Abstract
Purpose
This study evaluated the human factors affecting the ease of use of a disposable autoinjector developed for subcutaneous self-injections performed by patients with chronic diseases.
Materials and methods
This was a randomized, single-center study conducted with 65 patients with rheumatoid arthritis. Patients performed six simulated injections. Assessments of patient device acceptance and device usability were made by patient reports and independent observations of compliance with the device instruction for use (IFU) following single injections and repeated injections.
Results
A total of 390 simulated injections were performed. Patient device acceptance was high; more than 90% of patients found each of the tested criteria to be acceptable (>80% was required for statistical significance; P<0.016). Perceived ease of use and simplicity of the three-step process resulted in high acceptance scores: mean scores (± standard deviation) were 8.71 (±1.18) and 8.05 (±0.37), respectively, on a 0–10-point scale. Patients also expressed their acceptance with the ease and usefulness of the detection of the remaining drug in the autoinjector. In addition, 80% of patients declared that they would recommend the device to someone else. Globally, the human factors tested (age, sex, hand disability [Cochin score], extent of previous experience with self-injection [ie, expert or naïve]) had no impact on IFU device compliance. In particular, the lack of a Cochin score interaction indicated that the degree of hand disability is not a predictive factor of poor self-injection capability with this autoinjector.
Conclusion
This study demonstrated a high level of patient acceptance for self-injection with this autoinjector among patients with rheumatoid arthritis. In particular, patients with severe hand disability were able to successfully comply with device IFU.
Introduction
Chronic autoimmune inflammatory diseases, such as rheumatoid arthritis (RA), multiple sclerosis (MS), and Crohn’s disease are progressive conditions associated with disability, morbidity, and mortality.Citation1 The introduction of injectable, disease-modifying drugs a decade ago has had a considerable impact on the progression of such diseases. These drugs arrest joint destruction, increase physical activity, and improve quality of life in patients with RA, and they also arrest disease exacerbation for extended periods in MS.Citation2
Treatment nonadherence is considered to be a major issue in contemporary medicine, and the World Health Organization claimed in 2003 that improving patient adherence to long-term therapies would be more beneficial than any biomedical progress.Citation3 Consequently, patients’ adherence to treatment has become a major requirement for achieving optimal treatment efficacy and expected therapeutic outcomes.Citation4
Several studies have demonstrated that self-injection versus injection by health care workers can increase patient treatment adherence and reduce costs by decreasing the frequency of hospital visits, which benefits patients in terms of cost, time, ease of use, improved self-esteem, and greater independence in their social, domestic, and professional lives.Citation5,Citation6
A recent multicenter observational study in patients with MS reported that 25% of 2,566 patients for whom data were available were nonadherent (defined as missing at least one disease-modifying drug injection over a 4-week period).Citation7 Among the nonadherent patients, 32% reported injection-related reasons for their behavior. Limited prospective data on factors that influence adherence to disease-modifying drugs in MS identified the following factors: problems with injecting; reduced manual dexterity, which can make the correct self-injection procedure physically problematic; dependence on others; perceived lack of efficacy; and adverse events (injection site reactions and injection pain).Citation8,Citation9 Factors that influence compliance to injectable treatment in RA remain under-investigated, even though more newer injectable biologics are becoming available for use.Citation10 As in MS, patients with RA have reduced manual dexterity, which can make the correct self-injection procedure physically problematic.
In response to these potential problems with injections, various injection technologies (for example, autoinjection devices) have been introduced, which are designed to improve the ease of use, safety, and reliability of injections, and to reduce pain.Citation2 Autoinjectors automatically insert the needle and deliver a controlled dose of drug, such as the disease-modifying drugs used by patients with MS and RA. Autoinjectors have been shown to provide a number of benefits, including a reduced risk of injection site reactions, reduced discomfort, and greater ease of use compared with manual (syringe plus needle) injections.Citation11,Citation12 The BD Physioject (BD, Franklin Lakes, NJ, USA) has been specifically designed to help improve adherence to a subcutaneous (SC) injection schedule. Previous studies have demonstrated its performance and safety in healthy volunteersCitation11 and its acceptance in patients with MS.Citation13 This study evaluated its usability in a cohort of patients with RA because, among chronic diseases, RA can be considered as the worst case of hand disability that might affect the patient’s ability to deliver their dose of the drug. The primary study objectives were to evaluate patient device acceptance among naïve and experienced users of autoinjectors among patients with RA. Patients were asked to rate the perceived required force to press the button, the perceived required force to press the autoinjector onto the foam pad during injection, and the perceived visibility of the end of the injection process. These are the three steps required for successful injection: 1) correctly activate the system (force to press the button); 2) button can be activated only if the system is correctly pressed to injection site (force to press the autoinjector); 3) do not remove the system before the end of the injection (end of injection visibility). The secondary objectives were to evaluate usability (ie, compliance with the device instructions for use [IFU]) in this population under chronic treatment conditions (repeated simulated injections).
Materials and methods
Study design
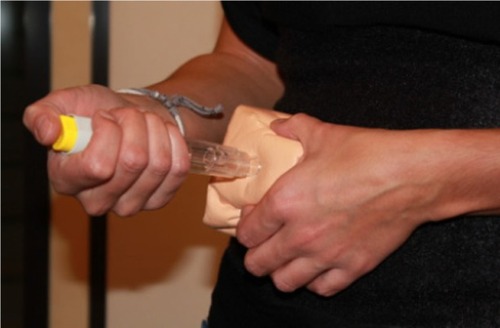
This was a randomized, single-center, study conducted in France in compliance with Good Clinical Practice according to European directives and French laws. Patients were recruited by the Rheumatology Department of the University Hospital of Grenoble between April 2010 and June 2010, and were observed by a clinical research associate employed by this hospital. Two observers were trained by BD. Instructions were written to help observers standardize their observations and communicate the same level of information to the patients. The testing environment simulated the environment of a physician’s office/clinic (for example, there was sufficient lighting, and only an observer was present with the patient in the testing room). Each patient performed a total of six simulated SC injections using a foam pad developed by BD (), which was applied to the usual injection sites (ie, the abdomen and thigh).
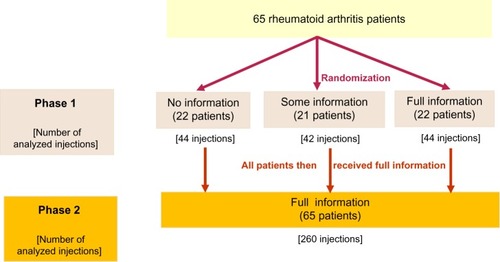
In phase 1 of the study, all patients were told that they were being given an autoinjection device meant to be used at home. Patients were randomized to receive full information (ie, device IFU), some information about the injector and injection process, or no information before first use of the device ( and ). The external observer was unmasked regarding the information level. Patients in the “no information” subgroup were asked to do the best job they could to figure out how to use the autoinjector without any instructions. Patients who received some information were asked to carefully study the instructions, which were comprised of pictures and minimally written explanations of the process. Patients’ questions were answered by repeating the provided instructions. Patients in the full information subgroup were asked to carefully read the complete instructions and their questions were fully answered by the observer. Injections were also randomized, as patients were instructed to inject a particular site on a particular side of the body. In phase 1, patients made two individual injections; they were monitored by an independent observer trained to be able to detect noncompliance with device IFU.
Table 1 Information received by patients in each subgroup
In phase 2, all patients were provided with full information about the device, and they were asked to make four repeated injections. Patients were again monitored by a trained independent observer.
As an exploratory objective at the end of study session to validate the foam pad, each patient performed two injections directly on his/her body using a needleless autoinjector. Compliance with device IFU and the patient’s level of acceptance of creating a skin fold during an injection were assessed.
Patients
Patients were recruited by the investigator (a rheumatologist) while they attended the clinic for treatment or for a medical consultation. If the investigator deemed that a patient was eligible for self-injection, he or she was invited to participate in the study. In order to be representative of the RA population, a 1:3 ratio of males and females, respectively, was expected. No other eligibility criteria (or ratios) were applied. Patient age, degree of hand disability, and previous self-injection experience were recorded after inclusion, but there were no limits on these parameters. Hand disability was assessed using the Cochin score, a validated instrument commonly used in clinical trials and epidemiological studies in the RA population. This scores responses to 18 questions on daily from 0 (activity performed without difficulty) to 5 (impossible to do).Citation14,Citation15 A minimum of 60 evaluable patients (ie, without missing data on the primary objective analysis) were required to be able to detect patient acceptance greater than 80% (with a power of 88% and a risk 1 error of 5%). Recruitment was halted after the 65th patient because 60 patients were evaluable. A total of 390 simulated injections were performed and analyzed.
Study assessments
The survey questionnaire is presented in . Three questions (Q1–Q3) were used to assess the perceived ease and comfort of use, as reported by the patient after each phase. Patients completed an 11-point Likert scale (scores ranging from 0–10). To complete the patient device acceptance assessment concerning the system’s ease of use, each patient answered four complementary questions (questions Q4–Q7). For their willingness to adopt the device, there were five specific questions in the survey addressing this parameter (Q8–Q12).
Table 2 Survey questionnaire
Usability (ie, patients’ compliance with device IFU) was evaluated by the independent observer who observed the handling of the autoinjector system during injections, while recording each error. Compliance with device IFU was assessed by analyzing the percentage of injections where all steps were correctly performed.
The effect of patients’ characteristics and human factors on patient device acceptance and device usability was assessed according to age, sex, Cochin score, previous practical experience in self-injection (naïve or expert patients), information subgroup (in phase 1), and side and site of the body used for simulated injections.
Data analysis
Data were analyzed using SAS version 9.2 (SAS Institute Inc, Cary, NC, USA). For the primary objective (ie, the detection of patient device acceptance >80% with a power of 88%), the type 1 error was set to 0.05 and a P-value of ≤0.05 was considered as statistically significant.
Eleven-point Likert scales were used to assess patient device acceptance across the three test criteria. Responses in the range of 0–5 on the Likert scale were classified as “not acceptable”, and responses in the range of 6–10 were classified as “acceptable.” A score of 8 points or above was considered as high acceptance. For each phase, an overall binary acceptability criterion, defined as an acceptable score (≥6) for the three Likert scales (Q1–Q3) was also computed.
For each criterion, the proportion of patients acceptant was compared to 80% using a one-sided exact binomial test. Binary criteria were also compared between the two phases using McNemar’s test. Compliance with device IFU (usability) was analyzed by the following descriptive statistics: the number of missing data, mean, standard deviation (SD), minimum and maximum, quartiles (quartile 1, quartile 2, and quartile 3), and two-sided 95% confidence intervals (CIs) for continuous variables; and the number of missing data, frequencies, percentages, and two-sided 95% CIs (using the Cloper–Pearson exact method) for categorical variables.
To assess the effect of human factors on patient device acceptance and device usability, mean acceptance scores were analyzed and compared between subgroups (age, sex, Cochin score, previous practical experience in self-injection, information subgroup [in phase 1], and side and site of the body used for simulated injections) using mixed models analysis of variance using an autoregressive correlation structure.
Results
Patient population
All 65 patients with RA included in this study had Cochin scores ≤79 at baseline, which correspond with severe hand disability (). Thirty-one patients were experienced (ie, had experiences with self-injection using a syringe and/or an autoinjector prior to inclusion in the study) and 34 were naïve. In phase 1, there were no major differences in patient profiles (ie, age, sex, Cochin score, and previous practical experience in self-injection) between the three study arms (). A total of 390 simulated injections were performed.
Figure 3 Distribution of Cochin scores (a measure of the severity of hand disability) in our study population (N=65).
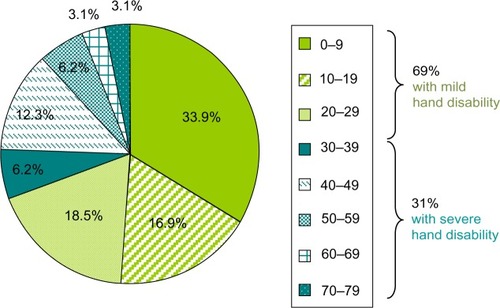
Table 3 Selected baseline characteristics of patients included in phase 1
Patient device acceptance
In phase 2, patient device acceptance was high (ie, >8). A statistically significant number of patients (>90%; P<0.016) found each of the three test criteria (Q1, Q2, and Q3) to be acceptable. Overall patient device acceptance (Q1, Q2, and Q3) was also high (81.5%) in phase 2, but these scores did not reach statistical significance (P=0.451) ().
Table 4 Acceptance scores for device functions in phase 2
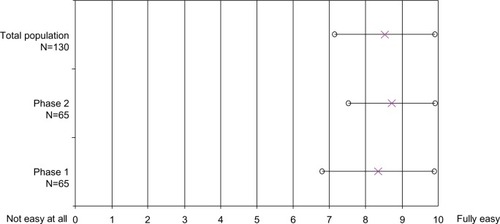
Perceived ease of use (Q4) and the simplicity of the three-step injection process (Q5) obtained high acceptance scores, with mean scores of around 9 out of 10. The mean score (±SD) for perceived ease of use (Q4) was 8.34 (±1.54) after phase 1 and 8.71 (±1.18) after phase 2. The perceived ease of use seemed to increase with utilization (). For the simplicity of the three-step process (Q5), the mean score was 8.65 (±1.18).
Figure 4 Patients’ acceptance about the ease of use of the autoinjector (Q4).
The mean score for the ease of detecting the remaining drug (Q6) in the autoinjector was 7.83 (±2.23), and the mean score for the usefulness of this feature (Q7) was 8.97 (±1.70). A total of 80% of patients declared that they would recommend the autoinjector to someone else (Q8) (ie, 80% of patients gave a score ≥6 for this question; the mean score ±SD was 8.05±1.96). The willingness to adopt the autoinjector for further SC treatment was also very high; 90.8% of patients gave a score ≥6 for further self-treatment (Q12), and 73.8% would accept further treatment from a nurse (Q10) ().
Table 5 Willingness to accept further subcutaneous treatment (four different treatment configurations)
The effect of human factors on patient device acceptance
The effects of different human factors are presented in . Hand disability (Cochin score) impacted patient device acceptance, and this was only in phase 1, where the percentage of patients who found the three test criteria to be “acceptable” was significantly higher in patients with lower degrees of hand disability (Cochin score <30; P=0.018). There was no statistically significant difference in phase 2.
Table 6 Effect of human factors on patient device acceptance and willingness to adopt
Concerning the other parameters of patient device acceptance (ie, perceived ease of use and simplicity of the process in three steps; ease and usefulness in detecting the remaining drug; and willingness to recommend the tested autoinjector to someone else and to accept a further treatment by self-injections with the tested autoinjector), age group seems to impact the mean device acceptance score to the greatest degree (see ), and previous experience in self-injection has an effect that approaches statistical significance (P=0.05) for ease of use.
Autoinjector usability in patients with RA
In phase 2, all patients were in “full information” conditions, so there was no difference in the information provided between the groups. A total of 85.3% of the eleven steps were properly performed by the patients ().
Figure 5 Compliance with device instructions for use.
Abbreviations: info, information; N, number; Abdo, abdomen.
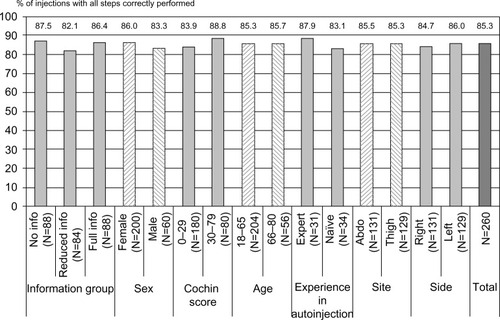
The effect of human factors on usability
An analysis of the effects of the same factors was performed for compliance with device IFU (ie, the percentage of injections where all of the steps were performed correctly), but no effect was detected. In particular, the Cochin score had no significant impact on the percentage of correct steps (83.9% versus 88.8% for Cochin scores of <30 and 30–79, respectively; P=0.088) (). These results indicate that the degree of hand disability was not a predictive factor of poor self-injection capability.
Device performance
During the study, all of the automated features and other functions of the device (button activation, full volume delivered, and needle cover automatically activated) worked correctly regardless of which human factors were being tested. The needle cover automatically activated on every occasion (390 injections) and no accidental activations were observed.
Foam pad validation
Compliance with device IFU and patient acceptance of the required skin fold during the injection with the needleless system used on the body were completely similar to that observed with the system used with the foam pad. These results validate the ability of the foam pad developed by BD to replace injection on the body, as simulated injection on this foam pad did not impact the study’s results.
Discussion
Reduced manual dexterity (which can make self-injection of drugs physically problematic), dependence on others, perceived lack of efficacy, and adverse events (such as injection site reactions and injection pain) are the main reasons for treatment noncompliance identified in patients suffering from chronic diseases such as RA.
Overall, the characteristics of patients enrolled in the study and the study results were in line with previously published data.Citation15–Citation19 The study met the design requirements for the numbers of patients enrolled (at least 60 patients were required in order to assess the primary objective), and the ratio of males to females (23.1% of patients were male, which reflects the range of males [10%–30%] in the typical population that have RA).Citation16,Citation17
In the study population, 74% of patients were aged 41–65 years old. Patients were directly recorded into age bands rather than by their actual ages, so it was impossible to calculate the mean age, but these descriptive results seem concordant with published data from three studies, which assessed the mean age of cohorts of patients with RA between 54 and 59 years of age.Citation15,Citation18,Citation19
In our study population, 31.8% of patients reported medium or severe hand disability (Cochin score >30), and more than 12% had a Cochin score >50. The investigator thought that he subconsciously filtered high hand disability (ie, those with a high Cochin score) when he had to assess RA patient eligibility for self-injection in his daily practice. On the contrary, the study population characteristics demonstrate that the proportion of patients with high hand disability, and yet who were also judged to be eligible for and successful at self-injection, was higher than expected by the investigator, and it was similar to that observed in the general RA population. Indeed, the distribution of Cochin scores is similar to that described in the literature in a cohort study that included 55 consecutive ambulatory and hospitalized patients with RA, 75% had a mild hand disability (Cochin score <30).Citation15 The mean Cochin score was 19.51 (±17.94; range: 0–65; quartile 1=6, quartile 3=30). In this cohort study, patients were not necessarily eligible for self-injection.
Among published studies concerning patient acceptance with autoinjectors, only one assessed the overall patient acceptance.Citation20 The others assessed isolated acceptance criteria such as ease of use, willingness to pursue the treatment with the tested autoinjector, or device preference.Citation13,Citation21–Citation24 One survey of overall patient acceptance in 61 patients measured acceptance of the utilization of an electronic autoinjector (Easypod®; EMD Serono, Rockland, MA, USA) to administer a hormone. After 15 days, 98% of responders had a good or very good opinion about the device, and 87% expressed a preference for using the device for a future treatment.Citation20 However, the method used in the study was questionable: each question had only three possible answers (bad, good, or very good). Moreover, the number of “very good” answers was not published, and the study addressed a pediatric population, so comparing these published data with the present study might not be useful.
The increase in perceived ease of use was also observed in a clinical trial assessing the effective use of an autoinjector (Avonex®; Biogen Idec Inc, Cambridge, MA USA) in 74 patients with MS.Citation23 The ease of use scores were similar to those observed here, and they increased from the first injection (mean score of 8.1) to the fourth score (mean score of 9.1).
In a recent survey, patients were asked to identify the characteristics of an ideal autoinjector.Citation22 The most frequently cited characteristics included the ability to carry out the injection simply and swiftly in just a few steps, drug release only upon skin contact, and prevention of accidental activation. This present study demonstrated high acceptance scores for ease of use and process simplicity.
The results of the present study agree with those of the more recent MOSAIC study,Citation13 which used a variant (RebiDose™; Merck Serono, Geneva, Switzerland) of the autoinjector used here. That 12-week, Phase IIIB clinical trial conducted with 121 patients with MS evaluated the ease of use of the autoinjector, as well as its functional reliability. After the first injection, 93.6% of patients answered that the injection was easy (36.7%) or very easy (56.9%), as measured on a five-point scale (with responses ranging from very difficult to very easy). The lack of accidental activations in this study also concurs with the findings from a previous study involving 480 real injections.Citation11
Results comparing patients’ willingness to recommend an autoinjector to someone else are difficult to find. The only results available in the literature concern a study conducted with real SC injections in a 1-year contraception study in which patients self-injected once every 12–14 weeks.Citation25 Fifty women were included after having undergone training in a simulated arm, and they made a first successful self-injection, which was observed by an investigator. A total of 94% of these women answered that they were (somewhat) likely to recommend self-injection.
The willingness to accept further SC treatment by self-injection with the BD Physioject™ was very high (90.8% of patients gave a score ≥6); this result is higher than that of the MOSAIC study, where 73% of patients said they would continue to use the experimental autoinjector if it became available, but it was similar to the findings of another study in which patients were using an autoinjector to take hormones (87% would like to pursue use of the device for a future treatment).Citation13,Citation20
Compliance with device IFU was excellent in both naïve and experienced patients, with 85% of patients able correctly to perform 100% of the injection steps. In phase 1, compliance with device IFU in patients in the “full information” group (mean: 97%; range: 64%–100%; 95% CI: 92.4%, 99.2%) was higher than in an earlier study in 40 healthy volunteers, where the mean compliance rate was 93% (range: 40%–100%; 95% CI; 79.6%, 98.4%).Citation11 Two reasons might explain this: first, the study in healthy volunteers was conducted with an earlier version of the IFU – the version used in the present study incorporated key learnings from this previous study; second, patients with RA felt more concerned by the RA treatment and they were more experienced in this type of treatment than healthy volunteers. The results pertaining to compliance with device IFU from the present study are similar to those for an autoinjector used in a Phase IIIB open clinical trial conducted among patients with MS where the overall success rate was 89%.Citation23
Globally, the effect of the human factors tested (age, sex, hand disability, previous practical experience with self-injection) did not significantly impact scores for autoinjector usability in this cohort of patients with RA. This absence of effect on the usability of the same device confirms the findings of the MOSAIC study, which found that neurological and cognitive deficits, as well as fatigue, generally had no influence on ratings of satisfaction, functional reliability, or ease of use among patients with MS.Citation13
A threshold of 80% was chosen for comparisons of patient acceptance because it was the threshold considered as satisfactory in several acceptance studies.Citation21,Citation28,Citation29
The robustness of the method used to assess the effect of human factors – and, hence, the confidence in the observed results – is high. Indeed, although this study was conducted prior to the publication of the US Food and Drug Administration’s draft guidance about human factor studies, the method used was compliant with this new guidance concerning sample size and user environement.Citation30 If users with distinctly different characteristics (for example, age, skill sets, or experience levels) are going to use a new device, this guidance indicates that validation testing activities should include 15 patients from each major user group. In the present study, the number of patients in each subgroup was close to or greater than 15, irrespective of the factor being tested (see ).
Limitations of the current study
The methods and tools used in this study were based on those used in other published studies; the most common tool used to assess patient satisfaction and acceptance is the Likert scale, either with a 1–5-point or 0–10-point format.Citation6,Citation22,Citation23,Citation25–Citation27 The 0–10-point scale is a tool commonly used in studies assessing acceptance of autoinjectors,Citation22,Citation23 and it was the scale used in a previous BD marketing study, which is the main reason why it was used in this study; however, for performance criteria such as ease of use, a 1–5-point scale could have been more powerful.
The questionnaires used to capture information relevant to the evaluation of patient device acceptance were not formally validated prior to this study. A self-injection assessment questionnaire using a 1–5-point Likert scale was published after the conduction of the present study, and it was demonstrated to be a valid and reliable tool for RA patients to use to assess their success in giving self-injections, while also determining the likelihood with which they would adhere to a self-injection regimen.Citation6 This tool could be used in a further study with this autoinjector to assess the perceived advantages and the potential barriers to self-injection (including the psychological, social, and physical barriers, as well as the level of acceptance of self-injection and willingness to continue the treatment by self-injection).
This study was a monocentric study conducted in France alone. Even if the sample size and RA population representativity were considered, a larger scale study may reinforce our results.
Conclusion
This study found high patient acceptance of the autoinjector in each of the tested criteria. In the full information group, compliance with device IFU was excellent in naïve and experienced patients, regardless of the patient’s age and degree of hand disability. This means that even patients with severe hand disability can comply with device IFU, while managing self-injection successfully. Therefore, the findings of this study add to the evidence supporting the use of autoinjectors to help improve treatment compliance among patients with chronic diseases.
Acknowledgments
The authors thank Frédéric Mistretta and Jean-François Ferlet (RCTs, Lyon, France) for their help in conducting data management and analysis; Sandrine Massicot, Fatah Tidadini (clinical research associates), and Mélanie Gilson (rheumatologist) of the University Hospital of Grenoble for their help in conducting the clinical study.
We thank the reviewers for their valuable comments, which helped to considerably improve the quality of the manuscript.
Disclosure
The study was funded and sponsored by Becton Dickinson – BD Medical Pharmaceutical Systems. FS, MDT, HA, JC, and CG are employed by BD Medical Pharmaceutical Systems, within the Medical Affairs and Research and Development departments. LG is employed by the University Hospital of Grenoble (Echirolles, France). PEL is employed by Creabio-ra SAS (Givors, France). The authors report no other conflicts of interest in this work.
References
- GabrielSEMichaudKEpidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseasesArthritis Res Ther200911322919519924
- LugaresiAAddressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivation?Expert Opin Drug Deliv200969995100219637982
- World Health OrganizationAdherence to Long-Term Therapies: Evidence for ActionGeneva, SwitzerlandWorld Health Organization2003
- DiMatteoMRVariations in patients’ adherence to medical recommendations: a quantitative review of 50 years of researchMed Care200442320020915076819
- ArthurABKlinkhoffAVTeufelASafety of self-injection of gold and methotrexateJ Rheumatol19992623023059972962
- KeiningerDCoteurGAssessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ)Health Qual Life Outcomes20119221232106
- DevonshireVLapierreYMacdonellRGAP Study GroupThe Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosisEur J Neurol2011181697720561039
- CostelloKKennedyPScanzilloJRecognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long termMedscape J Med200810922519008986
- TreadawayKCutterGSalterAFactors that influence adherence with disease-modifying therapy in MSJ Neurol2009256456857619444532
- MohrDCCoxDEpsteinLBoudewynATeaching patients to self-inject: pilot study of a treatment for injection anxiety and phobia in multiple sclerosis patients prescribed injectable medicationsJ Behav Ther Exp Psychiatry2002331394712389798
- BerteauCSchwarzenbachFDonazzoloYEvaluation of performance, safety, subject acceptance, and compliance of a disposable autoinjector for subcutaneous injections in healthy volunteersPatient Prefer Adherence2010437938821049090
- LugaresiADurastantiVGasperiniCCoSa Study GroupSafety and tolerability in relapsing-remitting multiple sclerosis patients treated with high-dose subcutaneous interferon-beta by Rebiject autoinjection over a 1-year period: the CoSa studyClin Neuropharmacol200831316717218520983
- WraySArmstrongRHerrmanCResults from the single-use autoinjector for self-administration of subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis (MOSAIC) studyExpert Opin Drug Deliv20118121543155322032264
- Lefevre-ColauMMPoiraudeauSFermanianJResponsiveness of the Cochin rheumatoid hand disability scale after surgeryRheumatology (Oxford)200140884385011511751
- PoiraudeauSLefevre-ColauMMFermanianJRevelMThe ability of the Cochin rheumatoid arthritis hand functional scale to detect change during the course of diseaseArthritis Care Res200013529630314635299
- TangKBeatonDEGignacMALacailleDZhangWBombardierCCanadian Arthritis Network Work Productivity GroupThe Work Instability Scale for rheumatoid arthritis predicts arthritis-related work transitions within 12 monthsArthritis Care Res (Hoboken)201062111578158720521332
- PoiraudeauSLefevre-ColauMMFermanianJMayoux-BenhamouMARevelMQualités métrologiques de l’indice d’incapacité fonctionnelle de Cochin adapté à la main rhumatoïdeAnn Réadaptation Med Phys200043106115 French
- JawaheerDOlsenJLahiffMQUEST-RAGender, body mass index and rheumatoid arthritis disease activity: results from the QUEST-RA StudyClin Exp Rheumatol201028445446120810033
- RossiniMMaddali BongiSLa MontagnaGVitamin D deficiency in rheumatoid arthritis: prevalence, determinants and associations with disease activity and disabilityArthritis Res Ther2010126R21621114806
- DahlgrenJVeimoDJohanssonLBechIPatient acceptance of a novel electronic auto-injector device to administer recombinant human growth hormone: results from an open-label user survey of everyday useCurr Med Res Opin20072371649165517559757
- FuchsGSMikkelsenSKnudsenTKKappelgaardAMEase of use and acceptability of a new pen device for the administration of growth hormone therapy in pediatric patients: an open-label, uncontrolled usability testClin Ther200931122906291420110030
- Verdun di CantognoERussellSSnowTUnderstanding and meeting injection device needs in multiple sclerosis: a survey of patient attitudes and practicesPatient Prefer Adherence2011517318021573048
- PhillipsJTFoxEGraingerWTuccilloDLiuSDeykinAAn open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex® prefilled syringe in multiple sclerosis subjectsBMC Neurol20111112621999176
- Hey-HadaviJPleilADeebLCEase of use and preference for a new disposable self-injection pen compared with a reusable pen for administering recombinant human growth hormone: a multicenter, 2-month, single-arm, open-label clinical trial in patient-caregiver dyadsClin Ther201032122036204721118739
- PrabhakaranSSweetASelf-administration of subcutaneous depot medroxyprogesterone acetate for contraception: feasibility and acceptabilityContraception201285545345722079605
- GreenJKleinmanLCieslaGHuangJWintfeldNRevickiDSubcutaneous injection survey: psychometric evaluation of a treatment satisfaction instrument associated with a novel HIV medicationHIV Clin Trials20023538739512407488
- CohenCHellingerJJohnsonMPatient acceptance of self-injected enfuvirtide at 8 and 24 weeksHIV Clin Trials20034534735714583851
- TierneyPASamuelDPatelKSThomasDMAudit of patient acceptance of nasal surgery as a day case procedureBr J Clin Pract19965073573599015905
- DevonshireVArbizuTBorreBPatient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, Phase IIIb studyBMC Neurol2010102820433746
- US Food and Drug Administration [webpage on the Internet]Draft guidance for industry and Food and Drug Administration staff: applying human factors and usability engineering to optimize medical device designSilver Spring, MDUS Food and Drug Administration2011Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm259748.htmAccessed March 12, 2013