Abstract
Glaucoma is one of the leading causes of blindness and is characterized by optic nerve damage that results in visual field loss. Elevated intraocular pressure (IOP) has been associated with glaucoma progression; thus, IOP-lowering medications are the standard of care for glaucoma. Guidelines suggest monotherapy with IOP-lowering agents such as β-blockers (eg, timolol), prostaglandin analogs, carbonic anhydrase inhibitors (eg, brinzolamide), and α2-receptor agonists (eg, brimonidine). However, monotherapy may provide insufficient IOP reduction in some patients, thereby necessitating the use of multiple IOP-lowering medications. Multidrug regimens may be complex, may increase the risk of preservative-related ocular symptoms, and may potentially reduce overall drug exposure as a consequence of drug washout during closely timed sequential administrations; these difficulties may reduce overall drug efficacy and decrease patient persistence and adherence with multidrug treatment regimens. Fixed-combination medications that provide two IOP-lowering therapies within a single solution are available and may overcome some of these challenges. However, all currently available fixed combinations combine timolol with another IOP-lowering agent, indicating that additional fixed-combination alternatives would be beneficial. To meet this demand, a novel fixed combination of brinzolamide 1% and brimonidine 0.2% (BBFC) has recently been developed. In two randomized, double-masked, multinational clinical trials, BBFC had greater IOP-lowering efficacy than brinzolamide or brimonidine monotherapy after 3 months of treatment in patients with open-angle glaucoma or ocular hypertension. In both studies, the overall safety profile of BBFC was consistent with that of brinzolamide and brimonidine. Comparative studies with BBFC versus other IOP-lowering monotherapy and fixed-combination medications are not available, but the IOP reductions observed with BBFC are similar to or greater than those reported in the literature for other glaucoma treatments; thus, BBFC provides an additional fixed-combination therapeutic option for patients who require further efficacious IOP reduction and improved convenience and tolerability versus concomitant administration of two separate medications.
Introduction
In 2010, glaucoma accounted for over 8 million incidences of blindness worldwide and was one of the leading causes of blindness.Citation1 By 2020, an estimated 79 million individuals worldwide will have been diagnosed with glaucoma.Citation1 Glaucoma is characterized by elevated intraocular pressure (IOP), progressive optic neuropathy, and corresponding visual field loss.Citation2,Citation3 Lowering IOP to an individualized target level (typically a ≥25% reduction from initial IOP) and maintaining that level reduces the risk of vision loss and improves outcomes,Citation4–Citation6 even among patients with normal-tension glaucoma.Citation4 Reduction of elevated IOP is currently the only therapeutic approach effective for the prevention of glaucoma progression.Citation7
A wide array of IOP-lowering agents with different mechanisms of action are available, including β-blockers (eg, timolol), prostaglandin analogs (eg, latanoprost), carbonic anhydrase inhibitors (CAIs; eg, brinzolamide), and α2-adrenergic agonists (eg, brimonidine).Citation5,Citation8 These medications reduce IOP by decreasing aqueous production,Citation5 increasing aqueous outflow,Citation5,Citation9 or both. β-blockers and CAIs reduce aqueous production by limiting blood flow to the iris root–ciliary bodyCitation10 or through inhibition of sulfonamide-susceptible carbonic anhydrase isozymes, respectively.Citation11 In contrast, prostaglandin analogs reduce IOP by increasing uveoscleral and trabecular meshwork outflow of aqueous humor,Citation7,Citation12 and α2-adrenergic agonists reduce aqueous production and augment aqueous outflow through the uveoscleral pathway.Citation13
Standard first-line treatment for glaucoma consists of treatment with a single IOP-lowering medication;Citation5,Citation14 however, one prospective study showed that approximately 40% of patients require multiple IOP-lowering medications to reach and maintain their target IOP.Citation15 Unfortunately, persistence (ie, continued use of medication over time) with IOP-lowering medications is low.Citation16–Citation19 A systematic review of 14 studies that evaluated persistence using survival analysis demonstrated that only 31% of patients remained on their initial therapy at the end of 12 months.Citation16 Persistence may be affected by the medication and regimen prescribed. A retrospective United States health claims database study showed that persistence with prostaglandins, α2-receptor agonists, and CAIs for 3 years was greater than that with β-blockers.Citation18 However, drug-related differences in persistence likely disappear within a specific drug class; for example, a retrospective, population-based review of a United States claims database showed that a similar percentage of patients were persistent with their prescribed prostaglandin analog medication during a 1-year period regardless of the specific agent prescribed (ie, latanoprost [69.4%], travoprost [70.6%], or bimatoprost [68.1%]).Citation19 In addition to the specific medication given, the dosing regimen prescribed for an individual may affect persistence; patients with complex therapeutic regimens requiring separate administration of several therapeutic agents tend to have lower persistence.Citation20,Citation21
Medication adherence (ie, following the agreed-upon treatment regimen)Citation18,Citation22–Citation37 is also less than optimal among patients with glaucoma, even though reduced adherence with IOP-lowering medication has been linked with progressive visual field loss.Citation22,Citation38 Rates of adherence to IOP-lowering treatment among patients with glaucoma across multiple studies are shown in . Lack of patient adherence to their therapeutic regimen may ultimately decrease drug effectiveness. In a retrospective analysis of patient adherence in an ophthalmology clinic, 26.8% of patients did not achieve their target IOP as a result of nonadherence.Citation39 The reasons for patient nonadherence are diverse. Treatment complexity (eg, treatment with >1 IOP-lowering drug) and patients’ attitude toward, and insufficient knowledge of, glaucoma have been associated with reduced adherence.Citation23,Citation24,Citation26,Citation28,Citation32,Citation36,Citation40–Citation42 Other factors that may disrupt medication use by patients include cost and insurance coverage, forgetting to take the medication, difficulty with instillation of drops, higher number of daily doses, initial medication drug class, and poor tolerability.Citation18,Citation24,Citation27,Citation29,Citation36,Citation41,Citation42
Table 1 Treatment adherence rates among patients with glaucoma
Patients who require multiple concomitant medications to achieve and maintain IOP control may be more likely to deviate from their prescribed medication regimen. In a retrospective, open-label database review, addition of a second medication to a monotherapy regimen increased the time between medication refills by >2 weeks in some patients.Citation43 Trouble remembering to take medication and having difficulty opening medication bottles were reported by more patients receiving multiple concomitant glaucoma treatments than those receiving one medication; these complaints were associated with reduced adherence.Citation27 The efficacy, cost, and tolerability of multidrug regimens may also affect persistence and adherence. Persistence can be related to treatment efficacy because lack of efficacy often results in a switch in treatment. With administration of multiple medications, administration of a second drug within 5 minutes of an initial medication may cause substantial reductions in the concentration of the first drug because of washout of the first drug,Citation44 thereby potentially reducing overall IOP-lowering efficacy.Citation44 In a survey of patients using topical glaucoma medications, 23.5% of patients administered a second drop of medication within 5 minutes of the first drop, and 14% waited less than 2 minutes before instilling the second drop.Citation45 Additionally, exposure to more than one preserved topical medication (and therefore a greater cumulative exposure to irritating preservatives) may increase ocular symptomsCitation46,Citation47 and may predispose patients to discontinue their therapy. Cost may also be a significant burdenCitation42 because each separate drug solution may be associated with an additional copay.Citation48
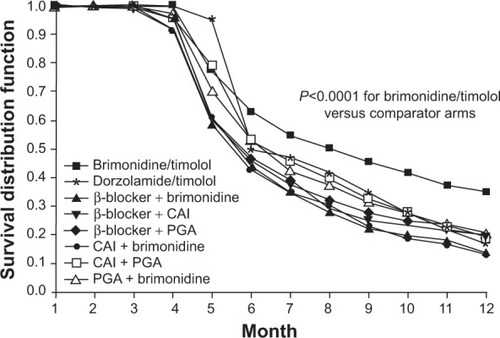
To address the barriers to optimal adherence and persistence with IOP-lowering therapy, several fixed-combination medications, which allow instillation of two medications in a single solution, have been developed. Fixed-combination medications reduce the number of medication bottles required, may reduce costs, and provide a simplified dosing regimen, all of which may increase persistenceCitation20,Citation21,Citation49 and adherence.Citation20,Citation21 In a 2008–2009 United States study, Kaplan–Meier survival analysis of a prescription database demonstrated increased persistence with fixed-combination IOP-lowering medications compared with concomitant administration of two separate drugs ().Citation21 The same study reported greater adherence with fixed combinations (40.6%–42.7%) than separate administration of two medications (23.3%–34.9%) after 1 year of treatment.Citation21
Figure 1 Kaplan–Meier analysis of treatment persistence among fixed and unfixed glaucoma medications.
Abbreviations: CAI, carbonic anhydrase inhibitor; PGA, prostaglandin analog.
Prospective trials have shown that switching from concomitant administration of multiple separate medications to a fixed-combination therapy increases patient adherence.Citation50,Citation51 For example, when patients were switched from separate administration of latanoprost 0.005% and timolol 0.5% to a fixed combination of latanoprost 0.005%/timolol 0.5%, the percentage of patients who reported never missing a dose was significantly greater after the switch (71.0%) compared with before the switch (59.3%; P=0.0115).Citation51 Because adherence relies on patients’ willingness to take their medication, it is important that patients prefer the medication they are prescribed over other equally efficacious alternatives. A reduction in ocular symptoms associated with the prescribed medication may have beneficial effects on patient preference and may increase adherence. Fixed combinations may have a better tolerability profile than concomitant administration of two agents with regard to ocular symptoms because cumulative exposure to irritating preservatives is reduced;Citation52 therefore, the reduced ocular symptoms associated with fixed-combination medications may improve overall adherence.
A fixed-dose combination of a CAI, brinzolamide 1%, and an α2-adrenergic agonist, brimonidine 0.2% (BBFC; Simbrinza®; Alcon Laboratories, Inc., Fort Worth, TX, USA), has recently been developed to provide improved IOP-lowering efficacy, with a safety profile similar to its individual components. BBFC is approved for 3-times-daily dosing in the United States and is indicated for the reduction of elevated IOP in patients with primary open-angle glaucoma or ocular hypertension. This review highlights the efficacy and safety of this new fixed-combination medication and discusses its practical implications for patients.
Efficacy of BBFC
In clinical trials, BBFC administered three times daily (in accordance with the approved dosing regimens of brinzolamide and brimonidine in the United States) had a greater IOP-lowering effect than brinzolamide 1% or brimonidine 0.2% after 3 months of treatment in patients with open-angle glaucoma or ocular hypertension.Citation53–Citation56 In these trials, baseline IOP values were similar among all treatment groups. Between-group differences in IOP from baseline were based on prespecified analyses of least squares (LS) means instead of arithmetic means. LS means differ from arithmetic mean values in that they account for covariates (eg, correlated IOP measurements within patients) and are less sensitive to missing data; therefore, LS means may be better estimates of the overall average IOP within this patient population. In a randomized, Phase III, double-masked clinical trial of BBFC versus brinzolamide or brimonidine in patients with open-angle glaucoma or ocular hypertension, the LS mean IOP after 3 months of treatment was significantly lower with BBFC (17.0–20.5 mmHg) than with brinzolamide (20.0–21.6 mmHg; P≤0.002 for all time points) or brimonidine (18.8–23.3 mmHg; P<0.001 for all time points) throughout the day (ie, 8 am, 10 am, 3 pm, and 5 pm; ).Citation53 Mean IOP reductions from baseline and percentage change in IOP from baseline were also greater with BBFC (5.7–8.8 mmHg; percentage reduction, 24.1%–34.9%) than with brinzolamide (4.1–6.2 mmHg; percentage reduction, 16.9%–22.6%) or brimonidine (3.5–6.5 mmHg; percentage reduction, 14.3%–25.8%).Citation53 Similar results were observed in a separate randomized, double-masked Phase III trial with a 3-month safety extension (LS mean IOP at 3 months: BBFC, 17.2–21.1 mmHg; brinzolamide, 20.4–22.0 mmHg, P≤0.005 versus [vs] BBFC; brimonidine, 18.9–23.2 mmHg, P<0.0001 vs BBFC; ) and a pooled analysis of both Phase III trials (LS mean IOP at 3 months: BBFC, 17.1–20.8 mmHg; brinzolamide, 20.2–21.8 mmHg, P<0.0001 vs BBFC; brimonidine, 18.8–23.2 mmHg, P<0.0001 vs BBFC).Citation54,Citation55 Greater reductions in mean IOP with BBFC compared with brinzolamide or brimonidine were observed at week 2 (the first post baseline evaluation day) in both Phase III trialsCitation53,Citation54 and continued for up to 6 months (mean reductions from baseline to month 6: 4.9–8.0 mmHg with BBFC, 4.1–5.8 mmHg with brinzolamide, and 3.0–6.3 mmHg with brimonidine).Citation56 Thus, the therapeutic benefit of BBFC occurs shortly after initial administration (ie, within the first 2 weeks) and continues for up to 6 months. Taken together, these data suggest that BBFC effectively lowers and maintains clinically relevant IOP reductions (ie, reductions ≥1 mmHg).Citation57 Although the IOP-lowering efficacy of some medications (eg, β-blockers, brimonidine) has been shown to fluctuate, often with decreased efficacy at night,Citation58 BBFC provides effective IOP reduction throughout the day. Some other fixed-combination glaucoma medications have also demonstrated 24-hour control of IOP. For example, fixed combinations of dorzolamide and timolol, and brimonidine and timolol both significantly decrease IOP from baseline (mean IOP reduction: 2.9 mmHg and 2.2 mmHg, respectively).Citation59 However, IOP reductions were greater during the night (6 pm and 2 am) with dorzolamide and timolol compared with brimonidine and timolol, which was to be expected given the decreased evening efficacy of both components.Citation58,Citation59 Given that significant IOP reductions with BBFC are still seen at 8 am (10 hours after dosing), it is likely that the nocturnal IOP-lowering efficacy of BBFC is being conferred by the brinzolamide, not the brimonidine, component.Citation60,Citation61 However, 24-hour IOP control studies need to be conducted to confirm this.
Figure 2 LS mean IOP during a 3-month clinical trial with a 3-month safety extension.
Abbreviations: BBFC, brinzolamide 1%/brimonidine 0.2% fixed combination; IOP, intraocular pressure; LS, least squares; SE, standard error.
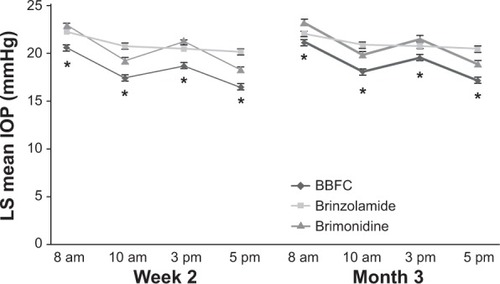
Table 2 Intraocular pressure across visits and time pointsTable Footnotea
The IOP reductions observed with BBFC in these clinical trials are similar to or greater than those observed with other monotherapy or fixed-combination treatments in other studies. Among IOP-lowering monotherapy treatments, prostaglandin analogs generally provide the greatest IOP-lowering efficacy (percentage IOP change from baseline at peak as determined in a meta-analysis of randomized clinical trials, 31%–33%), followed by β-blockers (23%–27%), an α2-adrenergic agonist (25%), and CAIs (17%–22%).Citation62 Similar trends in percentage IOP reduction from baseline have been observed among fixed-combination therapies combining timolol with prostaglandin analogs (peak IOP reduction as shown in a meta-analysis, 35%–36%), an α2-adrenergic agonist (32%), or CAIs (31%–34%).Citation63 With BBFC, peak percentage IOP reduction was approximately 32%–34%,Citation53,Citation54 which is similar to that previously published for prostaglandin analogs and greater than reports with α2-adrenergic agonist and CAI monotherapy.Citation62 In addition, mean IOP reduction from baseline with BBFC at 3 months (5.4–8.8 mmHg) was similar to reductions observed with fixed-dose combinations containing timolol after 3 months of treatment (prostaglandin analogs plus timolol, 2.6–10.2 mmHg; CAIs plus timolol, 3.7–9.0 mmHg; α2-agonists plus timolol, ~5.5–7.5 mmHg; ).Citation53,Citation54,Citation64–Citation75
Table 3 Mean 3-month IOP reductions with currently available fixed-combination glaucoma medications
Safety and tolerability of BBFC
Similar to other fixed-combination therapies,Citation48,Citation64,Citation66,Citation69,Citation72,Citation74–Citation76 the overall safety profile of BBFC is consistent with that of its individual components (brinzolamide 1% and brimonidine 0.2%).Citation53–Citation56 In clinical trials, ocular events were the most common treatment-related adverse events (TRAEs) associated with BBFC and occurred with similar frequency in the BBFC and brinzolamide or brimonidine groups ( and ).Citation53,Citation54,Citation56 In Phase III clinical trials, blurred vision (4.5%–6.1%) and eye irritation (2.8%–5.4%) were two of the most commonly reported ocular TRAEs with BBFC after 3 months of treatment.Citation53,Citation54 Blurred vision was the most common ocular TRAE observed with brinzolamide (6.2%–6.8%) at 3 months.Citation53,Citation54 The occurrence of blurred vision with BBFC and brinzolamide in some patients is unsurprising given that these medications are administered as ophthalmic suspensions. In contrast, the most frequently reported ocular TRAEs with brimonidine at 3 months were conjunctivitis (3.0%), dry eye (0.4%–2.7%), eye irritation (1.8%–2.6%), and ocular hyperemia (2.6%–4.1%).Citation53,Citation54 In both trials, the incidence of ocular hyperemia was more prevalent with brimonidine (2.6%–4.1%) than BBFC (0.9%–3.3%) or brinzolamide (0.4%–0.9%) at 3 months.Citation53,Citation54 After 6 months of treatment, eye irritation and eye allergy were the most common ocular TRAEs associated with BBFC (6.3% for both), whereas blurred vision (6.8%) and conjunctivitis (6.0%) were most frequent in the brinzolamide and brimonidine groups, respectively ().Citation56 Eye allergy rates were 0.4% with brinzolamide and 2.1% with brimonidine at 6 months.Citation56 The incidence of ocular hyperemia continued to be higher in the brimonidine group (3.8%) than the BBFC (2.7%) or brinzolamide (0.4%) groups after 6 months.Citation56 In the two Phase III clinical trials, discontinuations because of nonserious TRAEs were more common with BBFC (up to 11.3%) than with brinzolamide (up to 2.1%) or brimonidine (up to 9.4%).Citation53,Citation54 The slightly greater occurrence of some TRAEs and TRAE-related discontinuations with BBFC in these studies may be attributable to exposure to multiple therapeutic agents (ie, brinzolamide and brimonidine) versus monotherapy.
Table 4 Treatment-related adverse events (incidence ≥1% in any group) from a 3-month Phase III trial
Table 5 Treatment-related adverse events (incidence ≥1% in either group) from a 3-month clinical trial with a 3-month safety extension
The lack of head-to-head comparative studies of BBFC and other IOP-lowering monotherapies and fixed-combination medications prevents the assessment of BBFC tolerability in terms of other IOP-lowering therapies, and differences in study design preclude direct comparisons between IOP-lowering medications evaluated in different clinical trials. However, the incidence of eye burning/stinging/irritation (which are often associated with β-blockers) appeared to be similar with BBFC compared with previous reports for timolol at 3 months (up to 5.4% with BBFC vs up to 18.1% [burning and stinging] with timolol) and slightly greater with BBFC than timolol at 6 months (6.3% with BBFC vs 4.5% [burning and stinging] with timolol).Citation53,Citation54,Citation56,Citation64,Citation66,Citation75 The incidence of other AEs (eg, blurred vision, which is commonly associated with CAIs) was slightly greater at month 3 with BBFC (up to 6.1%) than that previously reported with dorzolamide (4.0%).Citation53,Citation54,Citation64 In general, the safety profile of BBFC appears to be similar to other currently marketed fixed-combination medications. Emergence or worsening of hyperemia was reported in up to 3.3% of patients receiving BBFC in two clinical trials,Citation53,Citation54 an incidence similar to that reported with prostaglandin analog/timolol fixed combinations across multiple studies (up to 2.8%).Citation68,Citation71,Citation72 Additionally, the incidence of blurred vision with BBFC (up to 6.1%)Citation53,Citation54 was only slightly greater than that previously observed with CAI fixed combinations (brinzolamide/timolol, 3.4%; dorzolamide/timolol, 4%).Citation64,Citation75
As with all fixed-combination medications, BBFC increases IOP-lowering efficacy by providing two medications with different mechanisms of action in a single drop, with a potential decrease in cumulative exposure to preservatives. Preservatives, particularly benzalkonium chloride (BAK), have been associated with a variety of ocular symptoms, including dry eye,Citation47,Citation77,Citation78 foreign body sensation in the eye,Citation77 stinging/burning,Citation77,Citation78 tearing,Citation77 reduced tear production,Citation78 and hyperemia;Citation77 thus, limiting exposure to preservatives by using fixed-combination medications instead of multiple individual medications may improve overall tolerability. For example, a recent systematic review and meta-analysis of randomized trials comparing fixed combinations of prostaglandins and timolol with concomitant administration of both medications showed that the relative risk of hyperemia was lower with the fixed combination than with the unfixed combinations (relative risk, 0.70; 95% confidence interval, 0.43–1.14).Citation79 In a pooled analysis of two 3-month clinical trials, ocular symptoms that have been associated with preservatives (eg, dry eye and ocular hyperemia) occurred at a similar rate with BBFC (1.4% and 2.1% for dry eye and ocular hyperemia, respectively) compared with individual administration of brinzolamide (0.9% and 0.7%) or brimonidine (1.5% and 3.3%).Citation55 Although it is possible that punctate keratitis, which was reported in only one of the Phase III trials (0.5%, 0.4%, and 1.4% with BBFC, brinzolamide, and brimonidine, respectively),Citation53 may have contributed to the incidence of these ocular symptoms, this association remains unclear. These data suggest that despite exposure to additional medications (ie, two therapeutic agents instead of one), BBFC does not elicit any greater risk of ocular symptoms than its individual components. This observation may be explained by the reduced exposure to preservatives with BBFC versus administration of two separate preservative-containing medications.
Some IOP-lowering agents (eg, topical β-blockers and α2-receptor agonists) have been associated with significant alterations in blood pressure.Citation80,Citation81 For example, in a head-to-head trial in 27 patients with newly diagnosed primary open-angle glaucoma, brimonidine and timolol, but not dorzolamide or latanoprost, significantly reduced systolic and diastolic blood pressure from baseline;Citation82 however, the clinical significance of these alterations is unknown. Interestingly, diastolic ocular perfusion pressure was low with timolol and brimonidine (53.0 mmHg and 46.2 mmHg, respectively), whereas values with dorzolamide (55.9 mmHg) and latanoprost (56.4 mmHg)Citation82 exceeded the threshold associated with progression of primary open-angle glaucoma (ie, <55 mmHg).Citation83 With BBFC, a slight decrease in mean systolic and diastolic blood pressure was observed in clinical studies; similar reductions were reported with brinzolamide and brimonidine and none were considered to be of clinical concern.Citation53–Citation56 Furthermore, individual blood pressure and pulse rate remained relatively stable (<1.5 bpm decrease in the BBFC, brinzolamide, and brimonidine groups).Citation53–Citation56 Some clinical studies of other available fixed-combination therapies, all of which contain timolol, have also reported no clinically significant changes in blood pressure from baseline.Citation65,Citation69 However, small but statistically significant mean alterations in heart rate and blood pressure from baseline have been reported with certain fixed-combination medications (eg, brimonidine/timololCitation66,Citation73 and latanoprost/timolol).Citation67
Additional considerations for BBFC
BBFC provides IOP-lowering efficacy greater than instillation of either of its components (brinzolamide or brimonidine), with potentially improved adherence and tolerability compared with concomitant administration of the separate medications. The increased convenience of dosing with one bottle instead of two may improve adherence and persistence and allow patients to achieve greater IOP control than dosing with separate components. IOP lowering may also be augmented with BBFC because it eliminates the potential of drug washout from sequential instillations of concomitant medications. In addition, reduced overall exposure to preservatives may increase patient comfort (and, as a result, potentially increase adherence to medication) and reduce the need for discontinuation or switching of therapies.
All currently available fixed-combination IOP-lowering medications provide similar IOP-lowering efficacy.Citation53,Citation54,Citation63 However, all of these medications, except BBFC, contain the β-blocker timolol. Because glaucoma incidence increases with age,Citation84 patients with glaucoma or ocular hypertension tend to have comorbid conditions or therapeutic regimens (eg, systemic β-blockers)Citation85 that make them vulnerable to adverse drug reactions (eg, depression of systemic cardiovascular function observed with β-blockers).Citation86–Citation90 By providing effective IOP reduction with brinzolamide and brimonidine instead of timolol, BBFC expands the available fixed-combination options for patients who require efficacious IOP lowering and for those in whom use of β-blockers is contraindicated.
Conclusion
Glaucoma affects millions of individuals worldwide and is a leading cause of blindness.Citation1 Reduction of IOP may prevent or delay visual field loss in patients with glaucoma or ocular hypertension;Citation4,Citation6 thus, monotherapy with IOP-lowering medications is standard-of-care treatment. However, many patients require multiple IOP-lowering therapies to reach their target IOP.Citation15,Citation91 Drug washout during concomitant administration of multiple medicationsCitation44 and low adherence and persistence with complex glaucoma therapeutic regimensCitation22,Citation24,Citation27 may reduce the effectiveness of multidrug regimens. Fixed-combination medications prevent drug washout, simplify dosing regimens, and may reduce costs,Citation92 thereby potentially increasing medication adherenceCitation21,Citation51,Citation93,Citation94 and persistence.Citation20,Citation21 BBFC provides IOP-lowering efficacy greater than or similar to various monotherapy and fixed-combination medications, with potentially improved convenience and better tolerability.
Acknowledgments
Medical writing support was provided by Jillian Gee, PhD, CMPP, of Complete Healthcare Communications, Inc. (Chadds Ford, PA, USA), and was funded by Alcon Laboratories, Inc.
Disclosure
Dr Nguyen is on the speakers bureau for Alcon Laboratories, Inc., and Allergan, Inc., (Irvine, CA, USA).
References
- QuigleyHABromanATThe number of people with glaucoma worldwide in 2010 and 2020Br J Ophthalmol200690326226716488940
- CassonRJChidlowGWoodJPCrowstonJGGoldbergIDefinition of glaucoma: clinical and experimental conceptsClin Experiment Ophthalmol201240434134922356435
- PetersDBengtssonBHeijlAFactors associated with lifetime risk of open-angle glaucoma blindnessActa Ophthalmol Epub2013710
- Collaborative Normal-Tension Glaucoma Study GroupComparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressuresAm J Ophthalmol199812644874979780093
- American Academy of Ophthalmology Glaucoma PanelPreferred Practice Pattern® Guidelines. Primary Open-Angle GlaucomaSan Francisco, CAAmerican Academy of Ophthalmology2010
- The AGIS InvestigatorsThe Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deteriorationAm J Ophthalmol2000130442944011024415
- WebersCABeckersHJNuijtsRMSchoutenJSPharmacological management of primary open-angle glaucoma: second-line options and beyondDrugs Aging200825972975918729546
- RealiniTA history of glaucoma pharmacologyOptom Vis Sci2011881363821131876
- NguyenQHThe role of prostaglandin analogues in the treatment of glaucoma in the 21st centuryInt Ophthalmol Clin2004442152715087728
- WatanabeKChiouGCAction mechanism of timolol to lower the intraocular pressure in rabbitsOphthalmic Res19831531601676314218
- MincioneFScozzafavaASupuranCTThe development of topically acting carbonic anhydrase inhibitors as anti-glaucoma agentsCurr Top Med Chem20077984985417504129
- TorisCBGabeltBTKaufmanPLUpdate on the mechanism of action of topical prostaglandins for intraocular pressure reductionSurv Ophthalmol200853Suppl 1S107S12019038618
- ArthurSCantorLBUpdate on the role of alpha-agonists in glaucoma managementExp Eye Res201193327128321524649
- European Glaucoma SocietyTerminology and Guidelines for Glaucoma3rd edBern, SwitzerlandEuropean Glaucoma Society Available at: http://www.eugs.org/eng/EGS_guidelines.aspAccessed January 24, 2014
- KassMAHeuerDKHigginbothamEJThe Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol2002120670171312049574
- ReardonGKotakSSchwartzGFObjective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic reviewPatient Prefer Adherence2011544146322003282
- AriasASchargelKUssaFCanutMIRoblesAYSanchezBMPatient persistence with first-line antiglaucomatous monotherapyClin Ophthalmol2010426126720463793
- NordstromBLFriedmanDSMozaffariEQuigleyHAWalkerAMPersistence and adherence with topical glaucoma therapyAm J Ophthalmol2005140459860616226511
- WilenskyJFiscellaRGCarlsonAMMorrisLSWaltJMeasurement of persistence and adherence to regimens of IOP-lowering glaucoma medications using pharmacy claims dataAm J Ophthalmol20061411 SupplS28S3316389058
- HigginbothamEJHansenJDavisEJWaltJGGuckianAGlaucoma medication persistence with a fixed combination versus multiple bottlesCurr Med Res Opin200925102543254719731993
- SchwartzGBurkCBennettTPatelVDAdherence and persistence with glaucoma therapy: brimonidine/timolol versus dorzolamide/timolol and various two-bottle combinationsJ Clin Exp Ophthalmol20123816
- RossiGCPasinettiGMScudellerLRadaelliRBianchiPEDo adherence rates and glaucomatous visual field progression correlate?Eur J Ophthalmol201121441041421140373
- DjafariFLeskMRHarasymowyczPJDesjardinsDLachaineJDeterminants of adherence to glaucoma medical therapy in a long-term patient populationJ Glaucoma200918323824319295380
- FriedmanDSOkekeCOJampelHDRisk factors for poor adherence to eyedrops in electronically monitored patients with glaucomaOphthalmology200911661097110519376591
- OkekeCOQuigleyHAJampelHDAdherence with topical glaucoma medication monitored electronically: the Travatan Dosing Aid studyOphthalmology2009116219119919084273
- ReesGLeongOCrowstonJGLamoureuxELIntentional and unintentional nonadherence to ocular hypotensive treatment in patients with glaucomaOphthalmology2010117590390820153902
- SleathBRobinALCovertDByrdJETudorGSvarstadBPatient-reported behavior and problems in using glaucoma medicationsOphthalmology2006113343143616458967
- UngCZhangEAlfaroTGlaucoma severity and medication adherence in a county hospital populationOphthalmology201312061150115723453512
- VandenbroeckSDe GeestSDobbelsFFieuwsSStalmansIZeyenTPrevalence and correlates of self-reported nonadherence with eye drop treatment: the Belgian Compliance Study in Ophthalmology (BCSO)J Glaucoma201120741442121048510
- HongSKangSYYoonJUKangUSeongGJKimCYDrug attitude and adherence to anti-glaucoma medicationYonsei Med J201051226126920191020
- OlthoffCMHoevenaarsJGvan den BorneBWWebersCASchoutenJSPrevalence and determinants of non-adherence to topical hypotensive treatment in Dutch glaucoma patientsGraefes Arch Clin Exp Ophthalmol2009247223524318802720
- LoonSCJinJJin GohMThe relationship between quality of life and adherence to medication in glaucoma patients in SingaporeJ Glaucoma Epub20131116
- ReesGChongXLCheungCYBeliefs and adherence to glaucoma treatment: a comparison of patients from diverse culturesJ Glaucoma Epub2013131
- BullerAJConnellBSpencerAFCompliance: clear communication’s criticalBr J Ophthalmol20058910137016170136
- DreerLEGirkinCMansbergerSLDeterminants of medication adherence to topical glaucoma therapyJ Glaucoma201221423424021623223
- KholdebarinRCampbellRJJinYPBuysYMMulticenter study of compliance and drop administration in glaucomaCan J Ophthalmol200843445446118711461
- NordmannJPBaudouinCRenardJPMeasurement of treatment compliance using a medical device for glaucoma patients associated with intraocular pressure control: a surveyClin Ophthalmol2010473173920689790
- ForsmanEKivelaTVestiELifetime visual disability in open-angle glaucoma and ocular hypertensionJ Glaucoma200716331331917438426
- VorwerkCThelenUBuchholzPKimmichFTreatment of glaucoma patients with insufficient intraocular pressure control: a survey of German ophthalmologists in private practiceCurr Med Res Opin20082451295130118366862
- FriedmanDSHahnSRGelbLDoctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency StudyOphthalmology20081158132013271327.e1e318321582
- RobinALNovackGDCovertDWCrockettRSMarcicTSAdherence in glaucoma: objective measurements of once-daily and adjunctive medication useAm J Ophthalmol2007144453354017686450
- TsaiJCMcClureCARamosSESchlundtDGPichertJWCompliance barriers in glaucoma: a systematic classificationJ Glaucoma200312539339814520147
- RobinALCovertDDoes adjunctive glaucoma therapy affect adherence to the initial primary therapy?Ophthalmology2005112586386815878067
- ChraiSSMakoidMCEriksenSPRobinsonJRDrop size and initial dosing frequency problems of topically applied ophthalmic drugsJ Pharm Sci19746333333384820359
- BullerAHerculesBLShould patients choose their own eyedrops?Acta Ophthalmol Scand200684115015116445459
- LiangHBrignole-BaudouinFPaulyARianchoLBaudouinCPolyquad-preserved travoprost/timolol, benzalkonium chloride (BAK)-preserved travoprost/timolol, and latanoprost/timolol in fixed combinations: a rabbit ocular surface studyAdv Ther201128431132521424577
- PisellaPJPouliquenPBaudouinCPrevalence of ocular symptoms and signs with preserved and preservative free glaucoma medicationBr J Ophthalmol200286441842311914211
- HigginbothamEJConsiderations in glaucoma therapy: fixed combinations versus their component medicationsClin Ophthalmol201041920169043
- HommerAThygesenJFerrerasAA European perspective on costs and cost effectiveness of ophthalmic combinations in the treatment of open-angle glaucomaEur J Ophthalmol200818577878618850558
- InoueKSetogawaAHigaRMoriyamaRWakakuraMTomitaGOcular hypotensive effect and safety of travoprost 0.004%/timolol maleate 0.5% fixed combination after change of treatment regimen from beta-blockers and prostaglandin analogsClin Ophthalmol2012623123522347794
- InoueKOkayamaRHigaRSawadaHWakakuraMTomitaGOcular hypotensive effects and safety over 3 months of switching from an unfixed combination to latanoprost 0.005%/timolol maleate 0.5% fixed combinationJ Ocul Pharmacol Ther201127658158722011049
- KitazawaYSmithPSasakiNKotakeSBaeKIwamotoYTravoprost 0.004%/timolol 0.5%-fixed combination with and without benzalkonium chloride: a prospective, randomized, doubled-masked comparison of safety and efficacyEye (Lond)20112591161116921701528
- KatzGDubinerHSamplesJVoldSSallKThree-month randomized trial of fixed-combination brinzolamide, 1%, and brimonidine, 0.2%JAMA Ophthalmol2013131672473023579344
- NguyenQHMcMenemyMGRealiniTWhitsonJTGoodeSMPhase III randomized 3-month trial with an ongoing 3-month safety extension of fixed-combination brinzolamide 1%/brimonidine 0.2%J Ocul Pharmacol Ther201329329029723425430
- RealiniTNguyenQHKatzGDubinerHFixed-combination brinzolamide 1%/brimonidine 0.2% vs monotherapy with brinzolamide or brimonidine in patients with open-angle glaucoma or ocular hypertension: results of a pooled analysis of two phase III studiesEye (Lond)201327784184723640612
- WhitsonJTRealiniTNguyenQHMcMenemyMGGoodeSMSix-month results from a phase III randomized trial of fixed-combination brinzolamide 1% + brimonidine 0.2% versus brinzolamide or brimonidine monotherapy in glaucoma or ocular hypertensionClin Ophthalmol201371053106023766627
- LeskeMCHeijlAHusseinMFactors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma TrialArch Ophthalmol20031211485612523884
- QuarantaLKatsanosARussoARivaI24-hour intraocular pressure and ocular perfusion pressure in glaucomaSurv Ophthalmol2013581264123217586
- KonstasAGQuarantaLYanDBTwenty-four hour efficacy with the dorzolamide/timolol-fixed combination compared with the brimonidine/timolol-fixed combination in primary open-angle glaucomaEye (Lond)2012261808721960068
- LiuJHMedeirosFASlightJRWeinrebRNComparing diurnal and nocturnal effects of brinzolamide and timolol on intraocular pressure in patients receiving latanoprost monotherapyOphthalmology2009116344945419157559
- IshikawaMYoshitomiTEffects of brinzolamide vs timolol as an adjunctive medication to latanoprost on circadian intraocular pressure control in primary open-angle glaucoma Japanese patientsClin Ophthalmol2009349350019750124
- van der ValkRWebersCASchoutenJSZeegersMPHendrikseFPrinsMHIntraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trialsOphthalmology200511271177118515921747
- ChengJWChengSWGaoLDLuGCWeiRLIntraocular pressure-lowering effects of commonly used fixed-combination drugs with timolol: a systematic review and meta-analysisPLoS ONE201279e4507923028770
- BoyleJEGhoshKGieserDKAdamsonsIAA randomized trial comparing the dorzolamide-timolol combination given twice daily to monotherapy with timolol and dorzolamide. Dorzolamide-Timolol Study GroupOphthalmology199810510194519519787368
- BrandtJDCantorLBKatzLJBimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertensionJ Glaucoma200817321121618414107
- CravenERWaltersTRWilliamsRBrimonidine and timolol fixed-combination therapy versus monotherapy: a 3-month randomized trial in patients with glaucoma or ocular hypertensionJ Ocul Pharmacol Ther200521433734816117698
- HigginbothamEJFeldmanRStilesMDubinerHFixed Combination Investigative GroupLatanoprost and timolol combination therapy vs monotherapy: one-year randomized trialArch Ophthalmol2002120791592212096962
- HigginbothamEJOlanderKWKimEEFixed combination of latanoprost and timolol vs individual components for primary open-angle glaucoma or ocular hypertension: a randomized, double-masked studyArch Ophthalmol2010128216517220142538
- HughesBABacharachJCravenERA three-month, multicenter, double-masked study of the safety and efficacy of travoprost 0.004%/timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma or ocular hypertensionJ Glaucoma200514539239916148589
- HutzelmannJOwensSSheddenAAdamsonsIVargasEComparison of the safety and efficacy of the fixed combination of dorzolamide/timolol and the concomitant administration of dorzolamide and timolol: a clinical equivalence study. International Clinical Equivalence Study GroupBr J Ophthalmol19988211124912539924327
- PalmbergPKimEEKwokKKTresslerCSCanada and United States Fixed Combination Latanoprost/Timolol Study GroupA 12-week, randomized, double-masked study of fixed combination latanoprost/timolol versus latanoprost or timolol monotherapyEur J Ophthalmol201020470871820099236
- PfeifferNEuropean Latanoprost Fixed Combination Study GroupA comparison of the fixed combination of latanoprost and timolol with its individual componentsGraefes Arch Clin Exp Ophthalmol20022401189389912486510
- SherwoodMBCravenERChouCTwice-daily 0.2% brimonidine-0.5% timolol fixed-combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12-month randomized trialArch Ophthalmol200612491230123816966616
- StrohmaierKSnyderEDuBinerHAdamsonsIThe efficacy and safety of the dorzolamide-timolol combination versus the concomitant administration of its components. Dorzolamide-Timolol Study GroupOphthalmology199810510193619449787367
- KabackMScoperSVArzenoGIntraocular pressure-lowering efficacy of brinzolamide 1%/timolol 0.5% fixed combination compared with brinzolamide 1% and timolol 0.5%Ophthalmology2008115101728173418538406
- DiestelhorstMLarssonLIEuropean Latanoprost Fixed Combination Study GroupA 12 week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertensionBr J Ophthalmol200488219920314736774
- JaenenNBaudouinCPouliquenPManniGFigueiredoAZeyenTOcular symptoms and signs with preserved and preservative-free glaucoma medicationsEur J Ophthalmol200717334134917534814
- KuppensEVde JongCAStolwijkTRde KeizerRJvan BestJAEffect of timolol with and without preservative on the basal tear turnover in glaucomaBr J Ophthalmol19957943393427742279
- QuarantaLBiagioliERivaIProstaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysisJ Ocul Pharmacol Ther201329438238923231442
- SchumanJSClinical experience with brimonidine 0.2% and timolol 0.5% in glaucoma and ocular hypertensionSurv Ophthalmol199641Suppl 1S27S378970247
- MelamedSDavidROngoing clinical assessment of the safety profile and efficacy of brimonidine compared with timolol: year-three results. Brimonidine Study Group IIClin Ther200022110311110688394
- QuarantaLGandolfoFTuranoREffects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucomaInvest Ophthalmol Vis Sci20064772917292316799034
- BonomiLMarchiniGMarraffaMBernardiPMorbioRVarottoAVascular risk factors for primary open angle glaucoma: the Egna-Neumarkt StudyOphthalmology200010771287129310889099
- HeijlABengtssonBOskarsdottirSEPrevalence and severity of undetected manifest glaucoma: results from the early manifest glaucoma trial screeningOphthalmology201312081541154523631945
- SchumanJSEffects of systemic beta-blocker therapy on the efficacy and safety of topical brimonidine and timolol. Brimonidine Study Groups 1 and 2Ophthalmology200010761171117710857839
- BauerKBrunner-FerberFDistlerathLMAssessment of systemic effects of different ophthalmic beta-blockers in healthy volunteersClin Pharmacol Ther19914966586641676358
- JavittJCSchiffmanRMClinical success and quality of life with brimonidine 0.2% or timolol 0.5% used twice daily in glaucoma or ocular hypertension: a randomized clinical trial. Brimonidine Outcomes Study Group IJ Glaucoma20009322423410877373
- KassMAGordonMOHoffMRTopical timolol administration reduces the incidence of glaucomatous damage in ocular hypertensive individuals. A randomized, double-masked, long-term clinical trialArch Ophthalmol198910711159015982818278
- UusitaloHKahonenMRopoAImproved systemic safety and risk-benefit ratio of topical 0.1% timolol hydrogel compared with 0.5% timolol aqueous solution in the treatment of glaucomaGraefes Arch Clin Exp Ophthalmol2006244111491149616628416
- WaldockASnapeJGrahamCMEffects of glaucoma medications on the cardiorespiratory and intraocular pressure status of newly diagnosed glaucoma patientsBr J Ophthalmol200084771071310873979
- McCartyCAMukeshBNKitchnerTEIntraocular pressure response to medication in a clinical setting: the Marshfield Clinic Personalized Medicine Research ProjectJ Glaucoma200817537237718703947
- HommerARole of fixed combinations in the management of open-angle glaucomaExpert Rev Pharmacoecon Outcomes Res2011111919921351861
- DicksonMPlauschinatCACompliance with antihypertensive therapy in the elderly: a comparison of fixed-dose combination amlodipine/benazepril versus component-based free-combination therapyAm J Cardiovasc Drugs200881455018303937
- TaylorAAShoheiberOAdherence to antihypertensive therapy with fixed-dose amlodipine besylate/benazepril HCl versus comparable component-based therapyCongest Heart Fail20039632433214688505
