Abstract
Purpose
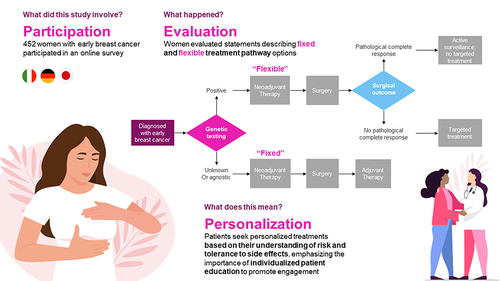
Patients with early breast cancer (eBC) are increasingly provided with different options, which may involve a sequence of different treatments and treatment modalities, and eligibility for certain adjuvant treatments depending upon pre-surgical and surgical outcomes. This study examined patient preferences around aspects of treatment decision-making in eBC.
Patients and Methods
A total of 452 patients with self-reported eBC in Germany (n=151), Italy (n=151), and Japan (n=150) completed an online survey about physician interactions and treatment side effects. The survey included best-worst scaling (BWS) to assess prioritization of 13 statements reflecting aspects of treatment decision-making. In a series of choice tasks, participants chose their most and least preferred options among subsets of 4 statements. Hierarchical Bayesian modeling was used to estimate BWS preference scores for each statement. BWS scores were based on the number of times a statement was chosen as most versus least preferred; scores total 100 for each patient.
Results
The most preferred aspects of treatment decision-making were “treatment aggressiveness matches personal risk” (mean BWS score = 13.49), “being told about what is coming” (13.18), deciding based on “own surgical outcome” (11.90), “avoiding unnecessary treatment” (10.35), and “involving in treatment decisions” (9.44). The least preferred aspects were “not being asked about treatment decisions along the way” (3.27) and “receiving the same treatment as other patients” (3.41). Patients in Japan preferred “being told about what is coming”, “deciding based on own surgical outcome”, “avoiding unnecessary treatment”, and being “involved in decisions” more than patients in Italy and Germany. Patients in Germany were more satisfied with their physician interactions and care, although their outcomes were not always better than those in Italy and Japan.
Conclusion
Patients value individualized treatment tailored to their risk of recurrence and tolerance of side effects, highlighting the need for focused patient education about options, to encourage their engagement.
Plain Language Summary
New treatment pathways based on promising biomarkers are being studied in early breast cancer. This study aimed to understand the importance that patients may place on different features describing how decisions are made along potential treatment pathways for early breast cancer. Participants in Italy, Germany, and Japan were asked to compare various aspects of treatment decision-making and choose those that were most and least important to them. Among the aspects tested, the top 4 were similar across countries: the desire to receive treatment with a level of aggressiveness that matches their individual prognosis, the need to receive adequate and timely information about their upcoming treatment, the need to tailor treatment decisions based on their individual surgery outcomes, and a desire to avoid overtreatment. Not being involved in treatment decisions was the least preferred of the aspects. Patients in Germany and Italy most valued the ability to tailor the aggressiveness of their treatment based on their individual risk of recurrence, whereas patients in Japan prioritized being knowledgeable and prepared for their treatment journey. The results from this study emphasize patients’ desire to be adequately informed about available treatment choices for early breast cancer, to avoid unnecessary treatments, and to be involved in treatment decisions.
Abbreviations
ANOVA, analyses of variance; BC, breast cancer; BWS, best-worst scaling; eBC, early breast cancer; EU-27, European Union-27; HER2, human epidermal growth factor receptor 2; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; IV, intravenous; PARP, poly (ADP-ribose) polymerase; SD, standard deviation.
Data Sharing Statement
Data underlying the findings described in this manuscript cannot be shared due to the content of the Informed Consent forms signed by the patients. Please visit our Disclosure Commitment page for guidance on the AstraZeneca Data Sharing Policy: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics Approval and Informed Consent
The authors state that this study received exemption status from full or expedited ethical review by Pearl IRB, that they have followed the principles outlined in the Declaration of Helsinki for all human or experimental investigations, and that informed consent was obtained electronically from the patients involved.
Acknowledgments
Medical writing support was provided by Shalini Vasantha, Ph.D., and Ramu Periyasamy, Ph.D., from Indegene Pvt. Ltd, Bangalore, India, on behalf of Cerner Enviza. The data have been presented previously as a poster at the 18th St.Gallen International Breast Cancer Conference, February 28, 2023 - March 1, 2023, Vienna, Austria.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
KB, EM, and DM are employees and/or stockholders of Cerner Enviza, an Oracle company, which provides consulting services to AstraZeneca. At the time of the study, XG was an employee and stockholder of Cerner Enviza. EF and SMcC are employees and/or stockholders of AstraZeneca. At the time of the study, SM was an employee and stockholder of AstraZeneca.