Abstract
Introduction
Health literacy is an important competency to make informed, shared decisions in line with patient’s preferences. On the other hand, lower health literacy is associated with poorer health outcomes. Evidence-based patient decision aids (EbPDA) are validated instruments to support informed medical decisions and empower patients for relevant involvement in their care. This study aimed to investigate the effect of a digital EbPDA for hypertension on health literacy.
Methods
In a randomized controlled trial, 124 participants were presented with a web-based scenario related to a newly diagnosed condition of arterial hypertension. The intervention group was provided with an online decision aid, while the control group was prompted to search for related information without support. Specific health literacy for hypertension was operationalized based on the European survey for health literacy (HLS-EU-Q47).
Results
The intervention group showed a statistically significant increase in subjectively perceived overall specific health literacy regarding hypertension (p=0.02, Cohen’s d=0.44). The effect was also statistically significant for the subcategories understanding, appraising, and applying health-related information (all p<0.05). At least equal results could be shown for participants with a lower level of education compared to participants with a high level.
Conclusion
The findings suggest that digital EbPDAs can be an effective and easily scalable instrument to improve populations’ specific health literacy. A possible advantage of the measure could be that patients are addressed concerning important and pressing personal decisions, fostering awareness of the individual’s need for health literacy to reflect one’s options and preferences. EbPDAs may also be a promising approach to target vulnerable populations, as the investigated EbPDA seems to perform equally in less versus more educated individuals. For future research, it may be interesting to investigate whether EbPDAs have effects on general health literacy that go beyond the disease specifically addressed.
Introduction
Patient engagement and patient participation in the medical decision-making process depend on the health practitioner’s efforts to respect and integrate the patient’s preferences and – particularly – on the patient’s capacity and motivation to understand and consider different medical options and consequences.Citation1,Citation2 Thus, patients’ empowerment in terms of general and specific health literacy is an essential goal of health communication and crucial to making relevant “healthy” decisions.Citation3 Shared-decision making (SDM) is the widely accepted standard for supporting good decisions: it integrates medical evidence, clinical experience, and the patient’s preferences.Citation4 The improvement of patient’s health literacy is an important foundation of improving SDM: general health literacy is of avail to gain disease-specific information, to pose relevant questions, and thus to engage in the decision-making process.Citation5–Citation9 On the other hand, improving health literacy is often an effect – if not a purpose – of interventions that foster shared decision-making.Citation10 Ultimately, SDM-related skills are an important part of health literacy.
Hypertension is one of the most significant contributors to the global burden of disease. Moreover, there are strong disparities in the prevalence and treatment of high blood pressure both between high- and low-income countries and within the individual countries. In general, the prevalence of high blood pressure is associated with a lower socio-economic status, especially concerning education.Citation11–Citation13 Overall, hypertension is among the most important public health challenges.Citation14
For public health interventions in general, it is especially challenging to support vulnerable populations that are at risk for developing bad health because of their relatively low socio-economic status. Thus, as many interventions more easily reach the better educated, they are at risk to even increase health inequity.Citation15 There is solid evidence that low health literacy is associated with poorer health outcomes – both in terms of hypertension and other diseases – and it is widely accepted that promoting health literacy can decrease health disparities.Citation16,Citation17 Nevertheless, the evidence for specific interventions to promote health literacy is heterogeneous, mainly supporting interventions that address specific needs and skills and often depend on highly motivated initiatives.Citation18–Citation21
Evidence-based patient decision aids (EbPDA) are not among the typically listed concepts for increasing health literacy.Citation22 Hence, they are an effective instrument to support patients that face critical health-related decisions.Citation23 It is conceivable that patients who are confronted with a new diagnosis and the need for a critical decision are more interested in improving their specific health literacy. As EbPDAs are conceptualizing a kind of a “crash course” for a specific health topic, they may be a promising instrument to target the health literacy of specifically involved patients, regardless of their education, and thus also contributing to health equity.Citation24
The primary objective of this study was to evaluate the impact of a newly developed digital evidence-based patient decision aid for hypertension on disease-specific health literacy within a typical setting. The secondary objective was to analyze if this impact varies between participants with different education levels.
Methods
Study Design, Participants and Randomization
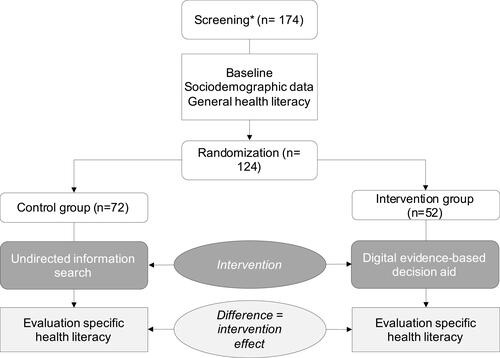
The investigation was performed in a virtual scenario that simulates the decision situation of a newly diagnosed condition of chronic arterial hypertension. It was designed as a between-subjects, double-blind, randomized controlled trial comparing two conditions (). Participants were recruited via public contacting, mailings, and social media to participate in a web-based survey. It was conducted at the University of Erfurt, Germany, in 2019. After inclusion criteria check (adults 40–70 years, no professional medical education), baseline data was assessed (socio-demographic data including the presence of hypertension, level of education, general health competency). Participants were presented with a scenario description that simulated a preventive routine-checkup. Herein, the physician informed the individual that the condition of hypertension was newly diagnosed. Afterwards, the physician announced the next appointment to decide on treatment options. The participants were then randomly assigned to the intervention or control group, using a digitally generated, blinded allocation. In preparation for the next appointment, the intervention group was then provided with a digital decision aid, while the control group was requested to get ready for the decision by searching the Internet or books or asking family members or friends for information or advice. The participants were prompted to return to the online scenario afterwards without a fixed time limit. At this point, the scenario ended, and the perceived specific health literacy was assessed with a questionnaire based on the HLS-EU-Q47 for measuring patient ́s specific health literacy. Finally, the control group was also offered to use the digital decision aid. Only entirely completed surveys were included; dropouts were recorded. The recruitment of new participants was continued until the calculated sample size for both groups was reached. Ethics approval was obtained by the ethics commission of the University of Erfurt, Germany.
Intervention
Patient decision aids are designed to facilitate shared decision-making and patient participation in medical decisions. The essential content here is the comprehensible and evidence-based presentation of information relevant to decision-making. The digital decision aid used in this study was developed as part of the implementation program for shared decision-making “SHARE TO CARE”, which also includes other modules, eg, online and face-to-face training of physicians and interventions to empower patients.Citation25 All modules apply an identical, plain six-step model of SDM, which refers to the “essential elements of SDM”.Citation26 The didactics and content of the digital evidence-based patient decision aid (EbPDA) apply the International Patient Decision Aid Standards (IPDAS).Citation27,Citation28 The whole program develops about 80 EbPDAs for different clinical decisions, including the decision aid for arterial hypertension treatment options, investigated in this study. All decision aids follow an identical concept, prompting the user through four chapters: (1) a description of the underlying health condition; (2) a description of the possible medical treatment decisions; (3) a comparing tabular overview; (4) a decision tool to weigh the individual’s preferences regarding the respective treatment options that can per exported for personal use, particularly for subsequent consultation. In the EbPDA for hypertension that was deployed in this study, the decision tool was based on the ARRIBA-method, an interactive risk-calculator and decision-making instrument for cardiovascular events.Citation29,Citation30 The provided medical content is based on a systematic review of current evidence for antihypertensive therapies.Citation25 It is presented with written, graphical, and video-based information, featuring interactive elements to illustrate the relation of personal preferences, possible choices, and experiences of affected patients, physicians, and related healthcare workers.Citation31 The complete EbPDA is peer-reviewed by medical and scientific experts and underwent user-testing by laypeople.
Baseline Data and Outcomes
Socio-demographic data regarding age, gender, condition of hypertension, general and professional medical education were collected. Items to assess general and specific health literacy were compiled based on the German translation of the HLS-EU questionnaire (HLS-EU-Q47):Citation32,Citation33 31 items refer to disease prevention and health promotion and do not account for the study questions of this article (item 17–47, numbering refers to the original version of the HLS-EU-Q47 as provided in the appendix of Sorensen et alCitation33). The first 16 items of the HLS-EU-Q47 refer to personal healthcare and are partitioned into the four subcategories access/obtain (item 1–4), understand (item 5–8), appraise/process (item 9–12), and apply information relevant to health (item 13–16). These 16 items were eligible to assess general health literacy before the intervention. For measuring specific health literacy, the same subcategories were covered: five items were eligible for the context and adapted to assess specific health literacy by replacing the general term “illness” with the specific medical condition of hypertension (item 1, 2, 10, 12, 13), one additional item was generated in form and content of the HLS-EU-Q47 to cover the subcategory understand information relevant to health in a patient-centered and disease-specific context (items 5 and 8 were integrated to the question “ … how easy would you say it is to understand information on treatments of hypertension?”, An adaption of item 2, Supplement 1). Following the methodology of HLS-EU-Q47, all questions were to be answered on a weighted Likert-type scale ranging from 1=very easy, 2=easy, 3=difficult to 4=very difficult. Specific health literacy served as the primary endpoint and was compared between the control group and intervention group. The primary research question was to determine the differences of mean specific health literacy. The secondary research question was to analyze differences in two subgroups, split by the participants’ level of education, eg, a subgroup with a higher education level and a subgroup with a lower level of education. A higher education level was defined as holding at least a matriculation standard, whereas a lower education level was defined as holding no matriculation standard. All data were collected via online forms.
Statistical Analysis and Sample Size Calculation
The analysis was conducted with a per-protocol approach since no test data could be assessed for dropouts. All data are expressed as mean with standard deviation (SD) and 95% confidence interval (CI) unless stated otherwise. General and specific health literacy data were checked for internal consistency with Cronbach’s alpha. Previous studies justified an equivalent weighting of the different items of HLS-EU-Q47; therefore, our data was subsumed as means for each category.Citation33 Mean difference (MD) between IG and CG of the primary endpoint-specific health literacy (total and subscale scores) and general health literacy were compared using two-sided, independent samples t-tests. A p‐value <0.05 was considered to indicate statistical significance.
A priori sample size calculation using G*POWER® suggested a sample size of 51 participants per group to show a mean effect of Cohen’s d=0.50 with a statistical power of 1-ß=0.80 for the primary endpoint. All analyses were performed using IBM SPSS Statistics 27.0 (SPSS, Chicago, IL).
Results
Sample Characteristics and Randomization Check
A total of n=174 participants were recruited and completed the assessment. After applying the exclusion criteria age and medical education, a total of 124 participants were randomized and ran completely through the scenario with n=52 for intervention and n=72 for the control group (mean age=53.63 yrs, SD 8.40; gender 64% female). Due to a higher dropout rate in the intervention group (versus control instruction), differences in sample sizes occurred. However, randomization check showed no apparent differences for age, gender, education, and the actual presence of hypertension (). Group split for the analysis of education effects resulted in the following four groups: lower education IG n=33, CG n=32; higher education IG n=19, CG n=40.
Table 1 Sociodemographic Data
Health Literacy
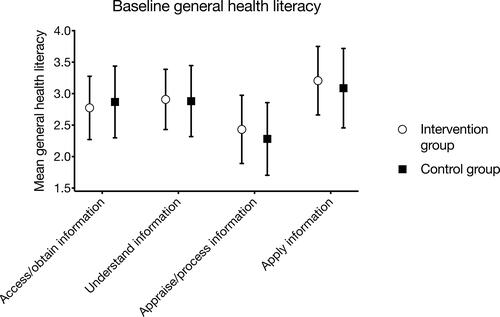
Reliability check for internal consistency showed a Cronbach ́s alpha of 0.89 for general health literacy, 0.86 for specific health literacy, and ≥.70 for all subgroups; therefore, a statistical calculation based on mean values was eligible. The baseline evaluation for general health literacy showed no significant differences between control group and intervention group in the four subcategories access (p=0.34), understand (p=0.78), appraise (p=0.14), and apply (p=0.27) information relevant to health ().
Figure 2 Assessment of general health literacy before intervention based on HLS-EU-QQ47 survey for health literacy. Control and intervention group showed no significant differences.
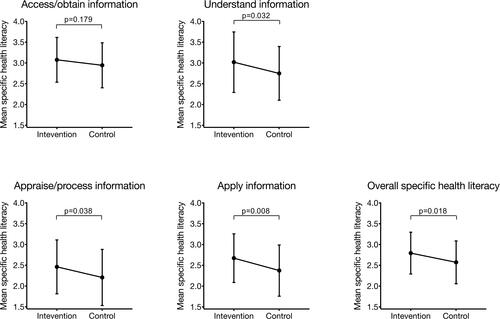
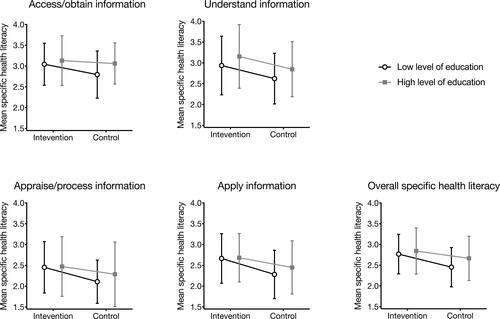
After the intervention, overall perceived specific health literacy for hypertension was significantly higher in the intervention as compared to the control group (intervention group: M=2.79, SD=0.50; control group: M=2.57, SD=0.51; p=0.018, Cohen ́s d=0.437; , ). Analysis of subdimensions showed significant increase for understand information (p=0.032; d=0.397), appraise/process information (p=0.038; d=0.381) and apply information (p=0.008; d=0.481) and no increase for access/obtain information (p=0.179) relevant to health (, ). The intervention effect was not different between participants with higher versus lower level of education, indicated by the absence of a significant interaction effect in any of the subscales and the total scale (all p>0.05). (, ).
Table 2 Specific Health Literacy in the Intervention and Control Group
Table 3 Specific Health Literacy in the Intervention and Control Group, Split into the Subgroups of Participants with Low vs High Level of Education
Discussion and Conclusion
Discussion
The results of this study demonstrate that a digital evidence-based patient decision aid (digital EbPDA) can increase specific health literacy, ie, the subjective evaluation of effective handling of health information. While the self-reported general health literacy in both control and intervention group showed no significant differences before intervention, after offering the decision aid, a significant increase for the participants’ self-reported specific health literacy on arterial hypertension could be achieved. Further analysis revealed significant positive effects on the subdimensions understand, appraise and apply health information, but no increase in the subdimension finding specific health information. Reasonably, having an EbPDA does not increase the ability to find information – as the EbPDA simply delivers it. Instead, it is helpful to process health information.
EbPDAs address decision situations of specific medical conditions – like therapeutic options for hypertension. To this effect, an EbPDA aims at populations with not only specific risks but even with existing conditions, thus possibly a group with increased personal motivation to process the presented information. It seems conceivable that aiming at pressing decisions of involved patients may be effective leverage to impact specific and concurrently also general health literacy: Reflecting on personal preferences for different options naturally creates a need to improve one’s health literacy. Real patients are likely to be even stronger motivated to engage with the presented EbPDA than our study’s participants. Additionally, the didactic concept of the investigated EbPDA is designed to benefit particularly people with lower educational backgrounds, thus vulnerable populations. The concept of this and several other EbPDAs developed within the SDM implementation program SHARE TO CARE is very similar.Citation25 The common subject employs the three questions (ASK3, sometimes also referred to as “three good questions” or similar) that improve the quality of health communication on medical decisions: what are my options? What are the benefits and harms? How likely are these benefits and harms to occur to me?Citation34,Citation35 For this purpose, the evidence-based information is presented in plain language, descriptive graphics, and video-statements that display the expertise and experience of real patients, physicians, and other healthcare workers.Citation31 Although each EbPDA refers to a specific medical decision situation, the presented content can serve as a generic example for other medical decisions and therefore contributes to general health literacy. This may be especially important for the didactical goal to engage participants in posing the three questions, an approach that was successfully taken also in a recent Australian study within the AskShareKnow-Network that aimed at health literacy and SDM.Citation36
Digital EbPDAs can be designed for different medical conditions, decision contexts, and options. They can be easily provided via the internet and, thus, may be a promising low-threshold approach to complement other eHealth-related activities that foster evidence-based medicine, patient involvement, and empowerment.
Higher income and higher education are associated with higher patient activation and engagement regarding shared decision-making.Citation37,Citation38 Contrary, lower socio-economic status is associated with reduced adherence, for example, for antihypertensive therapies.Citation39,Citation40 From a public health perspective, it is important to reflect whether the gains that result from an intervention apply to the whole population equally. Since people with higher socio-economic and educational resources often benefit more than people with respective disadvantages, some interventions seem to increase health inequality.Citation41 Different approaches are discussed to dissolve this unjust effect, aiming either on whole populations, populations at risk (eg, smoking population), or vulnerable populations. The latter refers to populations at risk for exposure to risk factors thus, especially people with reduced socio-economic and educational backgrounds.Citation15 Our study showed at least equal effects of the investigated EbPDA regarding different educational levels, indicating that digital decision aids may be a promising instrument also to target less educated people. Although the study was not powered for this sub-analysis, this result is consistent with recent studies that discussed a positive effect on health equality for decision aids and SDM interventions in general.Citation42–Citation44
Generalizability and Limitations
A possible limitation of this study is related to its methodological design: although the constructed online scenario simulates a typical situation of everyday healthcare, it is not possible to evoke the same urgency that evolves in real patients who are confronted with substantial, life-changing, or even life-threatening decisions. On the other hand, the voluntary participation may condition a potential selection bias towards more engaged attendees. However, both of these limitations may be mitigated under real-life conditions because real patients should be even more motivated to participate under conditions concerning their personal health – the voluntary participation is then no longer in question. The elevated dropout rate in the intervention group is likely caused by the effort that has to be expended to work through the digital EbPDA. It is conceivable, that the dropout rate may be lower in real-life patients with an actual need for health-related decisions and a higher motivation to learn about their disease. As neither socio-economic variables nor general health literacy at baseline differed substantially between the groups, it may be assumed that this has not biased the outcome. Nevertheless, usability and acceptance of EbPDAs remain an issue that should be investigated in specific studies. Further limitations pertain to the analysis of the influence of participants’ educational level, as the study is not powered in this regard a priori. These results have to be considered cautiously. Hence, the graphs in tend to show, if any, a larger benefit for the lower rather than for the higher educated participants. Nevertheless, future studies focusing specifically on the effect of EbPDAs on vulnerable populations are necessary to validate these conclusions. Some additional constraints result from the employment of the HLS-EU-Q47 questionnaire that investigates only self-perceived health literacy with limited items in the adaption for specific health literacy, and that does not survey long-term effects.Citation33
Conclusion
Practical strategies to improve health literacy are important to empower people to engage in their personal health, make well-informed decisions that fit personal preferences, and reduce health disparities. One of the most ambitious challenges for appropriate interventions is to effectively reach and involve people. Our findings suggest that EbPDAs are an effective measure to increase health literacy for hypertension, independent of the educational level. Thus, they may be helpful also for vulnerable populations and may therefore contribute to health equity. EbPDAs are applied in relevant medical decision situations that matter to the individual. This practical and intuitive connection to a patient’s actual need for information may be advantageous leverage to increase this intervention’s efficiency and effectiveness. The digital provisioning of the investigated EbPDA is an additional advantage to scale up appropriate interventions and may combine with other eHealth-related measures. Furthermore, EbPDAs directly increase shared decision-making. Thus, they substantially target critical situations and support patient’s autonomy and self-responsibility by providing relevant evidence-based information. We suggest that from a public health point of view, digital EbPDAs are a promising additional measure to empower and increase the population’s health and decrease health inequity. Further studies are needed to validate the presumed effects on vulnerable populations and further investigate the potential for general health literacy and the impact of EbPDAs in a large-scale application.
Abbreviations
CI, confidence interval; EbPDA, evidence-based patient decision aid; HLS-EU-Q47, European Health Literacy Survey (47 items); MD, mean difference; SD, standard deviation; SDM, shared decision-making.
Data Sharing Statement
The dataset used and analyzed during the current study is available from the corresponding author on request.
Ethics Approval and Consent to Participate
Ethics approval was given by the ethics council of the University of Erfurt, Germany (No. 20190615, 7/2019). The study was conducted in accordance with the Declaration of Helsinki. All participants have provided informed consent. Participants of the control group were offered to use the EbPDA after data were assessed.
Author Contributions
FK originated and conducted the study, conducted primary data analyses, and prepared a draft. KW supervised the study, conducted further data analysis, and wrote the manuscript. CB originated and supervised the study. FG supervised the study and conducted the statistical analysis. FS and JUR developed the intervention (decision aid). ND contributed to the intervention. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Michael Schipper for help with the EbPDA-login coding and the entire SHARE TO CARE working group. Kai Wehkamp and Felicia Beatrice Kiefer are co-first authors for this study.
Disclosure
CB and FBK declare no conflicts of interest. FG, FS, KW and JUR are shareholders of SHARE TO CARE, Patientenzentrierte Versorgung GmbH (Cologne/Germany). KW reports fee for a lecture on shared decision-making from Roche Germany GmbH. FG reports personal fees from Roche and Chugai Pharma. NDB is shareholder of the GPZK gGmbH/non-profit corporation (Rostock/Germany). The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Clayman ML, Bylund CL, Chewning B, Makoul G. The impact of patient participation in health decisions within medical encounters: a systematic review. Medical Decision Making. 2015;36:427–452. doi:10.1177/0272989X15613530
- Barr PJ, Scholl I, Bravo P, Faber MJ, Elwyn G, McAllister M. Assessment of patient empowerment - A systematic review of measures. PLoS One. 2015;10(5):e0126553. doi:10.1371/journal.pone.0126553
- Parker RM, Gazmararian JA. Health literacy: essential for health communication. J Health Commun. 2003;8(sup1):116–118. doi:10.1080/713851963
- Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344(jan271):e256–e256. doi:10.1136/bmj.e256
- Ishikawa H, Yano E. Patient health literacy and participation in the health-care process. Heal Expect. 2008;11(2):113–122. doi:10.1111/j.1369-7625.2008.00497.x
- Keij SM, van Duijn-bakker N, Stiggelbout AM, Pieterse AH. What makes a patient ready for shared decision making? A qualitative study. Patient Educ Couns. 2021;104(3):571–577. doi:10.1016/j.pec.2020.08.031
- Wigfall LT, Tanner AH. Health literacy and health-care engagement as predictors of shared decision-making among adult information seekers in the USA: a secondary data analysis of the health information national trends survey. J Cancer Educ. 2018;33(1):67–73. doi:10.1007/s13187-016-1052-z
- Ousseine YM, Durand M-A, Bouhnik A-D, et al. Multiple health literacy dimensions are associated with physicians’ efforts to achieve shared decision-making. Patient Educ Couns. 2019;102(11):1949–1956. doi:10.1016/j.pec.2019.05.015
- Chang HL, Li FS, Lin CF. Factors influencing implementation of shared medical decision making in patients with cancer. Patient Prefer Adherence. 2019;13:1995–2005. doi:10.2147/PPA.S217561
- Muscat DM, Shepherd HL, Nutbeam D, Trevena L, McCaffery KJ. Health literacy and shared decision-making: exploring the relationship to enable meaningful patient engagement in healthcare. J Gen Intern Med. 2020;1–4. doi:10.1007/s11606-020-05912-0
- Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. 2015;33(2):221–229. doi:10.1097/HJH.0000000000000428
- Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115mmHg, 1990–2015. JAMA - J Am Med Assoc. 2017;317(2):165–182. doi:10.1001/jama.2016.19043
- Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control. Circulation. 2016;134(6):441–450. doi:10.1161/CIRCULATIONAHA.115.018912
- Fisher NDL, Curfman G. Hypertension - a public health challenge of global proportions. JAMA - J Am Med Assoc. 2018;320(17):1757–1759. doi:10.1001/jama.2018.16760
- Frohlich KL, Potvin L. Transcending the known in public health practice: the inequality paradox: the population approach and vulnerable populations. Am J Public Health. 2008;98(2):216–221. doi:10.2105/AJPH.2007.114777
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107. doi:10.7326/0003-4819-155-2-201107190-00005
- McNaughton CD, Kripalani S, Cawthon C, Mion LC, Wallston KA, Roumie CL. Association of health literacy with elevated blood pressure a cohort study of hospitalized patients. Med Care. 2014;52(4):346–353. doi:10.1097/MLR.0000000000000101
- Visscher BB, Steunenberg B, Heijmans M, et al. Evidence on the effectiveness of health literacy interventions in the EU: a systematic review. BMC Public Health. 2018;18(1):1414. doi:10.1186/s12889-018-6331-7
- Sørensen K, Van Den Broucke S, Fullam J, et al. Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health. 2012;12(1):80. doi:10.1186/1471-2458-12-80
- Freedman AM, Miner KR, Echt KV, Parker R, Cooper HLF. Amplifying diffusion of health information in low-literate populations through adult education health literacy classes. Journal of Health Communication. 2011;16:119–133. doi:10.1080/10810730.2011.604706
- Tavakoly Sany SB, Behzhad F, Ferns G, Peyman N. Communication skills training for physicians improves health literacy and medical outcomes among patients with hypertension: a randomized controlled trial. BMC Health Serv Res. 2020;20(1):60. doi:10.1186/s12913-020-4901-8
- Huhta AM, Hirvonen N, Huotari ML. Health literacy in web-based health information environments: systematic review of concepts, definitions, and operationalization for measurement. J Med Internet Res. 2018;20(12):e10273. doi:10.2196/10273
- Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;2017(4). doi:10.1002/14651858.CD001431.pub5
- McCaffery KJ, Holmes-Rovner M, Smith SK, et al. Addressing health literacy in patient decision aids. BMC Med Inform Decis Mak. 2013;13(SUPPL. 2):S10. doi:10.1186/1472-6947-13-S2-S10
- Danner M, Geiger F, Wehkamp K, et al. Making shared decision-making (SDM) a reality: protocol of a large-scale long-term SDM implementation programme at a Northern German University Hospital. BMJ Open. 2020;10(10):e037575. doi:10.1136/bmjopen-2020-037575
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301–312. doi:10.1016/j.pec.2005.06.010
- Volk RJ, Llewellyn-Thomas H, Stacey D, Elwyn G. Ten years of the international patient decision aid standards collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak. 2013;13(S2):S1. doi:10.1186/1472-6947-13-S2-S1
- Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi:10.1136/bmj.38926.629329.AE
- Keller H, Krones T, Becker A, et al. Arriba: effects of an educational intervention on prescribing behaviour in prevention of CVD in general practice. Eur J Prev Cardiol. 2012;19(3):322–329. doi:10.1177/1741826711404502
- Krones T, Keller H, Sönnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med. 2008;6(3):218–227. doi:10.1370/afm.854
- De Looper M, Damman O, Smets E, Timmermans D, Van Weert J. Adapting online patient decision aids: effects of modality and narration style on patients’ satisfaction, information recall and informed decision making. J Health Commun. 2020;25(9):712–726. doi:10.1080/10810730.2020.1840674
- Schaeffer D, Vogt D, Berens EM, Hurrelmann K. Gesundheitskompetenz der Bevölkerung in Deutschland – Ergebnisbericht. 2017. doi:10.2390/0070-pub-29088450.
- Sorensen K, Van den Broucke S, Pelikan J, et al. Measuring health literacy in populations: illuminating the design and development process of HLS-EU-Q. BMC Public Health. 2013;13(1):948. doi:10.1186/1471-2458-13-948
- Shepherd HL, Barratt A, Trevena LJ, et al. Three questions that patients can ask to improve the quality of information physicians give about treatment options: a cross-over trial. Patient Educ Couns. 2011;84(3):379–385. doi:10.1016/j.pec.2011.07.022
- Garvelink MM, Jillissen M, Knops A, Kremer JAM, Hermens RPMG, Meinders MJ. Implementation of the three good questions—A feasibility study in Dutch hospital departments. Heal Expect. 2019;22(6):1272–1284. doi:10.1111/hex.12960
- Muscat DM, Morony S, Trevena L, et al. Skills for shared decision-making: evaluation of a health literacy program for consumers with lower literacy levels. HLRP Heal Lit Res Pract. 2019;3(3):S58–S74. doi:10.3928/24748307-20190408-02
- Smith SG, Pandit A, Rush SR, Wolf MS, Simon CJ. The role of patient activation in preferences for shared decision making: results from a national survey of U.S. Adults. J Health Commun. 2016;21(1):67–75. doi:10.1080/10810730.2015.1033115
- Osborn R, Squires D. International perspectives on patient engagement: results from the 2011 commonwealth fund survey. J Ambul Care Manage. 2012;35(2):118–128. doi:10.1097/JAC.0b013e31824a579b
- Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124(7):1124–1140. doi:10.1161/CIRCRESAHA.118.313220
- Carr-Lopez SM, Shek A, Lastimosa J, et al. Medication adherence behaviors of medicare beneficiaries. Patient Prefer Adherence. 2014;8:1277–1284. doi:10.2147/PPA.S64825
- Lorenc T, Petticrew M, Welch V, Tugwell P. What types of interventions generate inequalities? Evidence from systematic reviews. J Epidemiol Community Health. 2013;67(2):190–193. doi:10.1136/jech-2012-201257
- Skains RM, Kuppermann N, Homme JL, et al. What is the effect of a decision aid in potentially vulnerable parents? Insights from the head CT choice randomized trial. Heal Expect. 2020;23(1):63–74. doi:10.1111/hex.12965
- Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS One. 2014;9(4):e94670. doi:10.1371/journal.pone.0094670
- Muscat DM, Morony S, Shepherd HL, et al. Development and field testing of a consumer shared decision-making training program for adults with low literacy. Patient Educ Couns. 2015;98(10):1180–1188. doi:10.1016/j.pec.2015.07.023