Abstract
Excessive growth hormone (GH) is usually secreted by GH-secreting pituitary adenomas and causes gigantism in juveniles or acromegaly in adults. The clinical complications involving cardiovascular, respiratory, and metabolic systems lead to elevated morbidity in acromegaly. Control of serum GH and insulin-like growth factor (IGF) 1 hypersecretion by surgery or pharmacotherapy can decrease morbidity. Current pharmacotherapy includes somatostatin analogs (SAs) and GH receptor antagonist; the former consists of lanreotide Autogel (ATG) and octreotide long-acting release (LAR), and the latter refers to pegvisomant. As primary medical therapy, lanreotide ATG and octreotide LAR can be supplied in a long-lasting formulation to achieve biochemical control of GH and IGF-1 by subcutaneous injection every 4–6 weeks. Lanreotide ATG and octreotide LAR provide an effective medical treatment, whether as a primary or secondary therapy, for the treatment of GH-secreting pituitary adenoma; however, to maximize benefits with the least cost, several points should be emphasized before the application of SAs. A comprehensive assessment, especially of the observation of clinical predictors and preselection of SA treatment, should be completed in advance. A treatment process lasting at least 3 months should be implemented to achieve a long-term stable blood concentration. More satisfactory surgical outcomes for noninvasive macroadenomas treated with presurgical SA may be achieved, although controversy of such adjuvant therapy exists. Combination of SA and pegvisomant or cabergoline shows advantages in some specific cases. Thus, an individual treatment program should be established for each patient under a full evaluation of the risks and benefits.
Introduction
Pituitary adenoma accounts for 15% of primary intracranial tumors.Citation1 Growth hormone (GH)-secreting pituitary adenoma had a World Health Organization 2000-standardized incidence rate of 0.34 per 100,000. GH-secreting pituitary adenoma is the only type of adenoma that shows a male-dominant tendency, although the difference due to sex is not significant.Citation2 Excessive GH secretion can cause gigantism in juveniles, because of the active epiphyseal growth plates that allow linear growth, or acromegaly in adults.Citation3 The data of incidence, clinical presentations, and treatment strategy for patients with gigantism is limited, owing to its lower incidence rate relative to that of acromegaly;Citation3,Citation4 however, similar organomegaly and deteriorating glucose tolerance have been found in follow-up patientsCitation5 with either acromegaly or gigantism.Citation6 Recent studies have shown that therapy for acromegaly was also successful and safe in patients with gigantism.Citation3,Citation6–Citation8 Thus, in this review, we mainly summarize the clinical application of somatostatin analogs (SAs) for acromegaly, with the related data of gigantism being discussed in the last part of this review.
Background
Clinical manifestation
Acromegaly and gigantism usually manifest as coarsened facial features and hypertrophy of hands, feet, and soft tissue.Citation9 The characteristic clinical manifestations mainly derive from local mass effects and biological function of excessive secreted GH. First, the compression of local nerves causes temporal hemianopia of one or both eyes, ophthalmoplegia, and ptosis.Citation10 Further, the elevated intrasellar pressure could shut down the hypophyseal portal vein, which carries regulatory hormones from the hypothalamus, therefore leading to hypopituitarism and moderate hyperprolactinemia.Citation11 Second, the excessive secretion of GH and insulin-like growth factor (IGF) 1 will lead to severe complications in the cardiovascular, respiratory, metabolic, skeletal, and integumentary systems, which subsequently increases the risk of death significantly.Citation12 Cardiovascular complications, especially myocardial infarction, are the most common cause of death, while malignancy and cerebrovascular events occupy the second and third most common causes of mortality, respectively.Citation13 Although the biologic changes are usually more concerning, the mass effects of GH-secreting pituitary adenoma should not be ignored. Temporal hemianopia, ophthalmoplegia, ptosis, and hypopituitarism affect the quality of life in patients with pituitary adenoma to a variable extent, while hemorrhage in giant pituitary adenoma can lead to acute pituitary apoplexy, which is a life-threatening condition.
Treatment goals and strategies
A previous study has indicated that GH <2.5 ng/mL, younger age, and shorter duration of disease are the independent determinants of longer survival,Citation1 so the indication of medical intervention should be confirmed after diagnosis.Citation14 Sometimes, the serum GH and IGF-1 show a divergent relationship;Citation15 hence, if both the random serum GH <2.5 ng/mL and IGF-1 normalization for age and sex can be achieved, patients can be expected to have nearly the same duration of life as unaffected persons.Citation16
The current therapies of GH-secreting pituitary adenoma are surgery, medical treatment, and radiotherapy.Citation14 The main aim of these approaches is to remove the mass that compresses its surroundings and to normalize serum GH and IGF-1 levels.Citation12,Citation16 Although different treatments have their specific advantages and disadvantages, the ultimate goal of the treatment is to reduce the mortality and morbidity in acromegaly and gigantism patients. Transsphenoidal surgery is an optimal choice for microadenomas and noninvasive macroadenomas, especially for the resolution of compression. The biological control rates of GH and IGF-1 can reach 75%–95% in microadenomas and 40%–68% in noninvasive macroadenomas.Citation14 Patients with a tumor less than 2 cm and random serum GH level less than 50 ng/mL show higher remission rates after surgery;Citation16 however, about 40%–60% of macroadenomas are unable to be cured by surgery alone because of the invasion to cavernous sinus or third ventricle.Citation14 Although complete excision of such macroadenomas is impossible, surgical debulking could enhance the effect of SA.Citation17,Citation18 For medical therapy, SA, GH receptor antagonist (GHRA), and dopamine agonist (DA) are available. SAs are the strongly recommended medical treatment for GH-secreting pituitary adenoma when surgery fails to achieve GH and IGF-1 normalization.Citation14 Radiation therapy for GH-secreting pituitary adenoma is recommended as a third-line treatment to control excessive GH or IGF-1 when surgery and medical treatment are unsuccessful.Citation14 The main limitation of radiation therapy is safety, considering the high occurrence rate of hypopituitarism.Citation19
Overview of medical treatment
Patients with GH-secreting pituitary adenoma receiving SA treatment for more than 3 months show a reduced tumor volume and normalized serum GH and IGF-1.Citation20 There are two efficient, long-lasting formulations of SA, octreotide long-acting release (LAR) and lanreotide Autogel ([ATG]; Ipsen, Paris, France), which replace lanreotide slow release (SR) because of their higher rate of response.Citation21–Citation25 They are equivalent in the control of biological markers and tumor volume.Citation26,Citation27 Although GHRA shows high efficacy in improving quality of life and controlling IGF-1, tumor shrinkages are not observed in most patients.Citation28 Also, the guidelines of Melmed et al limit the strong recommendation of GHRA in the case of persistently elevated IGF-1 despite a maximal dose of SA treatment.Citation14 Recent data show that a combination of SA and GHRA is effective and can greatly reduce the costs of medical therapy.Citation29,Citation30 As per the findings of Abs et al in 1998, there was limited effect in monotherapy with cabergoline for acromegaly.Citation31 A recent meta-analysis, however, conducted by Sandret et al, who systematically reviewed all trials of cabergoline therapy for acromegaly, suggested that cabergoline could achieve better results than expected, either alone or in combination with SA.Citation32
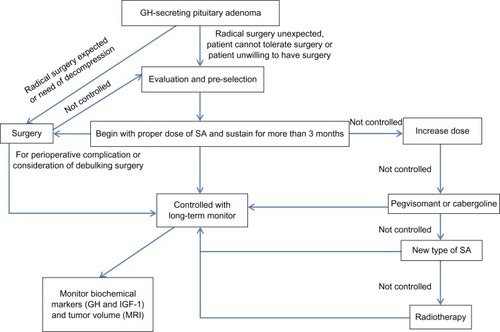
In this review, we will focus on the clinical predictors, preselection of SA, and the benefits from the stable blood concentration. The controversy of presurgical SA treatment and the different strategies of primary or secondary medical treatment will also be discussed ().
Table 1 Main content of this review
Optimization of SA treatment
Clinical predictors of medical treatment
As mentioned above, it is important to reduce both GH and IGF-1 to normal levels;Citation16 however, patients with GH-secreting pituitary adenoma show different susceptibilities to SA treatment. The full response to a 12-month SA treatment includes control of GH/IGF-1 and more than 20% tumor shrinkage in primary treatment or no tumor remnant on magnetic resonance imaging, while a partial response means that a more than 50% decrease of GH and/or failure to control IGF-1 levels with or without more than 20% tumor shrinkage is obtained ().Citation33 Taking the cost of SA treatment into account, it is necessary to access the benefit before the treatment. Clinical predictors of SA treatment that are already known include tumor size, serum GH before treatment, and the density of the somatostatin receptor (SSTR).Citation34–Citation36 Related studies have shown that, in patients with serum GH >16.7 ng/mL or 20 ng/mL, SA treatment is unsatisfactory for GH/IGF-1 control and tumor shrinkage.Citation34,Citation35 In the study by Colao et al, small, noninvasive tumor with low serum GH demonstrated a better response to lanreotide ATG.Citation37 According to the results of molecular biological researches, SSTRs, on which somatostatin binds, have five subtypes, of which SSTR 2 and 5 are predominantly expressed on the cell membrane of GH-secreting pituitary tumor cells.Citation38 High-density SSTR distribution on the tumor cell may result in a promising response to SA treatment;Citation36,Citation38 however, Bertherat et al showed that loss of SSTR could not explain the continued partial suppression of GH induced by SA and that the density of SSTR was poorly related to SA treatment in vivo.Citation39 In other words, factors apart from those mentioned also play a role in influencing SA treatment results. Several factors have been found to affect susceptibility to lanreotide and octreotide. For example, in female patients with hypogonadism, oral estrogens together with SA could facilitate better IGF-1 reduction compared with SA monotherapy.Citation40 In addition to this, elderly people who show obvious cardiovascular or respiratory complications at diagnosis are more sensitive to SA treatment.Citation41
Table 2 Definition of response to somatostatin analog treatment (12 months)
Preselection before medical treatment
With different responses to SA treatment, patients with GH-secreting pituitary adenoma need preselection before the medical treatment. To predict the long-term response to SA treatment in patients with GH-secreting pituitary adenoma, the acute octreotide suppression test and 111In-pentetreotide scintigraphy are applied clinically.Citation42–Citation44 The acute octreotide test involves the use of subcutaneous Sandostatina (Sandoz, Milano, Italy) 50/100 μg to suppress the secretion of GH. Serum GH is assayed before (30 minutes, 15 minutes, 5 minutes) and after (hourly intervals for 6 hours) the sandostatina treatment.Citation42 In the earliest study of the acute octreotide test, Lamberts et al found a close relationship between the mean serum GH measured 2–6 hours after a subcutaneous 50 μg sandostatina administration and the mean 24-hour serum GH after a 96-week sandostatina treatment.Citation44 Therefore, they hypothesized that the measurement of serum GH after an acute administration of 50 μg sandostatina might be a good method for dose adjustment;Citation44 however, the results differed in other studies. Colao et al, in 1996, conducted a similar study with octreotide subcutaneous 100 μg for suppression, and the data showed that the acute test could not determine whether the patients might respond to the long-term treatment or not.Citation42 With the use of depot long-acting somatostatin in clinic, the current standard for judging the response to SA treatment is stricter, and the value of the acute octreotide suppression test in predicting the long-term SA treatment response needs reassessment. Furthermore the criterion for the acute octreotide test response affects the results. In a study by Karavitaki, the data showed that, when the criterion of GH <2 ng/mL was adopted in the acute octreotide test, the sensitivity, specificity, positive predictive value, and negative predictive value of safe serum GH were 92%, 67%, 92%, and 67%, respectively, for the 6-month lanreotide therapy and 100%, 80%, 94%, and 100%, respectively, for the 6-month octreotide LAR therapy;Citation42 however, the results of IGF-1 normalization were not satisfied.Citation43 In a meta-analysis by Freda et al, the authors summarized the efficacy of two SA treatments and found that preselection was a positive predictor for IGF-1 and tumor shrinkage but not for serum GH.Citation45 Although the abovementioned studies demonstrated varying results, the acute octreotide test showed its advantages in the prediction of long-acting SA treatment response, especially for sensitivity, which means that patients who respond to the acute octreotide test would be more likely to benefit from long-acting SA treatment.
In the preselection procedure using 111In-pentetreotide scintigraphy, related data show discrepant results.Citation42,Citation46–Citation48 Plöckinger et al found that 111In-pentetreotide could be taken by pituitary adenoma cells regardless of the immunohistological subtypes,Citation46 although the pentetreotide was expected to combine with SSTR. However, the small numbers of patients, lack of long-term follow-up, and failure to fulfill the current criterion of response to SA treatment in the earlier study means that preselection by 111In-pentetreotide scintigraphy is not widely used.
Achieving a long-term stable blood concentration
Lanreotide ATG itself is a sustained-release profile, lanreotide ATG shows a half-life of 22 days and residence time of 30–32 days for a 40/60 mg single dose.Citation49 The mean steady-state serum concentration of 3.82, 5.69, 7.69 ng/mL were obtained in about 84 days following four doses of 60, 90, and 120 mg ATG every 4 weeks, respectively, in patients with acromegaly.Citation50 For octreotide LAR, the time to reach maximum drug concentration was 22 days for octreotide 20 mg and 12.6 days for 60 mg, while the steady state of long-acting octreotide 20 mg after three doses with 28-day intervals showed a mean concentration, minimum concentration, and maximum concentration of 1,216, 1,065, and 1,585 pg/mL, respectively, and that the concentration was maintained at a consistent level during the dose intervals.Citation51 Thus, to obtain a maximal and lasting clinical benefit, the SA treatment schedule of at least three to four doses with a 4-week interval is suggested. Such a treatment schedule was adopted in earlier studies concerning the effect of SA. Furthermore, longer treatment duration may bring higher response rates and better symptomatic improvements as outlined in the guidelines of Melmed et al.Citation14 In recent studies, the observation of serum GH, IGF-1, and tumor shrinkage was performed after a long-term treatment of no less than 12 months,Citation22,Citation52,Citation53 in which a better biochemical marker response and more obvious tumor shrinkage was achieved compared with after a 3-month treatment.Citation21,Citation53 Besides the increased percentage of patients who achieved safe serum GH and normalized IGF-1, the vascular, cardiac, and sleep parameters were improved with 6-month ATG treatment.Citation54
Clinical use in presurgical period and impact on surgery
The main purpose of presurgical application of SA treatment is to remit the complications involving cardiovascular, respiratory, and, probably, metabolic systems to further decrease the anesthetic and perioperative risksCitation54,Citation55 and to decrease serum GH and IGF-1 levels and reduce tumor volume, which possibly leads to higher rates of total tumor resection.Citation14 Cardiovascular complications are the major cause of acromegaly morbidity.Citation12 Longer duration of disease activity and older age may lead to a higher risk during the perioperative period. Therefore, though such negative events were not proven, in the study of Seidman et al, to be markedly higher in active acromegaly than in controls,Citation56 it is still worthwhile to emphasize the benefit of cardiovascular control during and after surgery,Citation55 since the presurgical usage of SA has been demonstrated to result in decreased complication rates and shortened durations of hospitalization.Citation57 Because of the varied durations of presurgical application of SA among studies,55,57,62,64,65,70,78 however, the potential cost benefit needs to be further assessed. The improvements in cardiovascular function could be achieved after a single dose of lanreotide,Citation58 whereas, in the study of Annamalai et al, a 6-month treatment might be needed.Citation54 As for respiratory complications, which mainly refer to change of lung volumes and the ventilation/perfusion relationship, long-term SA treatment could reduce their occurrence.Citation12,Citation14 Nevertheless, the adverse respiratory events caused by intubation difficulty during anesthesia, derived from laryngeal and pharyngeal soft tissue hypertrophy or vocal cord swelling, could not be prevented by presurgical SA treatment.Citation55,Citation56 For metabolic complications, there is no report suggesting that metabolic complications can cause severe perioperative problems in acromegaly, although patients with uncontrolled blood sugar levels were detected in 30% in acromegaly.Citation12 In a previous report, diabetic patients were shown to be at increased risk for poor wound healing and susceptibility to infection;Citation59 therefore, the control of blood sugar might bring benefits for acromegaly patients who are prepared for surgery.Citation55 In a study of octreotide by Colao et al, 6-month SA treatment decreased the insulin demand in patients receiving an insulin treatment and normalized the blood glucose in patients taking oral glucose control drugs.Citation57 Similar studies of lanreotide were also conducted, and a stable blood glucose status could be achieved.Citation60,Citation61
Controversy persists among neurosurgeons regarding whether presurgical SA treatment could lead to a significant improvement of total resection of radical operation.Citation55,Citation57,Citation62–Citation72 Surgery provides the biochemical cure of normalized IGF-1 in 75%–95% of patients with microadenomas and 40%–68% of patients with noninvasive macroadenomas.Citation68,Citation73–Citation77 In patients with invasive pituitary adenomas, a partial removal of tumor replaces the radical operation.Citation68,Citation74 For microadenomas, the cure rate by surgery is already high, so the presurgical SA treatment increases the cure rate slightly but not significantly.Citation63 For invasive adenomas, although tumor shrinkage was observed in most studies, the long-term biological control of serum GH and IGF-1 levels could not be achieved.Citation64 In the study of Mao et al, a higher rate of biochemical cure was observed in patients with presurgical SA treatment in 1–3-month follow-ups;Citation78 however, this may have resulted from the cumulative effect of presurgical SA rather than from an actual increase of total resection. Considering the high cure rate of microadenomas by surgery and the difficulties of total resection for invasive macroadenomas, even with presurgical SA treatment, the assumption that a more satisfactory surgical outcome of noninvasive macroadenomas could be achieved through tumor shrinkage resulting from the presurgical SA treatment is reasonable.
Comparison of lanreotide ATG and octreotide LAR
As mentioned in the guidelines of Melmed et al, in well-designed trials, the current long-acting SAs, lanreotide ATG and octreotide LAR, show equivalence in their control of symptoms and no significant difference in their ability to normalize serum GH and IGF-1.Citation14 Although a number of studies concerning presurgical treatment with lanreotide ATG or octreotide LAR exist, there are few prospective large-scale randomized trials comparing the effects of lanreotide ATG and octreotide LAR, and thus no definite conclusion has yet been achieved.Citation62,Citation64,Citation67,Citation69,Citation70,Citation78 Lanreotide ATG and octreotide LAR may therefore have similar effects in clinic according to the current data.
Different strategies: primary and secondary therapy
Medical treatment with long-acting SA allows for convenient application and shows potential advantages in controlling serum GH and IGF-1 levels; however, the cost–benefit effects of SA treatment should be carefully considered, whether intended as primary or secondary therapy. The indications of SA for acromegaly management recommended in the 2009 guidelines of Melmed et al are as follows: low probability of surgical cure; failure to control serum GH and IGF-1 surgically; and the aim of improving severe perioperative comor-bidities before surgery and maintain disease control between each two adjacent administrations of radiation treatment.Citation14 That is to say, both primary and secondary SA treatments are suggested in appropriate conditions. In the investigation by Giustina et al, the data showed that neurosurgery was the treatment of choice, for microadenomas and macroadenomas with compression to the optic nerve, in most pituitary adenoma centers in the world.Citation79 Further, SAs were chosen as the primary treatment in the condition of macroadenomas with lateral extensions.Citation79 In this case, both primary SA therapy and secondary SA therapy following surgery debulking were optional.Citation80 Increased response percentage of patients with secondary lanreotide was found if more than 75% tumor deb-ulking was achieved.Citation17 Prospective trials comparing secondary SA treatment following surgery and primary SA treatment are few, although both therapies have been proven effective in the treatment of GH-secreting pituitary adenoma Citation17,Citation80
Drug combinations
As the only GHRA available, pegvisomant has shown its effectiveness in the control of IGF-1.Citation81 Recent studies have shown that the combination of SA and pegvisomant in patients who could not achieve IGF-1 normalization was safe and aided improved quality of life in acromegaly.Citation82–Citation85 Further, the combination of pegvisomant and SA could reduce the dose of SA that is required.Citation84 There is, however, no evidence adequate to prove the significant benefits obtained from combination.Citation84 In Melmed et al’s guidelines, such combination is recommended on the condition that patients are resistant to other treatments.Citation14 In combination with cabergoline, the combination of SA and cabergoline might provide effective treatment in patients with mixed pituitary adenomas in whom simultaneously elevated prolactin (PRL) and GH are observed,Citation86 while, in patients who are partially responsive to the maximum SA dose, additive therapy with cabergoline could normalize IGF-1 in about half of the patients, including those without prolactinemia.Citation32
New SAs
Pasireotide, as a new SA,Citation33 is reported to show high affinity to SSTR 1, 2, 3, and 5.Citation87 Therefore, it has the potential to be effective in the control of GH, IGF-1, and tumor volume, as shown in a long-term trial investigating the efficacy and safety of pasireotide in acromegaly (Phase II extension study),Citation88 and pasireotide is expected in the treatment of Cushing’s disease.Citation89 There is, however, no evidence proving that pasireotide is valuable when the tumors are resistant to other SAs, despite its special utility in treating tumors resistant to SSTR 2-preferential analogs, which is owing to its high-affinity binding to SSTR 1, 2, 3, and 5.Citation90,Citation91 Dopastatin (BIM23A760) is reported to bind to SSTR 2 and 5 and dopamine D2(DAD2) compound, and somatropin (DG3173) is a novel SA with additional binding to SSTR 4 and low insulin-suppressing activity in preclinical studies; these SAs are therefore expected to be effective and supplementary in the treatment of acromegaly with octreotide and lanreotide.Citation92–Citation94
SAs in the treatment of gigantism
For the treatment of gigantism, the optimal choices include transsphenoidal surgery, medical therapy, and radiation therapy.Citation3 In the case of microadenomas and well-circumscribed macroadenomas, transsphenoidal surgery may provide a curative effect.Citation95 Radiation therapy can induce the normalization of GH, but the rate of hypopituitarism after treatment is high.Citation3 In recent years, the development of long-acting SAs has provided a highly effective method by which to control serum GH and normalize IGF-1.Citation6,Citation7 Lanreotide or octreotide as the primary therapy,Citation7 or combined with surgery,Citation6 are effective and safe for gigantism. However, the numbers of patients were limited in the studies concerning medical treatment of gigantism,Citation6–Citation8 so conclusions concerning the effect of medical treatment for gigantism are still unclear.
Conclusion
Long-acting lanreotide and octreotide provide an effective medical treatment whether as primary or secondary therapy for the treatment of GH-secreting pituitary adenoma. Following the development of therapeutic strategies for GH-secreting pituitary adenoma, better prognosis with higher control rates of serum GH and IGF-1 has been achieved. Considering, however, the high cost of SA drugs, several points should be considered before medical treatment. A proposed strategy of treatment for patients with acromegaly is summarized in . The complete assessment of medical treatment should contain the observation of clinical predictors and preselection, if necessary. To achieve a long-term stable blood concentration, a treatment process of more than 3 months should be implemented. Although controversy regarding presurgical adjuvant therapy exists, more satisfactory surgical outcomes of noninvasive macroadenomas may be achieved. Primary and secondary therapy of SA treatment show no obvious differences with limited data. Thus, in conclusion, a individualized treatment program should be established for each patient after a full evaluation of the risks and benefits to achieve maximum effectiveness with minimum costs.
Acknowledgments
This research was supported in part by two grants from the National Natural Science Foundation of China (No 41076092, 30971538) and one grant from the Guangdong Provincial 125 key laboratory foundation of medicine (K970401302).
Disclosure
The authors report no conflicts of interest in this work.
References
- MelmedSMedical progress: acromegalyN Engl J Med2006355242558257317167139
- RaappanaAKoivukangasJEbelingTPirilaTIncidence of pituitary adenomas in Northern Finland in 1992–2007J Clin Endocrinol Metab20109594268427520534753
- EugsterEAPescovitzOGigantismJ Clin Endocrinol Metab199984124379438410599691
- EtxabeJGaztambideSLatorrePVazquezJAAcromegaly: an epidemiological studyJ Endocrinol Invest19931631811878514973
- GelberSJHeffezDSDonohouePAPituitary gigantism caused by growth hormone excess from infancyJ Pediatr199212069319341593354
- ColaoAPivonelloRDi SommaCTauchmanovàLSavastanoSLombardiGGrowth hormone excess with onset in adolescence: clinical appearance and long-term treatment outcomeClin Endocrinol (Oxf)200766571472217388794
- ShimatsuATeramotoAHizukaNKitaiKRamisJChiharaKEfficacy, safety, and pharmacokinetics of sustained-release lanreotide (lanreotide Autogel) in Japanese patients with acromegaly or pituitary gigantismEndocr J201360565166323337477
- Näntö-SalonenKKoskinenPSonninenPToppariJSuppression of GH secretion in pituitary gigantism by continuous subcutaneous octreotide infusion in a pubertal boyActa Paediatr1999881293310090543
- MelmedSAcromegaly pathogenesis and treatmentJ Clin Invest2009119113189320219884662
- AndersonDFaberPMarcovitzSHardyJLorenzettiDPituitary tumors and the ophthalmologistOphthalmology19839011126512706664664
- ArafahBMPruntyDYbarraJHlavinMLSelmanWRThe dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomasJ Clin Endocrinol Metab20008551789179310843153
- ColaoAFeroneDMarzulloPLombardiGSystemic complications of acromegaly: epidemiology, pathogenesis, and managementEndocr Rev200425110215214769829
- HoldawayIMRajasooryaRCGambleGDFactors influencing mortality in acromegalyJ Clin Endocrinol Metab200489266767414764779
- MelmedSColaoABarkanAAcromegaly Consensus GroupGuidelines for acromegaly management: an updateJ Clin Endocrinol Metab20099451509151719208732
- AlexopoulouOBexMAbsRT’SjoenGVelkeniersBMaiterDDivergence between growth hormone and insulin-like growth factor-i concentrations in the follow-up of acromegalyJ Clin Endocrinol Metab20089341324133018230660
- HoldawayIMBollandMJGambleGDA meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegalyEur J Endocrinol20081592899518524797
- ColaoAAttanasioRPivonelloRPartial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegalyJ Clin Endocrinol Metab2006911859216263832
- PetrossiansPBorges-MartinsLEspinozaCGross total resection or debulking of pituitary adenomas improves hormonal control of acromegaly by somatostatin analogsEur J Endocrinol20051521616615762188
- JenkinsPJBatesPCarsonMNStewartPMWassJConventional pituitary irradiation is effective in lowering serum growth hormone and insulin-like growth factor-I in patients with acromegalyJ Clin Endocrinol Metab20069141239124516403824
- MaizaJCVezzosiDMattaMLong-term (up to 18 years) effects on GH/IGF-1 hypersecretion and tumour size of primary somatosta-tin analogue (SSTa) therapy in patients with GH-secreting pituitary adenoma responsive to SSTaClin Endocrinol (Oxf)200767228228917524029
- LucasTAstorgaRSpanish-Portuguese Multicentre Autogel Study Group on AcromegalyEfficacy of lanreotide Autogel administered every 4–8 weeks in patients with acromegaly previously responsive to lanreotide microparticles 30 mg: a phase III trialClin Endocrinol (Oxf)200665332032616918950
- CaronPBeckersACullenDREfficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in the management of acromegalyJ Clin Endocrinol Metab20028719910411788630
- CaronPBexMCullenDRGroup for Lanreotide Autogel Long-Term Study on AcromegalyOne-year follow-up of patients with acromegaly treated with fixed or titrated doses of lanreotide AutogelClin Endocrinol (Oxf)200460673474015163338
- GuttBBidlingmaierMKretschmarKDieterleCSteffinBSchopohlJFour-year follow-up of acromegalic patients treated with the new long-acting formulation of Lanreotide (Lanreotide Autogel)Exp Clin Endocrinol Diabetes2005113313914415789272
- CaronPMorange-RamosICogneMJaquetPThree year follow-up of acromegalic patients treated with intramuscular slow-release lanreotideJ Clin Endocrinol Metab199782118228989225
- MurrayRDMelmedSA critical analysis of clinically available somatostatin analog formulations for therapy of acromegalyJ Clin Endocrinol Metab20089382957296818477663
- AndriesMGlintborgDKvistborgAHagenCAndersenMA 12-month randomized crossover study on the effects of lanreotide Autogel and octreotide long-acting repeatable on GH and IGF-I in patients with acromegalyClin Endocrinol (Oxf)200868347348017941902
- van der LelyAJBillerBMBrueTLong-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDYJ Clin Endocrinol Metab20129751589159722362824
- FeenstraJde HerderWWten HaveSCombined therapy with somatostatin analogues and weekly pegvisomant in active acromegalyLancet200536594711644164615885297
- NeggersSJde HerderWWJanssenJAFeeldersRAvan der LelyAJCombined treatment for acromegaly with long-acting somatostatin analogs and pegvisomant: long-term safety for up to 4.5 years (median 2.2 years) of follow-up in 86 patientsEur J Endocrinol2009160452953319141604
- AbsRVerhelstJMaiterDCabergoline in the treatment of acro-megaly: a study in 64 patientsJ Clin Endocrinol Metab19988323743789467544
- SandretLMaisonPChansonPPlace of cabergoline in acromegaly: a meta-analysisJ Clin Endocrinol Metab20119651327133521325455
- ColaoAAuriemmaRSLombardiGPivonelloRResistance to soma-tostatin analogs in acromegalyEndocr Rev201132224727121123741
- NewmanCBMelmedSSnyderPJSafety and efficacy of long-term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients – a clinical research center studyJ Clin Endocrinol Metab1995809276827757673422
- BevanJSAtkinSLAtkinsonABPrimary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I, and tumor sizeJ Clin Endocrinol Metab200287104554456312364434
- ReubiJCLandoltAMThe growth hormone responses to octreotide in acromegaly correlate with adenoma somatostatin receptor statusJ Clin Endocrinol Metab19896848448502537844
- ColaoAAuriemmaRSReboraASignificant tumour shrinkage after 12 months of lanreotide Autogel-120 mg treatment given first-line in acromegalyClin Endocrinol (Oxf)200971223724519094074
- HoflandLJLambertsSWThe pathophysiological consequences of somatostatin receptor internalization and resistanceEndocr Rev2003241284712588807
- BertheratJChansonPDewaillyDSomatostatin receptors, adenylate cyclase activity, and growth hormone (GH) response to octreotide in GH-secreting adenomasJ Clin Endocrinol Metab1993776157715837903312
- ColaoAPivonelloRCappabiancaPEffect of gender and gonadal status on the long-term response to somatostatin analogue treatment in acromegalyClin Endocrinol (Oxf)200563334234916117824
- ColaoAPivonelloRSpinelliLA retrospective analysis on biochemical parameters, cardiovascular risk and cardiomyopathy in elderly acromegalic patientsJ Endocrinol Invest200730649750617646725
- ColaoAFeroneDLastoriaSPrediction of efficacy of octreotide therapy in patients with acromegalyJ Clin Endocrinol Metab1996816235623628964877
- KaravitakiNBotusanIRadianSCoculescuMTurnerHEWassJAThe value of an acute octreotide suppression test in predicting long-term responses to depot somatostatin analogues in patients with active acromegalyClin Endocrinol (Oxf)200562328228815730408
- LambertsSWUitterlindenPSchuijffPCKlijnJGTherapy of acromegaly with sandostatin: the predictive value of an acute test, the value of serum somatomedin-C measurements in dose adjustment and the definition of a biochemical ‘cure’Clin Endocrinol (Oxf)19882944114203251673
- FredaPUKatznelsonLvan der LelyAJReyesCMZhaoSHRabinowitzDLong-acting somatostatin analog therapy of acromegaly: a meta-analysisJ Clin Endocrinol Metab20059084465447315886238
- PlöckingerUBäderMHopfenmüllerWSaegerWQuabbeHJResults of somatostatin receptor scintigraphy do not predict pituitary tumor volume- and hormone-response to octreotide therapy and do not correlate with tumor histologyEur J Endocrinol199713643693769150695
- LegoviniPDe MenisEBilleciDContiBZoliPConteN111 Indium-pentetreotide pituitary scintigraphy and hormonal responses to octreotide in acromegalic patientsJ Endocrinol Invest19972074244289309542
- GoergesRBockischACordesUCorrelation between pituitary In-111-pentetreotide uptake and growth hormone (GH) response to octreotide in acromegalyEur J Endocrinol199626Suppl 1A55
- CendrosJMPeraireCTrocónizIFObachRPharmacokinetics and population pharmacodynamic analysis of lanreotide AutogelMetabolism200554101276128116154424
- BronsteinMMusolinoNJalladRPharmacokinetic profile of lanreotide Autogel in patients with acromegaly after four deep subcutaneous injections of 60, 90 or 120 mg every 28 daysClin Endocrinol (Oxf)200563551451916268802
- AstrucBMarbachPBouterfaHLong-acting octreotide and prolonged-release lanreotide formulations have different pharmacoki-netic profilesJ Clin Pharmacol200545783684415951474
- CroxtallJDScottLJLanreotide Autogel: a review of its use in the management of acromegalyDrugs200868571172318370450
- CaronPSomatuline(R) Autogel(R), a new formulation of lanreotide for the treatment of acromegalic patientsAnn Endocrinol (Paris)2002632 Pt 32S192S24 French12037499
- AnnamalaiAKWebbAKandasamyNA comprehensive study of clinical, biochemical, radiological, vascular, cardiac, and sleep parameters in an unselected cohort of patients with acromegaly undergoing presurgical somatostatin receptor ligand therapyJ Clin Endocrinol Metab20139831040105023393175
- Ben-ShlomoAMelmedSClinical review 154: The role of pharma-cotherapy in perioperative management of patients with acromegalyJ Clin Endocrinol Metab200388396396812629068
- SeidmanPAKofkeWAPolicareRYoungMAnaesthetic complications of acromegalyBr J Anaesth200084217918210743450
- ColaoAFeroneDCappabiancaPEffect of octreotide pretreatment on surgical outcome in acromegalyJ Clin Endocrinol Metab19978210330833149329359
- ManelliFDesenzaniPBoniECardiovascular effects of a single slow release lanreotide injection in patients with acromegaly and left ventricular hypertrophyPituitary19992320521011081155
- HoogwerfBJPostoperative management of the diabetic patientMed Clin North Am20018551213122811565495
- CoutureEBongardVMaizaJCBennetACaronPGlucose status in patients with acromegaly receiving primary treatment with the somatostatin analog lanreotidePituitary201215451852522058008
- SteffinBGuttBBidlingmaierMDieterleCOltmannFSchopohlJEffects of the long-acting somatostatin analogue Lanreotide Autogel on glucose tolerance and insulin resistance in acromegalyEur J Endocrinol20061551737816793952
- Pita-GutierrezFPertega-DiazSPita-FernandezSPlace of preoperative treatment of acromegaly with somatostatin analog on surgical outcome: a systematic review and meta-analysisPLoS One20138e6152323634209
- AbeTLüdeckeDKEffects of preoperative octreotide treatment on different subtypes of 90 GH-secreting pituitary adenomas and outcome in one surgical centreEur J Endocrinol2001145213714511454508
- ShenMShouXWangYEffect of presurgical long-acting octreotide treatment in acromegaly patients with invasive pituitary macroadenomas: a prospective randomized studyEndocr J201057121035104421099129
- ColaoAThe importance of presurgical somatostatin analogue therapy in acromegalyEndokrynol Pol200758435636018058729
- LosaMMortiniPUrbazLRibottoPCastrignanóTGiovanelliMPresurgical treatment with somatostatin analogs in patients with acromegaly: effects on the remission and complication ratesJ Neurosurg2006104689990616776333
- OshinoSSaitohYKasayamaSShort-term preoperative octreotide treatment of GH-secreting pituitary adenoma: Predictors of tumor shrinkageEndocr J200653112513216543682
- LudeckeDKAbeTTranssphenoidal microsurgery for newly diagnosed acromegaly: a personal view after more than 1,000 operationsNeuroendocrinology2006833–423023917047388
- YinJSuCBXuZQEffect of preoperative use of long-acting octreotide on growth hormone secreting pituitary adenoma and transsphenoidal surgeryChin Med Sci J2005201232615844307
- PlöckingerUQuabbeHJPresurgical octreotide treatment in acromegaly: no improvement of final growth hormone (GH) concentration and pituitary function. A long-term case-control studyActa Neurochir (Wien)2005147548549315806331
- LucasTAstorgaRCataláMSpanish Multicentre Lanreotide Study Group on AcromegalyPreoperative lanreotide treatment for GH-secreting pituitary adenomas: effect on tumour volume and predictive factors of significant tumour shrinkageClin Endocrinol (Oxf)200358447148112641631
- StevenaertABeckersAPresurgical Octreotide: treatment in acromegalyMetabolism1996458 Suppl 172748769388
- DePReesDADaviesNTranssphenoidal surgery for acromegaly in wales: results based on stringent criteria of remissionJ Clin Endocrinol Metab20038883567357212915637
- NomikosPBuchfelderMFahlbuschRThe outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’Eur J Endocrinol2005152337938715757854
- KaltsasGAIsidoriAMFlorakisDPredictors of the outcome of surgical treatment in acromegaly and the value of the mean growth hormone day curve in assessing postoperative disease activityJ Clin Endocrinol Metab20018641645165211297598
- ShimonICohenZRRamZHadaniMTranssphenoidal surgery for acromegaly: endocrinological follow-up of 98 patientsNeurosurgery200148612391243 discussion 1244–124511383725
- BeauregardCTruongUHardyJSerriOLong-term outcome and mortality after transsphenoidal adenomectomy for acromegalyClin Endocrinol (Oxf)2003581869112519417
- MaoZGZhuYHTangHLPreoperative lanreotide treatment in acromegalic patients with macroadenomas increases short-term postoperative cure rates: a prospective, randomised trialEur J Endocrinol2010162466166620061334
- GiustinaABronsteinMDCasanuevaFFCurrent management practices for acromegaly: an international surveyPituitary201114212513321063787
- GrassoLFPivonelloRColaoASomatostatin analogs as a first-line treatment in acromegaly: when is it appropriate?Curr Opin Endocrinol Diabetes Obes201219428829422627686
- NeggersSJvan AkenMOde HerderWWQuality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomantJ Clin Endocrinol Metab200893103853385918647806
- FendriSKaracaPTievEBuchfelderMLalauJControl of disease activity and tumor size after introduction of pegvisomant in a lanreotide-resistant acromegalic patientAnn Endocrinol (Paris)2013741495223337021
- NeggersSJde HerderWWFeeldersRAvan der LelyAJConversion of daily pegvisomant to weekly pegvisomant combined with long-acting somatostatin analogs, in controlled acromegaly patientsPituitary201114325325821221818
- MadsenMPoulsenPLOrskovHMøllerNJørgensenJOCotreatment with pegvisomant and a somatostatin analog (SA) in SA-responsive acromegalic patientsJ Clin Endocrinol Metab20119682405241321632808
- van der LelyABernabeuICapJCoadministration of lanreotide Autogel and pegvisomant normalizes IGF1 levels and is well tolerated in patients with acromegaly partially controlled by somatostatin analogs aloneEur J Endocrinol2011164332533321148630
- Sowiń skiJSawickaNPiatekKZybekARuchalaMPharmacoeconomic aspects of the treatment of pituitary gland tumoursContemp Oncol (Pozn)201317213714323788980
- LewisIBauerWAlbertRA novel somatostatin mimic with broad somatotropin release inhibitory factor receptor binding and superior therapeutic potentialJ Med Chem200346122334234412773038
- PetersennSFarrallAJBlockCLong-term efficacy and safety of subcutaneous pasireotide in acromegaly: results from an open-ended, multicenter, Phase II extension studyPituitary Epub3262013
- ColaoAPetersennSNewell-PriceJA 12-month phase 3 study of pasireotide in Cushing’s diseaseN Engl J Med20123661091492422397653
- HoflandLJvan der HoekJvan KoetsveldPMThe novel somatostatin analog SOM230 is a potent inhibitor of hormone release by growth hormone- and prolactin-secreting pituitary adenomas in vitroJ Clin Endocrinol Metab20048941577158515070915
- MurrayRDKimKRenSGThe novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretionJ Clin Endocrinol Metab20048963027303215181094
- GruszkaACullerMDMelmedSSomatostatin analogs and chimeric somatostatin-dopamine molecules differentially regulate human growth hormone and prolactin gene expression and secretion in vitroMol Cell Endocrinol20123621–210410922705877
- CunyTMohamedAGraillonTSomatostatin receptor sst2 gene transfer in human prolactinomas in vitro: impact on sensitivity to dopamine, somatostatin and dopastatin, in the control of prolactin secretionMol Cell Endocrinol2012355110611322348806
- PlöckingerUHoffmannUGeeseMDG3173 (somatoprim), a unique somatostatin receptor subtypes 2-, 4- and 5-selective analogue, effectively reduces GH secretion in human GH-secreting pituitary adenomas even in Octreotide non-responsive tumoursEur J Endocrinol2012166222323422065857
- LuPWSilinkMJohnstonICowellCTJimenezMPituitary gigantismArch Dis Child1992678