% vs 56% “very convenient”; 75% vs 67% “very confident”; 71% vs 57% “very satisfied”). All HCPs were confident in their patients’ competence to self-inject and thought all patients had overall positive experiences.
Conclusion
Australian patients with chronic rheumatic disease reported high levels of confidence and satisfaction following initial use of the CZP PFP. The availability of devices with patient-centered design innovations may help overcome barriers to self-injection for improved adherence/outcomes.
Introduction
Biological disease-modifying anti-rheumatic drugs (bDMARDs), including anti-tumor necrosis factor (anti-TNFs), are used for the treatment of chronic inflammatory diseases.Citation1 Certolizumab pegol (CZP) is the only Fc-free, PEGylated anti-TNF biologic.Citation2,Citation3 CZP is approved in Australia for the treatment of active ankylosing spondylitis (AS), moderate to severe chronic plaque psoriasis, active non-radiographic axial spondyloarthritis (nr-axSpA), active psoriatic arthritis (PsA), and moderate to severe active rheumatoid arthritis (RA).Citation3 It is administered subcutaneously, and is available for self-injection in a single-use pre-filled syringe or a pre-filled pen (PFP).Citation3 For AS, nr-axSpA, RA and PsA, CZP is initiated with a recommended loading dose of 400 mg for adult patients, given as two subcutaneous injections of 200 mg each at Weeks 0, 2 and 4, followed by maintenance doses of 200 mg every other week or 400 mg every 4 weeks.Citation3
Many patients with chronic rheumatic diseases demonstrate poor medication adherence, limiting the benefits they may derive from treatment.Citation4 Self-injection may improve adherence for patients as they can have increased independence and control over the injection process and when the medication is administered, compared with infusion therapy.Citation5,Citation6 However, there are barriers to self-injection using a pre-filled syringe, including needle phobia, problems with manual dexterity due to pain and joint swelling in the hands, and a lack of confidence in the self-injection process.Citation5,Citation7 User-friendly self-injection devices may overcome some of these challenges, improving treatment outcomes.Citation5
The CZP PFP was developed in collaboration with OXO, with input from patients and healthcare providers.Citation6,Citation8 It aims to address patients’ unmet needs with features such as a button-free delivery system; a wide, non-slip grip to support impaired dexterity; a large viewing window to check medication visually and monitor injection progress; a hidden and retractable needle to reduce the impact of needle phobia; and audible clicks at the start and finish to give patients confidence that a full dose has been administered (Figure S1).Citation1,Citation6,Citation8 In a previous comparative usability study, patients with RA ranked the CZP PFP as their preferred device over other anti-TNF PFPs, noting its ease of use and intuitive design.Citation8 Results from a patient satisfaction survey conducted in the UK demonstrated that patients had high confidence and satisfaction with self-injection using the CZP PFP.Citation6
This market research study aimed to determine the usability of the CZP PFP auto-injection device in Australian patients with active AS, active PsA or moderate to severe RA, including both patients who were biologic-naïve or had previously received a biologic treatment. It explored: patients’ perspectives and preferences regarding self-injection; ease of use of the CZP PFP, including, for biologic-experienced patients, comparisons with their previous biologic administration method; the perceived confidence and willingness of patients to use (and continue use of) CZP PFP before and after initial use, including review of the training materials provided with the device; and healthcare professional (HCP) perceptions of patients’ confidence levels before and after initial use of the CZP PFP.
Materials and Methods
Participants
Eligible patients were at least 18 years of age, had active AS, active PsA or moderate to severe RA diagnosed by a rheumatologist, and were eligible for treatment with CZP on the Australian Pharmaceutical Benefits Scheme, but had not been previously treated with CZP. Patients were excluded if they could not complete the questionnaire on the tablet device, or if they were unable to administer at least one injection by themselves at each study visit. Since this was a market research study, prior approval of the protocol by an ethics committee was not required. The study was conducted in accordance with relevant market research guidelines.
Patients were recruited from six participating clinical centers in Australia. Prospective patients were approached by their rheumatologists and were provided with a Participant Information Sheet and Consent Form. All participants provided written informed consent prior to or at Visit 1.
Study Design
The study was conducted between November 2018 and May 2019. An overview of the study design is provided in . Patients and the HCP (rheumatologists or biologic nurse specialists) responsible for assisting patients completed four sets of short questionnaires throughout their “on-boarding” experience with the CZP PFP, consisting of three visits to clinical centers at Week 0, Week 2 and Week 4. Loading doses of CZP were administered as two injections at each visit.
Figure 1 Overview of study design.
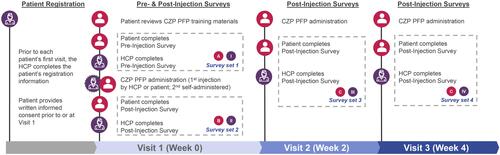
At Week 0, patients reviewed the CZP PFP training materials (a video and a step-by-step printed guide). Separate pre-injection surveys were then completed by the patient and the HCP. Following this, the first injection of the CZP PFP was administered, either by the HCP or by the patient themselves. The second injection had to be self-administered by the patient. After the injections, the patient completed a post-injection survey about their experience using the CZP PFP for the first time, and the HCP completed a survey on their perspective of the participant outcome.
At Week 2, patients self-administered at least one of the two second loading-dose injections with the CZP PFP and completed the second post-injection survey set, which asked about their satisfaction with and convenience of the CZP PFP. HCPs completed a survey about the participants’ overall experience using the CZP PFP device and their perception of patients’ willingness to continue with self-administration. At Week 4, patients self-administered at least one of the two third loading-dose injections with the CZP PFP, and both the HCP and the patient completed another set of post-injection surveys similar to that from Week 2.
Survey Instrument
Items from the self-injection assessment questionnaire version 2.0 (SIAQv2.0) were adapted for this study.Citation9 To manage the length of the surveys and reduce the burden of participation in research, some domains deemed less relevant to the research objectives were excluded. The pre-injection surveys used in this research included questions on patients’ feelings about injections, self-confidence about giving themselves an injection, and feelings about their previous medication (if applicable); post-injection surveys asked about ease of use of the device, satisfaction with self-injection, and willingness to continue to self-inject (Table S1). HCP surveys asked about the nurse or physician’s opinion of their patients’ confidence and competence to self-inject (Table S2).
Results
Patient Disposition and Baseline Characteristics
A total of 70 patients opted to participate in the study, of whom 65 completed all three visits. The mean age of the total opt-in cohort was 53.3 (standard deviation: 15.1) years and the majority were female (71%; ). About two-thirds were diagnosed with moderate disease (n=47). Almost half of participants were biologic-experienced (n=33), of whom 42% were previously treated with adalimumab ().
Table 1 Baseline Demographics
Evaluation of Training Materials and Pre-Injection Perceptions
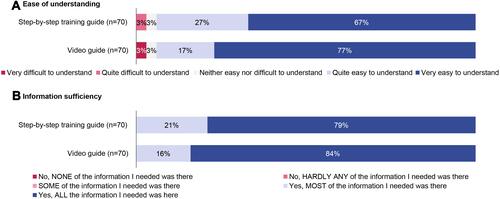
The majority of patients (94%) found the training materials quite easy or very easy to understand; all patients agreed that the materials provided all or most of the information they needed (). Overall, 77% of HCPs thought patients had a good understanding of the training materials and 81% thought patients could confidentially self-inject using the PFP following training. When stratified by prior injection experience, HCPs reported biologic-naïve patients as having lower levels of understanding and confidence (Figure S2).
Figure 2 Patient evaluations of training materials.
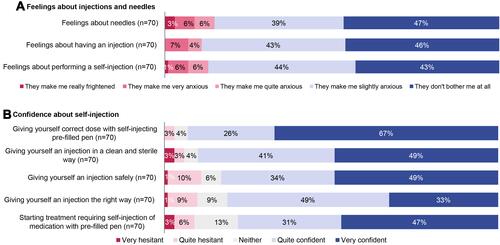
Patients generally had mild or no anxiety about injections or needles (), regardless of prior biologic experience (Figure S3). Most patients felt confident about self-injection after reviewing the training materials (), though a higher percentage of biologic-experienced patients reported feeling very confident (Figure S3).
Figure 3 Patients’ pre-injection perceptions.
Post-Injection Questionnaires
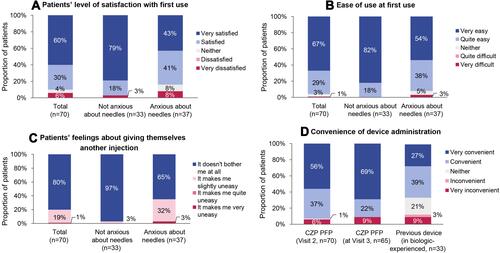
Two-thirds of patients administered the first injection themselves at the first visit (n=46), all of whom felt that it was very easy (n=35, 76%) or quite easy (n=11, 24%) to administer without any help. While most patients (90%) reported being satisfied or very satisfied with their first experience using the CZP PFP, those with pre-injection needle anxiety reported lower satisfaction and ease of use scores when compared with patients who were not anxious about needles ( and ). After the first injection, 80% of patients were comfortable about self-administering their next injection; patients with initial anxiety about needles were more likely to have remaining unease (35% versus 3% in those without needle anxiety; ). At Visit 3, 69% of patients reported finding the CZP PFP “very convenient”, while only 27% of biologic-experienced patients felt that their previous devices were “very convenient” ().
Figure 4 Patients’ post-injection perceptions.
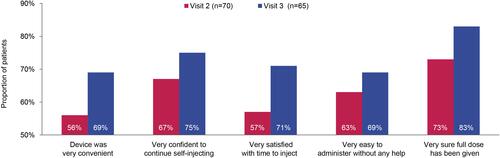
Patients’ confidence and satisfaction with self-injection using the CZP PFP increased as visits progressed. A higher proportion of patients reported that the device was “very convenient” at Visit 3 (69%) compared with Visit 2 (56%; ). From Visit 2 to Visit 3, more patients also became “very confident to continue self-injecting” (67% versus 75%), “very satisfied with time to inject” (57% versus 71%), and were “very sure the full dose had been given” (73% versus 83%; ). By Visit 3, no patients reported finding the CZP PFP “difficult” or “very difficult” to administer; 69% of patients felt it was “very easy to administer without any help” (63% at Visit 2; ).
Figure 5 Patient confidence and satisfaction with self-injection at Visits 2 and 3.
After observing their patients’ first self-administration, 100% of HCPs agreed that their patients were competent to self-inject; at Week 4, all HCPs were 100% confident in their patients’ ability to continue to self-injections, and thought that all patients had an overall positive experience (Figure S4).
Discussion
For patients with chronic inflammatory disease who are prescribed subcutaneous anti-TNF therapy, the usability of self-injection devices can have a direct positive impact on medication adherence, which in turn may improve the effectiveness of treatment.Citation4,Citation5,Citation8–Citation10 As non-adherence to medication may be unintentional and driven by difficulties that patients may face when taking their medication, such as dexterity issues or needle phobia,Citation5 improvements in medication delivery devices can be associated with higher rates of compliance and persistence.Citation11
The CZP PFP was designed with enhanced features to address specific challenges associated with self-injection among patients with impaired dexterity such as RA patients, as well as to address commonly-reported concerns by patients regarding the self-injection process.Citation1,Citation5 The aim of this market research was to explore patient and HCP responses to the CZP PFP device through an evaluation of the on-boarding process for CZP self-injection among a sample of RA, PsA and AS patients in Australia.
Patient training and education have been found to be important factors in improving rates of adherence to self-injection as they can provide patients with greater confidence and empowerment, and increase levels of independence.Citation5 In the UK CZP PFP patient experience study, nurses reported higher levels of confidence in patients’ ability to self-inject safely than were reported by the patients themselves.Citation6 A similar trend was observed in this sample of Australian patients; after the first visit, 100% of HCPs were confident about their patients’ competence, whereas 20% of patients had remaining unease regarding further self-injections, particularly in those with pre-injection needle anxiety. As noted by Bailey et al,Citation6 this could indicate a gap between the patients’ confidence in their abilities and their actual competence to correctly self-inject. This further highlights the importance of adequate training, communication and support at treatment initiation in order to reassure patients and manage any injection-related anxiety or uncertainty.Citation6,Citation10
Needle fear may cause anxiety about self-injections, reducing confidence and negatively impacting the self-injection process.Citation10 The CZP PFP features a hidden retractable needle which remains non-visible to patients during the injection process, aiming to reduce fear related to needle phobia.Citation6 However, as demonstrated in the current study, pre-existing fear of needles could still lead to lower satisfaction with self-injection, and higher perception of difficulty at first use of the self-injection device. It is thus particularly important for patients with pre-injection needle anxiety to receive adequate support which acknowledges and manages their emotional response during the injection process.Citation10 Comprehensive and easy-to-understand training materials, as well as an intuitive, user-friendly device, can help to overcome this by increasing confidence prior to, during, and after the first self-injection.Citation6,Citation8
The results from this market research survey builds on previous patient experience research on the device.Citation6,Citation8 In a similar market research survey conducted in a UK population of patients with RA, PsA or axSpA, high confidence and satisfaction scores were reported when using the CZP PFP.Citation6 This study supports those findings and provides evidence that high levels of patient satisfaction with CZP PFP may also be found among Australian RA, PsA and AS patients.
In a previous comparative usability study, patients with RA ranked the CZP PFP as their preferred device over adalimumab, etanercept, and golimumab PFPs.Citation1,Citation8 In these patients who may have impaired dexterity or poor hand strength, features such as an ergonomic handle and button-free activation were rated as highly important as it enabled them to hold the device confidently and with a good grip.Citation1,Citation8 Likewise, in the current survey, a higher proportion of patients rated the CZP PFP as “very convenient” compared with the administration of their previous medications.
Patients’ confidence and satisfaction with self-injection generally improved between Visits 2 and 3, indicating that, with increasing experience, patients felt more comfortable using the CZP PFP. Patient experience studies have reported on the importance of setting up “routines”, where the association of self-injection with familiar repeated “rituals” helps to mitigate patients’ fear and anxiety by giving patients confidence and control over the administration process.Citation10
Although generally high satisfaction scores were reported by the end of the study, it should be noted that a small remaining proportion of patients were not completely satisfied with the CZP PFP. Studies suggest that multiple factors can affect patient satisfaction;Citation5 patients often have personal preferences in the treatment of their own disease, and empowering patients to play a more active role in their disease management can improve treatment experience and adherence.Citation5,Citation10 To address these differences in patient preferences, a portfolio of self-injection solutions will be needed: for example, some patients may prefer the control provided by the use of a pre-filled syringe, whereas others may prefer some of the functions associated with the PFP. The availability of a range of devices is thus essential in providing choice, and allows patients and physicians to discuss and adapt treatment administration options according to individual needs.Citation5
Limitations
Due to the lack of a comparator in the study, we are unable to comment on direct comparisons of CZP PFP with other self-injection devices. The SIAQ was amended for this survey, and some domains such as “injection site reactions” or questions within domains were not included in the version used in this study. The exclusion of these elements meant that SIAQ domain scores, which provide a validated evaluation of patients’ self-injection experience,Citation9 could not be calculated.
Aside from biologics, prior experience with other injected medications may also have impacted patients’ expectations of and satisfaction with the CZP PFP; however, a detailed history of non-biologic medications was outside the scope of this market research study. Other factors that were not assessed, such as differences in clinical efficacy or symptom improvement experienced during visits after the first injection (Visits 2 and 3), may also impact overall satisfaction with therapy.Citation10
It was a requirement that a HCP be present during each visit to ensure completion of patient obligations for the market research. It is unclear whether the physical presence of HCPs during self-injection had any impact on patients’ perception of and confidence with the CZP PFP. In previous studies, patients have reported that, regardless of the usefulness of the provided training materials, they found the presence of a nurse during their first injection reassuring.Citation6
Finally, the study recruited only 70 patients, with 65 patients remaining at Visit 3, and may not be representative of the entire population of Australian CZP PFP patients. In particular, most of the patients opting into the study had mild or no injection or needle anxiety; patients who were most afraid of self-injection may have opted out of the study, reducing the generalizability of these results to the wider patient population.
Conclusions
Australian patients with AS, PsA and RA generally reported high levels of confidence and satisfaction following their initial use of the CZP PFP. With the provision of holistic training and support, particularly among patients reporting some level of pre-initiation needle anxiety, the availability of a range of devices with patient-centered design innovations could help to overcome barriers to self-injection, increasing adherence and improving outcomes among patients with chronic rheumatic disease.
Data Sharing Statement
Data from non-clinical studies are outside of UCB’s data sharing policy and are unavailable for sharing.
Ethics Approval and Informed Consent
Since this was a market research study, prior approval of the protocol by an ethics committee was not required. The study was conducted in accordance with relevant market research guidelines, including the obtaining of informed consent and adherence to ethical reporting standards.
Author Contributions
Substantial contributions to study conception and design: JA, HG, JZ, AL, AJ; substantial contributions to analysis and interpretation of the data: JA, HG, JZ, AL, AJ; drafting the article or revising it critically for important intellectual content: JA, HG, JZ, AL, AJ; final approval of the version of the article to be published: JA, HG, JZ, AL, AJ; agreed on the journal to which the article will be submitted: JA, HG, JZ, AL, AJ; agreed to take responsibility and be accountable for the contents of the article: JA, HG, JZ, AL, AJ.
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Wee Yan Ran, BSocSci (Hons), from Costello Medical, Singapore for medical writing and editorial assistance based on the authors’ input and direction.
Disclosure
JZ has received speaker honoraria from UCB, Lilly, Janssen-Cilag, Novartis and AbbVie. AL is an employee of UCB Pharma and participates in the UCB Stock Award Plan. JA has received sponsorship for conference attendance from UCB. AJ has received sponsorship for educational activities from UCB. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Lyseng-Williamson KA. Certolizumab pegol administration devices: a profile of their use and usability. Drugs Ther Perspect. 2017;33(11):515–522.
- Hyrich KL, Verstappen SM. Biologic therapies and pregnancy: the story so far. Rheumatology. 2014;53(8):1377–1385.
- Cimzia®. (Certolizumab Pegol) 200 Mg/Ml Injection [Australian Prescribing Information]. Malvern: UCB Pharma; 2019.
- Marengo MF, Suarez-Almazor ME. Improving treatment adherence in patients with rheumatoid arthritis: what are the options? Int J Clin Rheumtol. 2015;10(5):345–356.
- van den Bemt BJ, Gettings L, Domańska B, Bruggraber R, Mountian I, Kristensen LE. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Delivery. 2019;26(1):384–392.
- Bailey K, Mountian I, Bruggraber R, Sunderland K, Tilt N, Szegvari B. Patient Satisfaction with CIMZIA® (Certolizumab Pegol) AutoClicks® in the UK. Adv Ther. 2020;37(4):1522–1535.
- Domańska B, Stumpp O, Poon S, Oray S, Mountian I, Pichon C. Using patient feedback to optimize the design of a certolizumab pegol electromechanical self-injection device: insights from human factors studies. Adv Ther. 2018;35(1):100–115.
- Domańska B, VanLunen B, Peterson L, Mountian I, Schiff M. Comparative usability study for a certolizumab pegol autoinjection device in patients with rheumatoid arthritis. Expert Opin Drug Deliv. 2017;14(1):15–22.
- Keininger D, Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ). Health Qual Life Outcomes. 2011;9(1):2.
- Schiff M, Saunderson S, Mountian I, Hartley P. Chronic disease and self-injection: ethnographic investigations into the patient experience during treatment. Rheumatol Ther. 2017;4(2):445–463.
- Maniadakis N, Toth E, Schiff M, et al. A targeted literature review examining biologic therapy compliance and persistence in chronic inflammatory diseases to identify the associated unmet needs, driving factors, and consequences. Adv Ther. 2018;35(9):1333–1355.
