Abstract
Heart failure (HF) is a progressive condition with periods of apparent stability and repeated worsening HF events. Over time, unless optimization of HF treatment, worsening HF events become more frequent and patients enter into a cycle of recurrent events with high morbidity and mortality. In patients with HF there is an activation of deleterious neurohormonal pathways, such as the renin angiotensin aldosterone system and the sympathetic system, and an inhibition of protective pathways, including natriuretic peptides and guanylate cyclase. Therefore, HF burden can be reduced only through a holistic approach that targets all neurohormonal systems. In this context, vericiguat may play a key role, as it is the only HF drug that activates the nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate system. On the other hand, it has been described relevant disparities in the management of HF population. Consequently, it is necessary to homogenize the management of these patients, through an integrated patient-care pathway that should be adapted at the local level. In this context, the development of new technologies (ie, video call, specific platforms, remote control devices, etc.) may be very helpful. In this manuscript, a multidisciplinary group of experts analyzed the current evidence and shared their own experience to provide some recommendations about the therapeutic optimization of patients with recent worsening HF, with a particular focus on vericiguat, and also about how the integrated patient-care pathway should be performed.
Introduction
Heart failure (HF) is very common in clinical practice and it is associated with high morbidity and mortality. The HFA Atlas survey showed that in Europe in 2018–2019, although with relevant differences between countries, the incidence of HF was 3.20 cases per 1000 person-years, the HF prevalence was 17.20 cases per 1000 people, and the number of HF hospitalizations was 2671 per million people annually, with a length of hospital stay of 8.50 days.Citation1 Of note, the RECALCAR project showed that in Spain, between 2007 and 2019, whereas the crude mortality rate during hospitalization for acute coronary syndrome has progressively decreased over time, this has remained stable for HF.Citation2,Citation3 On the other hand, among patients with acute HF admission, the 15- and 30-day readmission rates due to cardiovascular reasons are high (7% and 14%, respectively),Citation4 although these numbers could be even greater.Citation5 In addition, a large portion of the annual health-care budget is devoted to HF patients, with unplanned hospitalization representing the main source of healthcare-related expenditure (around 75%), and only a small proportion of total costs corresponding to medication costs (7%).Citation6,Citation7
Worsening Heart Failure (WHF): Relevance and Needs
HF is a progressive condition that punctuates periods of apparent stability with repeated WHF events. WHF includes all inpatient or outpatient decompensation events requiring intravenous diuretic therapy.Citation8 Over time, unless optimization of HF treatment, WHF events become more frequent and patients enter into a cycle of recurrent events.Citation9 This is very relevant, as each HF hospitalization is not only associated with a significant reduction of quality of life, but also with an increased risk of death that raises with each HF hospitalization.Citation10,Citation11 In fact, WHF is associated with a four-fold increase in 1-year mortality risk compared with chronic HF, from 6% to 28%.Citation12 However, the risk of cardiovascular death or HF hospitalization is highest in the early phase after hospitalization. For example, a substudy of the PARADIGM-HF trial showed that compared with patients with no prior hospitalization, whereas the risk of cardiovascular death or HF hospitalization was 26% higher in patients with a prior HF hospitalization >12 months, this was a 46% greater in those with a recent hospitalization (<6 months).Citation13
As a result, it is necessary to optimize the management of patients with HF, with the early use of disease-modifying treatment.Citation14 However, it has been described relevant disparities regarding mortality and readmission rates among patients hospitalized for HF between those specialties that attend the HF patient (ie, cardiology, internal medicine, etc.), mainly due to differences in the clinical profile and management of these patients, and also between different Autonomous Communities in Spain.Citation2,Citation3 In this context, it is important to stratify and identify those patients at high risk of decompensation, particularly those with a recent WHF episode in order to provide a better approach through an integrated patient-care pathway.
A multidisciplinary group of experts about the management of patients with HF analyzed the current evidence and shared their own experience to provide some recommendations about how the integrated patient-care pathway should be performed. In addition, this group of experts analyzed the role of vericiguat in the management of patients with recent WHF.
Patient Pathway and Health Care Transition
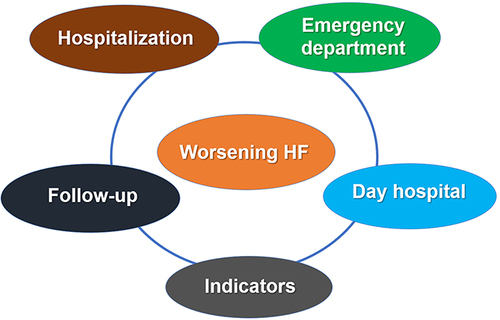
After an acute HF decompensation, which can be either in an outpatient or in patient setting (ie, hospitalization, emergency department, outpatient clinic/day hospital), the patient pathway can be very heterogenous, as it currently depends on the specialist that treat the patient and also the specific health-care system in which the patient is attended.Citation15–17 Consequently, it is necessary to homogenize the management of these patients. The main components of the health-care organization among patients with WHF should include all settings in which the patients can be assisted (ie, hospitalization, emergency department, day hospital, office clinic), in order to optimize the management of the patient flow. Only through a multidisciplinary (ie, physicians and nurses) and patient-centered approach, an adequate continuity of care can be assured, reducing readmissions and further complications. This approach should focus on the early intervention during all phases of the patient´s journey, including the inpatient phase, discharge planning, early post-discharge visits and structured follow-up.Citation18,Citation19 Remarkably, this should be adapted to the local health-care system, considering the actual material and human resources available. In addition, specific indicators should be established to analyze the impact of the actions developed at the local levelCitation20–22 ().
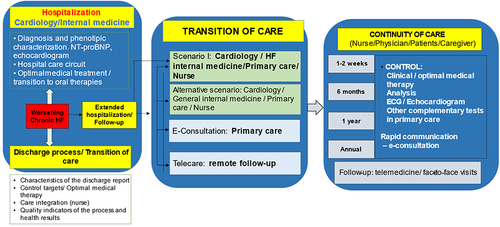
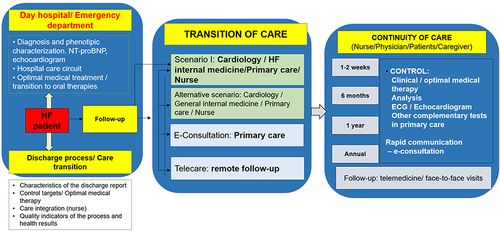
When the patient presents with WHF and require intravenous diuretics, the patient can be attended in the day hospital, emergency department or may require hospitalization, according to the severity of the decompensation. After stabilization, the discharge process and transition of care should be adequately organized not only from a global level, but mainly from a local point of view. In this context, the characteristics of the discharge report have to be well defined, indicating the diagnostic and therapeutic procedures performed during the decompensation, as well as the therapeutic targets (ie, blood pressure, heart rate, etc.). Additionally, the therapeutic plan, as well as the follow-up visits should also be included. During the transition of care, an appropriate communication and integration between different health-care providers, including physicians (internal medicine, cardiology, primary care, etc.), nurse and patients/caregivers should be promoted.Citation22 In this context, the European guidelines recommend that before discharge, persistent signs of congestion should be excluded and, when possible, optimization of oral treatment (disease-modifying therapies) should start during hospitalization or at least at early post-discharge phase. In addition, an early follow-up visit is recommended at 1–2 weeks after discharge.Citation14 However, the Spanish Society of Cardiology considers that it would be desirable to have a first telephonic contact <24–48 hours after discharge with nurse and an office visit with the primary care physician and the specialist (cardiology or internal medicine), within the first 7–10 days and 30 days after discharge, respectively.Citation22
In addition, telemedicine offers a unique opportunity that facilitates the transition and continuity of care of patients with HF. There are different formats of telemedicine consultation that can be used in this population, according to the specific characteristics of the population and the material and human resources that are available in each sanitary area. These formats may include telephone, video call, specific platforms, and remote control devices. These formats are fully complementary.Citation23–25 In fact, different studies have demonstrated that within the comprehensive management of patients with HF, the addition of telemedicine may result in better outcomes, including the prevention of further HF events and reduction of costs, regardless frailty status of patients.Citation26–31 Therefore, both, telemedicine and face-to-face visits are useful tools that can be adapted to the need that the patient require at each moment.Citation23–25 Additionally, electronic consultations are an excellent instrument that facilitates the communication between different health-care providers, promoting the comprehensive management of patients with HF.
Finally, it is necessary to develop specific quality indicators of the process and to analyze the health results to determine areas of improvement in order to optimize the patient pathway, with a particular focus on the transition and continuity of care and the early implementation and uptitration of disease-modifying therapies.Citation22 In summary, to increase the early use of guideline-directed medical therapies, it is necessary the development of multidisciplinary HF management programs, locally adapted, through the use of electronic patient awareness tools, telehealth and electronic medical interventions for providers.Citation32 and represent general examples of the health-care pathways of the patients with HF, according to the settings they are attended.
Vericiguat and the VICTORIA Trial
In patients with HF there is an activation of deleterious neurohormonal pathways, such as the renin angiotensin aldosterone system (RAAS) and the sympathetic system, and an inhibition of some protective pathways, including the systems of natriuretic peptides and guanylate cyclase. Angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB) and angiotensin II and neprilysin antagonists (ARNI) effectively inhibit RAAS, whereas beta blockers the sympathetic system. In contrast, ARNI stimulates the natriuretic peptide system through the neprilysin inhibition. Additionally, although the main mechanisms are not well known, the sodium-glucose co-transporter 2 inhibitors (SGLT2i) provide a positive impact in patients of HF, with a reduction of cardiovascular events. However, none of the previous HF therapies acts on the guanylate cyclase system.Citation33–36
Remarkably, oxidative stress and endothelial dysfunction lead to a decreased activity of the nitric oxide and soluble guanylate cyclase and ultimately, to a reduction of the production and activity of the cyclic guanosine monophosphate (cGMP). The impairment of this system is associated with important negative effects on the cardiovascular and renal systems. Vericiguat is an oral soluble guanylate cyclase stimulator that may reverse, or at least improve these alterations, in the heart, by reducing myocardial stiffening, fibrosis, and ventricular hypertrophy and remodeling, in the kidneys, by decreasing fibrosis and improving renal blood flow and in the blood vessels, by reducing vasoconstriction and enhancing endothelial function.Citation37,Citation38 Therefore, to actually reduce HF burden, it is necessary to provide a complete approach that targets all the different neurohormonal systems, particularly in those patients at high risk, such as those with a recent WHF episode.Citation39 In this context, vericiguat provides important benefits, as the VICTORIA trial has demonstrated.Citation40
Victoria was a Phase 3, randomized, double-blind, placebo-controlled trial that included 5050 patients with symptomatic chronic HF, a left ventricular ejection fraction <45%, elevated natriuretic peptide levels and evidence of WHF, defined as HF hospitalization within the 6 months before randomization or receiving intravenous diuretic therapy, without hospitalization, within the previous 3 months. Patients received vericiguat (target dose of 10 mg once daily) or placebo, in addition to standard therapy. The majority of patients were in NYHA functional class II (59%) or III (40%), 67% and 17% had been hospitalized within 3 months and 3–6 months before randomization, respectively, and 16% required intravenous diuretic for HF, without hospitalization.Citation40
In the VICTORIA trial, treatment with vericiguat was associated with a significant reduction in the risk of the primary outcome, the composite of death from cardiovascular causes or first HF hospitalization by 10% (HR 0.90; 95% CI 0.82–0.98) after a median follow-up of 10.8 months. In addition, vericiguat significantly reduced total HF hospitalizations by 9% (HR 0.91; 95% CI 0.84–0.99) and the composite of death from any cause or HF hospitalization by 10% (HR 0.90; 95% CI 0.83–0.98).Citation40 These results were consistent regardless of the previous use of HF treatment, renal function, or index event, and were particularly effective in those patients with NT-proBNP levels <8000 pg/mL.Citation40–44 In addition, vericiguat was very well tolerated, with similar rates of adverse events and serious adverse events than placebo. With regard to systolic blood pressure, there was a discrete decrease with vericiguat that occurred in the first weeks of treatment before returning to baseline, but rates of symptomatic hypotension or syncope were similar between groups. Of note, vericiguat did not modify the renal function or sodium or potassium levels.Citation40
Vericiguat in the Comprehensive Pharmacological Management of WHF
Vericiguat in Perspective of Other Contemporary Clinical Trials
To actually understand the relevance of the results of the VICTORIA trial, it is important to know the design and the results of the more contemporary clinical trials ().Citation40,Citation45–47 The PARADIGM-HF trial included patients with symptomatic HF with reduced ejection fraction (≤35%), 71% in NYHA functional class II, median NT-proBNP 1608 pg/mL, and 31% hospitalized for HF within the 6 months before inclusion. Compared with placebo, sacubitril/valsartan reduced the risk of the primary endpoint (first HF hospitalization or cardiovascular death) by 20%.Citation45 The DAPA-HF included patients with symptomatic HF with reduced ejection fraction (≤40%), 68% in NYHA functional class II, median NT-proBNP 1437 pg/mL and 16% hospitalized for HF within the 6 months before inclusion. Compared with placebo, dapagliflozin reduced the risk of the primary endpoint (WHF or cardiovascular death) by 26%.Citation46 The EMPEROR-Reduced included patients with symptomatic HF with reduced ejection fraction (≤40%), 75% in NYHA functional class II, median NT-proBNP 1906 pg/mL. Compared with placebo, empagliflozin reduced the risk of the primary endpoint (first HF hospitalization or cardiovascular death) by 25%.Citation47 In the VICTORIA trial, 59% of patients were in NYHA functional class II, median NT-proBNP was 2816 pg/mL and 84% hospitalized for HF within the 6 months before inclusion. Compared with placebo, vericiguat reduced the risk of the primary endpoint (first HF hospitalization or cardiovascular death) by 10%.Citation40
Figure 4 Baseline clinical characteristics and results of the primary outcome in the PARADIGM-HF, DAPA-HF, EMPEROR-Reduced and Victoria trials.
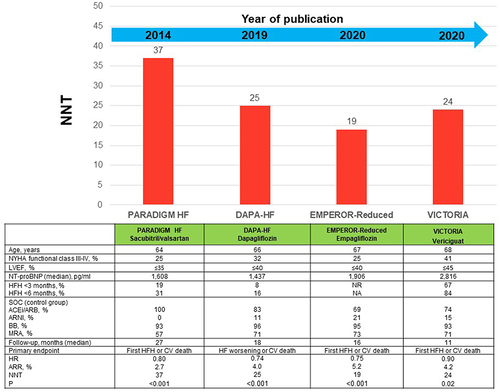
Therefore, there are important differences regarding inclusion criteria and baseline clinical characteristics. As a result, direct comparisons cannot be performed between these studies. Despite that, absolute differences between these contemporary studies were very close (NNT ranged from 19 to 37).Citation40,Citation45–47 However, it is important to emphasize that only the VICTORIA trial specifically analyzed those patients with WHF as inclusion criteria.
Contemporary Guideline-Directed Medical Therapies
International guidelines recommend that unless contraindicated, patients with HF with reduced ejection fraction should be treated with a RAAS inhibitor, preferably ARNI, a beta blocker, a mineralocorticoid receptor antagonist (MRA) and a SGLT2i to reduce the risk of HF hospitalization and death.Citation14,Citation48,Citation49 These drugs should be prescribed as early as possible, trying to achieve the target doses if tolerated.Citation39
In the light of the evidences with vericiguat, mainly provided from the VICTORIA trial, international guidelines are in general very homogeneous in their recommendations, and they state that vericiguat may be considered to reduce HF hospitalization and cardiovascular death in patients with HF with reduced ejection fraction and recent WHF already on maximum tolerated optimal HF therapy ().Citation14,Citation48,Citation49 As the pathogenesis of HF is complex, with the implication of numerous neurohormonal systems, a holistic approach is mandatory to reduce the morbidity and mortality among patients with HF. In this context, vericiguat may add benefits over the rest of disease-modifying therapies.Citation50 Thus, in a systematic network meta-analysis, of 75 relevant trials representing 95,444 participants, the combination that most effectively reduced all-cause death was ARNI, beta blockers, MRA, and SGLT2i, followed by ARNI, beta blockers, MRA and vericiguat. The combination of ARNI, beta blockers, MRA and vericiguat significantly reduced the risk of cardiovascular death and first HF hospitalization by 57% and all-cause mortality by 59%.Citation51 Therefore, vericiguat represents an added value in the management of patients with HF with reduced ejection fraction.
Table 1 Recommendations of International Guidelines and Expert Consensus About the Use of Vericiguat in Patients with Heart Failure with Reduced Ejection Fraction
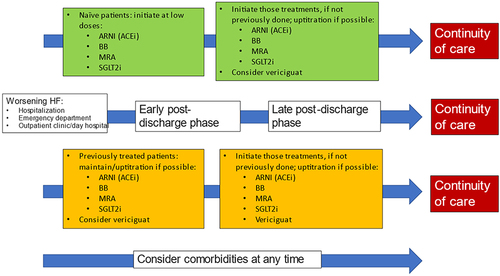
On the other hand, as guidelines recommend, evidence-based oral medical treatment should be administered as soon as possible, preferably before discharge and uptitration of disease-modifying therapies should start during the early post-discharge phase.Citation14,Citation48,Citation49 A HF therapeutic optimization pathway of patients with recent WHF episode is presented in . WHF includes not only those patients with hospitalization for HF, but also those that require intravenous diuretics for decompensated HF, either treated in the emergency department or in an outpatient setting (day hospital). In addition, two algorithms according to whether patients have been previously treated with oral disease-modifying therapies are presented. Of note, the initial prescription of these drugs is mandatory, but also uptitration during the follow-up. Assuring an adequate continuity of care through an appropriate communication between health-care levels is required.
Figure 5 Therapeutic optimization of patients with recent WHF episode.
Adherence and Optimization
A comprehensive pharmacological approach may be limited by side effects/tolerability concerns. A recent systematic review of 37 studies showed that in patients with HF and reduced ejection fraction, the proportion of patients that reached the target doses for ACEi/ARB, ARNI, beta-blockers, and MRA ranged from 4% to 55%, 11% to 87%, 4% to 60%, and 22% to 80%, respectively. Elderly, worsening renal function, hyperkalemia and hypotension were associated with non-use or sub-therapeutic dose.Citation52 In this context, it is necessary to know the side effects associated with each drug. Thus, ACEi/ARB/ARNI/MRA may cause hypotension, worsening renal function and hyperkalemia, beta blockers may produce symptomatic deterioration, asthenia, bradycardia, or hypotension, and the use of SGLT2i may be associated with an increased risk of genitourinary infections, hypoglycemia, dehydration, hypotension and prerenal renal failure.Citation14 With regard to vericiguat, no dose adjustment is required according to renal function (avoid if estimated glomerular filtration rate <15 mL/min/1.73 m2), and has a minimal impact on systolic blood pressure (mean reduction 1–2 mmHg), renal function or electrolytes. In case of symptomatic hypotension or systolic blood pressure <90 mmHg), temporary down-titration or discontinuation is recommended.Citation53
To reduce the possibility of adverse events, some parameters according to the type of HF drug, such as blood pressure, heart rate, renal function or potassium, should be monitored for the initiation or uptitration ().Citation14 In this context, among patients with HF with reduced ejection fraction, the initiation and uptitration of guideline-directed medications should be individualized according to patient’s symptoms, vital signs, tolerance, renal function, electrolytes, and comorbidities. As a result, initiation/uptitration should be performed according to these characteristics, rather than the standard recommended sequence, in order to provide the best and early therapeutic approach in each patient with HF.Citation14,Citation39,Citation48,Citation49 Despite the recommendations performed by guidelines, we consider that the prescription of vericiguat can be extended to other subgroups of patients, according to their clinical characteristics ().
Table 2 Parameters to Be Considered for Initiation/Uptitration of HF Drugs
Conclusion
The best approach to reduce HF morbidity and mortality is the early implementation of disease-modifying therapies. This is even more important among patients with a recent WHF episode, in which the risk is more marked. As the pathogenesis of HF is complex, with the implication of different neurohormonal systems (ie, RAAS and sympathetic, natriuretic peptides and guanylate cyclase), a holistic approach is mandatory to reduce the morbidity and mortality among patients with HF. In this context, vericiguat may play a key role. On the other hand, in patients with WHF it is necessary to homogenize the management of patients with HF through an integrated patient-care pathway that should be adapted at the local level.
Disclosure
JRGJ reports fees for conferences and for participating in advisory boards for Bayer. JCC reports fees as a speaker from Bayer. DPF received personal fees, non-financial support and/or research grants from Bayer, Novartis, Astra Zeneca, Boehringer Ingelheim, Roche, Rovi, Vifor, Abbot, Pfizer, Servier and Medtronic. JMC reports fees for participating in advisory boards for Bayer. AGQ reports fees for lectures and conferences from Bayer, Daiichi-Sankyo, Pfizer, AstraZeneca, Boehringer Ingelheim, Novartis, and Rovi. LM reports fees for lectures and conferences from Novartis, Bayer, AstraZeneca and Pfizer. JLZ has reports fees for lectures from Bayer and Daiichi- Sankyo. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Seferović PM, Vardas P, Jankowska EA, et al. The Heart Failure Association atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail. 2021;23(6):906–914. doi:10.1002/ejhf.2143
- Rodríguez-Padial L, Bertomeu V, Elola FJ, et al. Quality improvement strategy of the Spanish Society of Cardiology: the RECALCAR registry. J Am Coll Cardiol. 2016;68(10):1140–1142. doi:10.1016/j.jacc.2016.07.723
- RECALCAR Registry 2020. Healthcare for patients with heart disease in the national health system. Available from: https://secardiologia.es/images/institucional/sec-calidad/sec-recalcar/RECALCAR_2020_FINAL.pdf. Accessed March 13, 2023.
- Delgado JF, Ferrero Gregori A, Fernández LM, et al. Patient-associated predictors of 15- and 30-day readmission after hospitalization for acute heart failure. Curr Heart Fail Rep. 2019;16(6):304–314. doi:10.1007/s11897-019-00442-1
- Butler J, Yang M, Manzi MA, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(8):935–944. doi:10.1016/j.jacc.2018.11.049
- Farré N, Vela E, Clèries M, et al. Medical resource use and expenditure in patients with chronic heart failure: a population-based analysis of 88 195 patients. Eur J Heart Fail. 2016;18(9):1132–1140. doi:10.1002/ejhf.549
- Escobar C, Varela L, Palacios B, et al. Costs and healthcare utilisation of patients with heart failure in Spain. BMC Health Serv Res. 2020;20(1):964. doi:10.1186/s12913-020-05828-9
- Greene SJ, Mentz RJ, Felker GM. Outpatient worsening heart failure as a target for therapy: a review. JAMA Cardiol. 2018;3(3):252–259. doi:10.1001/jamacardio.2017.5250
- Greene SJ, Fonarow GC, Butler J. Risk profiles in heart failure: baseline, residual, worsening, and advanced heart failure risk. Circ Heart Fail. 2020;13(6):e007132. doi:10.1161/CIRCHEARTFAILURE.120.007132
- Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266. doi:10.1016/j.ahj.2007.01.041
- Rechenberg K, Cousin L, Redwine L. Mindfulness, anxiety symptoms, and quality of life in heart failure. J Cardiovasc Nurs. 2020;35(4):358–363. doi:10.1097/JCN.0000000000000630
- Tavazzi L, Senni M, Metra M, et al. Multicenter prospective observational study on acute and chronic heart failure: one-year follow-up results of IN-HF (Italian network on heart failure) outcome registry. Circ Heart Fail. 2013;6(3):473–481. doi:10.1161/CIRCHEARTFAILURE.112.000161
- Solomon SD, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan relative to a prior decompensation: the PARADIGM-HF trial. JACC Heart Fail. 2016;4(10):816–822. doi:10.1016/j.jchf.2016.05.002
- McDonagh T, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
- Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1(1):CD002752. doi:10.1002/14651858.CD002752.pub4
- Takeda A, Taylor SJ, Taylor RS, Khan F, Krum H, Underwood M. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;9:CD002752. doi:10.1002/14651858.CD002752.pub3
- Girerd N, Von Hunolstein JJ, Pellicori P, et al. Therapeutic inertia in the pharmacological management of heart failure with reduced ejection fraction. ESC Heart Fail. 2022;9(4):2063–2069. doi:10.1002/ehf2.13929
- Comín-Colet J, Enjuanes C, Lupón J, Cainzos-Achirica M, Badosa N, Verdú JM. Transitions of care between acute and chronic heart failure: critical steps in the design of a multidisciplinary care model for the prevention of rehospitalization. Rev Esp Cardiol. 2016;69(10):951–961. doi:10.1016/j.rec.2016.05.001
- Comín-Colet J, Verdú-Rotellar JM, Vela E, et al. Efficacy of an integrated hospital-primary care program for heart failure: a population-based analysis of 56,742 patients. Rev Esp Cardiol. 2014;67(4):283–293. doi:10.1016/j.rec.2013.12.005
- McCambridge J, Keane C, Walshe M, et al. The prehospital patient pathway and experience of care with acute heart failure: a comparison of two health care systems. ESC Heart Fail. 2021;8(2):1076–1084. doi:10.1002/ehf2.13089
- Medical Advisory Secretariat. Community-based care for the specialized management of heart failure: an evidence-based analysis. Ont Health Technol Assess Ser. 2009;9(17):1–42.
- SEC-PRIMARIA. Heart failure process. Available from: https://secardiologia.es/images/institucional/sec-calidad/sec-primaria/SEC-AP_IC_2021_20210922_DEFINITIVO.pdf. Accessed March 13, 2023.
- Barrios V, Cosín-Sales J, Bravo M, et al. Telemedicine consultation for the clinical cardiologists in the era of COVID-19: present and future. Consensus document of the Spanish society of cardiology. Rev Esp Cardiol. 2020;73(11):910–918. doi:10.1016/j.rec.2020.06.032
- Alvarez P, Sianis A, Brown J, Ali A, Briasoulis A. Chronic disease management in heart failure: focus on telemedicine and remote monitoring. Rev Cardiovasc Med. 2021;22(2):403–413. doi:10.31083/j.rcm2202046
- Imberti JF, Tosetti A, Mei DA, Maisano A, Boriani G. Remote monitoring and telemedicine in heart failure: implementation and benefits. Curr Cardiol Rep. 2021;23(6):55. doi:10.1007/s11886-021-01487-2
- Silva-Cardoso J, Juanatey JRG, Comin-Colet J, Sousa JM, Cavalheiro A, Moreira E. The future of telemedicine in the management of heart failure patients. Card Fail Rev. 2021;7:e11. doi:10.15420/cfr.2020.32
- Yun S, Enjuanes C, Calero E, et al. Study design of Heart failure Events reduction with Remote Monitoring and eHealth Support (HERMeS). ESC Heart Fail. 2020;7(6):4448–4457. doi:10.1002/ehf2.12962
- Yun S, Enjuanes C, Calero-Molina E, et al. Usefulness of telemedicine-based heart failure monitoring according to ‘eHealth literacy’ domains: insights from the iCOR randomized controlled trial. Eur J Intern Med. 2022;101:56–67. doi:10.1016/j.ejim.2022.04.008
- Yun S, Enjuanes C, Calero-Molina E, et al. Effectiveness of telemedicine in patients with heart failure according to frailty phenotypes: insights from the iCOR randomised controlled trial. Eur J Intern Med. 2022;96:49–59. doi:10.1016/j.ejim.2021.09.021
- Comín-Colet J, Enjuanes C, Verdú-Rotellar JM, et al. Impact on clinical events and healthcare costs of adding telemedicine to multidisciplinary disease management programmes for heart failure: results of a randomized controlled trial. J Telemed Telecare. 2016;22(5):282–295. doi:10.1177/1357633X15600583
- Jiménez-Marrero S, Yun S, Cainzos-Achirica M, et al. Impact of telemedicine on the clinical outcomes and healthcare costs of patients with chronic heart failure and mid-range or preserved ejection fraction managed in a multidisciplinary chronic heart failure programme: a sub-analysis of the iCOR randomized trial. J Telemed Telecare. 2020;26(1–2):64–72. doi:10.1177/1357633X18796439
- Brownell NK, Ziaeian B, Fonarow GC. The gap to fill: rationale for rapid initiation and optimal titration of comprehensive disease-modifying medical therapy for heart failure with reduced ejection fraction. Card Fail Rev. 2021;7:e18. doi:10.15420/cfr.2021.18
- Sayer G, Bhat G. The renin-angiotensin-aldosterone system and heart failure. Cardiol Clin. 2014;32(1):21–32. doi:10.1016/j.ccl.2013.09.002
- Antoine S, Vaidya G, Imam H, Villarreal D. Pathophysiologic mechanisms in heart failure: role of the sympathetic nervous system. Am J Med Sci. 2017;353(1):27–30. doi:10.1016/j.amjms.2016.06.016
- Hubers SA, Brown NJ. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation. 2016;133(11):1115–1124. doi:10.1161/CIRCULATIONAHA.115.018622
- Nightingale B. A review of the proposed mechanistic actions of sodium glucose cotransporter-2 inhibitors in the treatment of heart failure. Cardiol Res. 2021;12(2):60–66. doi:10.14740/cr1221
- Sandner P, Zimmer DP, Milne GT, Follmann M, Hobbs A, Stasch JP. Soluble guanylate cyclase stimulators and activators. Handb Exp Pharmacol. 2021;264:355–394. doi:10.1007/164_2018_197
- Sandner P, Follmann M, Becker-Pelster E, et al. Soluble GC stimulators and activators: past, present and future. Br J Pharmacol. 2021. doi:10.1111/bph.15698
- McMurray JJV, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction? A redefinition of evidence-based medicine. Circulation. 2021;143(9):875–877. doi:10.1161/CIRCULATIONAHA.120.052926
- Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–1893. doi:10.1056/NEJMoa1915928
- Lam CSP, Mulder H, Lopatin Y, et al. Blood pressure and safety events with vericiguat in the Victoria trial. J Am Heart Assoc. 2021;10(22):e021094. doi:10.1161/JAHA.121.021094
- Voors AA, Mulder H, Reyes E, et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: insights from the Victoria (Vericiguat global study in subjects with HFrEF) trial. Eur J Heart Fail. 2021;23(8):1313–1321. doi:10.1002/ejhf.2221
- Lam CSP, Giczewska A, Sliwa K, et al. Clinical outcomes and response to vericiguat according to index heart failure event: insights from the Victoria trial. JAMA Cardiol. 2021;6(6):706–712. doi:10.1001/jamacardio.2020.6455
- Ezekowitz JA, O’Connor CM, Troughton RW, et al. N-terminal pro-B-type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail. 2020;8(11):931–939. doi:10.1016/j.jchf.2020.08.008
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi:10.1056/NEJMoa1409077
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi:10.1056/NEJMoa1911303
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi:10.1056/NEJMoa2022190
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi:10.1016/j.jacc.2021.12.012
- McDonald M, Virani S, Chan M, et al. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol. 2021;37(4):531–546. doi:10.1016/j.cjca.2021.01.017
- González-Juanatey JR, Anguita-Sánchez M, Bayes-Genís A, et al. Vericiguat in heart failure: from scientific evidence to clinical practice. Rev Clin Esp. 2022;222(6):359–369. doi:10.1016/j.rceng.2021.12.006
- Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73–84. doi:10.1016/j.jchf.2021.09.004
- Greene SJ, Tan X, Yeh YC, et al. Factors associated with non-use and sub-target dosing of medical therapy for heart failure with reduced ejection fraction. Heart Fail Rev. 2022;27(3):741–753. doi:10.1007/s10741-021-10077-x
- Vericiguat. Summary of product characteristics; 2021. Available from: https://www.ema.europa.eu/en/documents/assessment-report/verquvo-epar-public-assessment-report_en.pdf. Accessed March 13, 2023.