Abstract
Targeting sphingosine-1-phosphate pathway with orally available immune-modulatory fingolimod (Gilenya™) therapy ameliorates relapsing–remitting multiple sclerosis (RRMS) by decreasing relapse rate as shown in FREEDOMS and TRANSFORMS. Fingolimod has also been shown to be superior to interferon-beta therapy as evidenced by TRANSFORMS. Albeit multiple benefits in treatment of multiple sclerosis including high efficacy and ease of administration, potential untoward effects such as cardiotoxicity, risk of infection, and cancer exist, thus mandating careful screening and frequent monitoring of patients undergoing treatment with fingolimod. This review outlines mechanism of action, observations, side effects, and practice guidelines on use of fingolimod in treatment of RRMS.
Introduction
Fingolimod (Gilenya™) is the first US Food and Drug Administration (FDA)-approved oral therapy for treatment of multiple sclerosis (MS) based on two Phase III pivotal trials, FREEDOMS and TRANSFORMS.Citation1–Citation4 Fingolimod targets the sphingosine-1-phosphate (S1P) pathway by regulation of lymphocyte trafficking from secondary lymphoid organs into the systemic circulation ().Citation5–Citation8 Interaction of the sphingolipid ligand, S1P, in the blood or lymph with the G protein-coupled receptor S1P receptor 1 (S1PR1) on lymphocytes is necessary for lymphocyte egress from lymph nodes into blood and lymph.Citation9–Citation11 The critical role played by S1P–S1PR1 interaction in immune trafficking is perturbed by fingolimod, a functional antagonist of S1PR.Citation12,Citation13 Fingolimod sequesters lymphocytes in the spleen and lymph nodes by inducing receptor internalization and degradation, causing lymphopenia and sparing the central nervous system from immune attack by myelin-reactive lymphocytes.Citation11 Fingolimod has been shown to effectively decrease relapse rate up to 50% and is superior to interferon-beta (IFNβ) therapy.Citation14–Citation17 However, since fingolimod signals via most of the S1PRs (S1PR1 and 3–5), untoward effects in systems expressing these receptors, including cardiovascular and visual systems (such as cardiac rhythm abnormalities and macular edema), have been observed in patients treated with fingolimod.Citation18–Citation21 Furthermore, due to fingolimod’s action on lymphopenia, side effects related to serious infections and cancer risk, possibly by interfering immune surveillance function of lymphocytes, are also observed.Citation18 In the post-market experience, rebound disease activity (most likely due to reversing fingolimod’s effect on lymphocyte egress) is observed upon discontinuation of the therapy.Citation22–Citation25 Thus, careful patient selection with rigorous and frequent monitoring and pre-consideration of optimal treatment sequencing are required for patients undergoing fingolimod therapy.Citation26–Citation29 This review article presents a comprehensive review of screening, monitoring, side effects, and efficacy in the clinical practice utilizing fingolimod for the treatment of relapsing–remitting MS (RRMS).
Table 1 S1PR in the immune system
Screening before initiating therapy
Baseline screening and ongoing monitoring are required for fingolimod treatment to avoid potential serious side effects ().Citation29,Citation30 The recommended baseline screening includes electrocardiogram (ECG) for cardiac rhythm abnormalities, ophthalmologic examination to evaluate for macular edema, serological test for immunity to varicella zoster virus (VZV) infection, complete blood count, liver function tests, blood pressure, and urine pregnancy test for females during reproductive age.Citation30 Additional recommendations include pulmonary function test, dermatological examination, and serological test for viral hepatitis and tuberculosis, when clinically indicated. A detailed evaluation such as referral to cardiology (for abnormal ECG) and dermatology proceeds if baseline screening is abnormal. Active immunization against VZV is recommended for nonimmune cases.Citation30,Citation31 Review of past medical history and medication list for potential drug interactions is also recommended as part of prescreening. Special attention is paid to patients with diabetes and uveitis due to the increased risk of developing macular edema with fingolimod therapy.Citation32,Citation33 Cardiac rhythm abnormalities such as torsades de pointes, bradycardia, or conduction block could occur, especially in patients who are concurrently on medications that can cause prolonged QT interval (eg, citalopram, chlorpromazine, haloperidol, methadone, erythromycin), or interfere with cardiac conduction (eg, beta blockers, diltiazem, verapamil, digoxin).Citation34,Citation35 Interval monitoring of follow-up ophthalmologic examination (at 3–4 months following treatment initiation), complete blood counts, and hepatic function tests are recommended.Citation1,Citation2 Continuous monitoring of infection is recommended until 2 months after discontinuation of fingolimod. Women of childbearing potential should use effective contraception during and for 2 months after stopping therapy, since fingolimod therapy may cause fetal abnormalities.Citation1,Citation2 Regular monitoring for hypertension is also recommended throughout the duration of therapy.Citation1,Citation2,Citation30
Figure 1 Proposed algorithm for patient management upon screening, FDO, and long-term follow-up for fingolimod therapy.
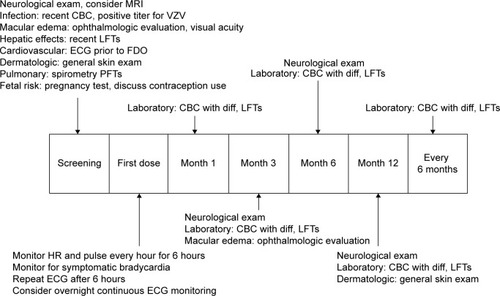
Patients undergoing fingolimod therapy receive more rigorous screening compared to those undergoing other disease-modifying therapies (DMTs). This may delay the initiation of therapy; however, patients in our practice have expressed satisfaction with thorough screening prior to starting therapy.
First-dose observation
The first-dose observation (FDO) is a 6-hour monitoring session assessing for cardiac rhythm abnormalities, especially bradycardia, after initiation of fingolimod therapy. FDO includes monitoring for heart rate, cardiac rhythm, and blood pressure every hour, and ECG at 0 hour and 6 hours after taking first dose of fingolimod.Citation36 The cardiovascular side effects during FDO from FREEDOMS and TRANSFORMS studies are bradycardia as being the most common cardiovascular event followed by first-degree atrioventricular (AV) block, second-degree AV block Mobitz type 1, sinus arrhythmia, and ventricular premature beats.Citation36 The new FDA-revised FDO monitoring includes a repeat ECG prior to discharge based on potential cardiac rhythm abnormalities following first dose of fingolimod.Citation37,Citation38 FDO is carried out as an outpatient procedure in most facilities where patients are observed on a cardiac monitor for 6 hours with access to a rapid response team. Extended monitoring is recommended for those patients who demonstrate the following: 1) heart rate <45 beats per minute, 2) a continued downward trend, 3) new-onset second-degree or more severe conduction block, 4) symptomatic bradycardia, and 5) prolonged QTc interval (QTc >470 ms in females and >450 ms in males) following 6-hour FDO monitoring.Citation37 Overnight continuous cardiac monitoring in a medical facility for FDO is recommended for patients with a preexisting cardiac condition or on concurrent medication that can interfere with cardiac conduction; patients with a prior cardiac history have not shown increased incidence of adverse events with FDO.Citation39–Citation41 Common complaints during FDO in our practice include headache, mild nausea, and symptomatic bradycardia in order of frequency. The guidelines for patients requiring repeat FDO are discontinuation within first 2 weeks of therapy, interruption of 1 or more days during weeks 3–4 of therapy, interruption of 7 or more days after 4 weeks of therapy, and interruption of 14 or more days anytime during treatment.Citation1,Citation37
Untoward effects associated with fingolimod use
Side effects associated with fingolimod reported in FREEDOMS and TRANSFORMS are abnormal laboratory finding, infections, cardiovascular side effects, macular edema, malignancies, pulmonary side effects, and rare cases of posterior reversible encephalopathy syndrome. Less serious side effects include headache and hypertension.Citation1,Citation2
Laboratory test abnormalities
Lymphopenia was the most common abnormal laboratory test leading to drug discontinuation in the FREEDOMS studies.Citation42,Citation43 Peripheral blood lymphocyte counts decreased up to 20%–30% from baseline within the first month of initiation of fingolimod therapy.Citation20 Lymphopenia is often reversible and normalized approximately 45–135 days following discontinuation of fingolimod.Citation44 Elevated alanine aminotransferase (ALT) (up to three or more times the upper limit of normal) was observed as the second most common abnormal laboratory finding.Citation1,Citation2 ALT levels also normalized spontaneously after discontinuation of fingolimod without permanent hepatic dysfunction.Citation1,Citation2
Risk of infection
The overall incidence of infections in FREEDOMS and TRANSFORMS studies was similar in the treatment and placebo groups; however, a slightly higher incidence of bronchitis, influenza, and herpes viral infections (including herpes zoster infection) was observed in the fingolimod treatment groups.Citation30 Other common infections included upper respiratory tract infections, nasopharyngitis, urinary tract infections, and sinusitis.Citation30 Two fatal cases of reactivation of latent herpes virus (in the fingolimod 1.25 mg treatment group), a case of fatal disseminated VZV infection, and a case of fatal herpes simplex virus type 1 encephalitis in TRANSFORMS study were also observed.Citation1–Citation3,Citation30 Other infections such as reactivation of human papilloma virus, John Cunningham virus (in a post-natalizumab case), tuberculosis, and cytomegalovirus were also reported; however, post-marketing data did not reveal an increased rate of occult infections.Citation45 About 20% of our patient cohort on fingolimod (n=50) have reported symptoms of vaginitis, recurrent upper respiratory, and sinus infections; however, the etiology is unclear.
Cardiovascular side effects
Fingolimod binds to S1PR1 in the heart, which can result in heart rhythm abnormalities such as bradycardia, therefore necessitating FDO.Citation36–Citation41 Maximal decrease in heart rate occurs at 4–6 hours, which is the basis of rationale for the 6-hour monitoring period.Citation20 Symptomatic (eg, dizziness, chest discomfort, palpitations, and/or fatigue) bradycardia was observed in less than 1% of subjects.Citation43,Citation46 In FREEDOMS and TRANSFORMS studies, symptomatic bradycardia during FDO resolved within 24 hours without pharmacological interventions.Citation1,Citation2 Fingolimod is also known to induce cardiac conduction abnormalities, including first- and second-degree AV block, on the 6-hour post-dose ECG; patients who continued on treatment did not have persistent cardiac conduction abnormalities.Citation34,Citation35 We have had infrequent cases of symptomatic bradycardia leading to discontinuation of fingolimod as seen with patients in our practice.
Macular edema
Another potential side effect associated with the upregulation of S1PR in the vascular endothelial cells of the macula is fingolimod-associated macular edema (FAME).Citation28–Citation32 Most of the cases with FAME in FREEDOMS and TRANSFORMS studies were asymptomatic; the overall incidence of FAME was 0.5% in the 0.5 mg group, with complete resolution following discontinuation of therapy.Citation1,Citation2 FAME generally occurred within 3–4 months of fingolimod initiation, although there has been a case report of early bilateral macular edema following fingolimod therapy within the first 3 months.Citation32,Citation33 Therefore, a follow-up ophthalmologic examination at 3–4 months post-fingolimod initiation is recommended. Few cases of unresolved macular edema were identified in the higher dose (1.25 mg) fingolimod group.Citation20 In our patient cohort, we have seen two cases of symptomatic FAME which appeared 3–4 months after fingolimod initiation. Both cases had complete resolution of visual disturbance within 2–3 months after discontinuing fingolimod.
Risk of malignancy
An increased risk of malignancies was found in association with fingolimod therapy in the TRANSFORMS and FREEDOMS studies. Most common malignancies found in association with fingolimod use are dermatological malignancies (Bowen’s disease, n=1; basal cell carcinoma, n=10; and malignant melanoma, n=4).Citation20,Citation21 Other malignancies reported in the studies are breast cancer (n=5) with a fatal case of metastatic breast cancer in a patient who died 10 months after discontinuing fingolimod.Citation47 Although there were at least three case reports of lymphoma in the fingolimod treatment group during drug development, a general consensus has not been reached on the risk of lymphoma with fingolimod.Citation48
Pulmonary side effects
Respiratory effects including mild reductions in 1-second forced expiratory volume and diffusion capacity for carbon monoxide were observed in FREEDOMS and TRANSFORMS.Citation1,Citation2 Spirometry and diffusion lung capacity tests are recommended if clinically indicated.Citation1 We have a standard protocol assessing for pulmonary function status through spirometry testing prior to FDO.
Pregnancy
Although fingolimod is classified as pregnancy category C, there have been cases of teratogenicity in live births during fingolimod clinical development.Citation49 Exposure to fingolimod in the first trimester resulted in five cases of abnormal fetal development in 66 pregnancies.Citation49,Citation50 The available pregnancy registry data continue to provide important information regarding use of fingolimod in women of childbearing potential with the known risk of possible fetal malformation and teratogenic effects.Citation51,Citation52 The current recommendation for women of childbearing potential is to use effective contraception during fingolimod therapy and for at least 2 months after discontinuation of fingolimod.Citation1
Tumefactive MS and rebound relapses
To date, 16 case reports in the literature describe worsening MS disease activity or even development of tumefactive demyelinating lesions (TDL) following fingolimod therapy.Citation53–Citation55 TDL are extremely rare and were observed in patients with established diagnosis of MS leading to the suspicion of a causal relationship between the use of fingolimod and development of TDL.Citation55,Citation56 The diagnosis of progressive multifocal leukoencephalopathy (PML) was entertained in this particular patient with TDL, since the patient was on natalizumab therapy before starting fingolimod; however, the patient was found to have “rebound” disease activity along with the development of TDL.Citation57–Citation60 It would seem prudent to monitor clinical progression and magnetic resonance imaging (MRI) activity for worsening disease activity in fingolimod-treated patients regardless of disease duration and prior DMT history.
Post-market experience
Sudden death in a hypertensive patient on calcium-channel blockers and beta blockers within 24 hours following first-dose fingolimod prompted the FDA and the European Medicines Agency (EMA) to recommend a modification in the FDO to include hourly heart rate and blood pressure monitor and an ECG (either continuous, according to the EMA, or pre-dose and 6 hours post-dose, according to the FDA) as well as excluding patients on medications that can cause cardiac rhythm abnormalities.Citation37,Citation38 Despite the recommendations, two open-label studies on fingolimod treatment initiation resulted in overall satisfactory safety and tolerability in patients with concomitant diseases, and no cardiac adverse events were observed in association with fingolimod use.Citation34,Citation39 On the contrary, a case report in 2013 identified that three (out of 59) patients without known cardiovascular disease were found to have cardiac rhythm abnormalities (eg, sinus bradycardia with idioventricular escape rhythm that lasted 45 seconds and second-degree AV block Mobitz type 1).Citation34 Additional post-marketing reports have raised concern over the risk for PML in patients who were originally treated with natalizumab.Citation60 A confirmed case of PML was reported in 2012 in a patient who was treated with natalizumab for 42 months prior to fingolimod therapy.Citation61 The second case of PML was observed in a patient treated with fingolimod, who did not have prior exposure to natalizumab.Citation62 The third reported case of PML was observed in a patient 3.5 months after fingolimod initiation and 4.5 months after natalizumab discontinuation.Citation61,Citation62
Efficacy
Fingolimod met the primary end point of annualized relapse rate reduction across both Phase III trials. With the exception of time to disability progression in TRANSFORMS, secondary end point measures including MRI data were statistically significant in FREEDOMS and TRANSFORMS.Citation63,Citation64 More importantly, TRANSFORMS showed greater efficacy in relapse rate reduction over IFNβ in a 12-month study ().Citation1,Citation2 Brain volume loss has been specifically studied in FREEDOMS, which found that fingolimod 0.5 mg dose significantly reduced brain volume loss up to 24 months vs placebo irrespective of the presence or absence of gadolinium-enhancing lesions, T2 lesion load, previous treatment status, or level of disability.Citation65 Long-term data to support ongoing reduction in disability progression and brain volume loss are not available at this time, but studies to assess fingolimod safety and tolerability continue ().Citation66,Citation67
Table 2 Summary of pivotal trials
Table 3 Summary of other clinical trials
Suggested treatment algorithm
Clinicians and patients across MS centers continue to struggle with selecting the most effective MS therapy for a particular patient with RRMS, and to assess whether drug benefits outweigh risks of treatment ().Citation68–Citation70 As the first of three first-line oral therapies for the treatment of RRMS, fingolimod presents a suitable option for patients with recent diagnosis of MS or those with suboptimal response and/or compliance to injectable first-line immune therapies.Citation71 Clinicians tend to switch patients to fingolimod (from natalizumab) based on the patients’ risk for development of PML based on serological status for John Cunningham virus, and those with neutralizing antibodies against natalizumab.Citation72 A washout period of approximately 3 months has been recommended when switching from natalizumab to fingolimod, but duration of washout period depends on the disease activity and other comorbidities such as the immune status of the patient.Citation73
Figure 2 Proposed algorithm to start fingolimod for treatment-naïve patients and prior disease-modifying therapy use.
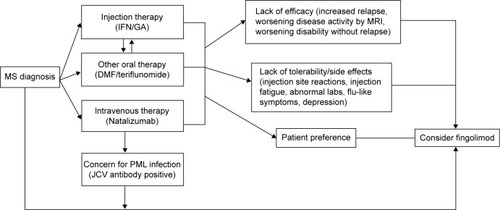
Patient adherence
It is well recognized that medication adherence is not always 100% with either oral or injectable DMTs for multiple reasons.Citation74,Citation75 Fingolimod provides an attractive alternative to injectable DMTs due to the ease of administration. However, a retrospective study on medication compliance showed that approximately 27% of fingolimod users discontinued within 1 year of treatment initiation.Citation75 Socioeconomic factors play a role in patient adherence to drug treatment with increasing out-of-pocket and copayments and lack of insurance coverage.Citation76,Citation77 The Consortium of MS Centers recently outlined that the main reasons for patients switching DMTs in MS included efficacy, safety, prescriber- or payer-related reasons, and patient-related reasons which included difficulty with adherence, desire to try different administration methods, and perceived lack of efficacy.Citation78 Most of the patients in our practice have discontinued fingolimod primarily due to side effects of the medication and perceived lack of efficacy. Typically, patients who have switched from injection therapy are pleased with the ease of oral administration of fingolimod, despite the lack of comprehensive long-term safety data.
Conclusion
Fingolimod is the first FDA-approved oral therapy for the treatment of RRMS as shown by two large Phase III studies, FREEDOMS and TRANSFORMS.Citation1 Clinical efficacy of fingolimod was observed to be superior to placebo and IFNβ-1a in reducing relapse rate and MRI activity. However, thorough screening, FDO, and long-term follow-up are recommended in order to avoid potential side effects associated with fingolimod therapy. Fingolimod presents as a treatment option as a first-line therapy for patients with new-onset RRMS or those switching therapies due to intolerability and/or lack of efficacy of prior DMTs. However, studies on long-term efficacy, safety, and mechanism of action of fingolimod remain to be further pursued for therapeutic optimization and to avoid undesirable side effects.
Disclosure
Jong-Mi Lee has received consulting agreements or service as speaker (Biogen Idec, Teva Pharmaceuticals, and Genzyme). The other author reports no conflicts of interest in this work.
References
- Novartis PharmaceuticalsFingolimod Prescribing Information2014 Available from: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdfAccessed December 8, 2014
- European Medicines AgencyEuropean Public Assessment Report (EPAR) for Gilenya2014 Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002202/human_med_001433.jsp&mid=WC0b01ac058001d125Accessed December 8, 2014 Gilenya: EPAR: Summary report for the public
- KapposLRadueEWO’ConnorPA placebo-controlled trial of oral fingolimod in relapsing multiple sclerosisN Engl J Med2010362387401 Pivotal Trial FREEDOMS20089952
- CohenJABarkhofFComiGTRANSFORMS Study GroupOral fingolimod or intramuscular interferon for relapsing multiple sclerosisN Engl J Med2010362402415 Pivotal Trial FREEDOMS20089954
- HlaTBrinkmannVSphingosine 1-phosphate (S1P): physiology and the effects of S1P receptor modulationNeurology201176S3S821339489
- BrinkmannVFTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous systemBr J Pharmacol20091581173118219814729
- BrinkmannVDavisMDHeiseCEThe immune modulator FTY720 targets sphingosine 1-phosphate receptorsJ Biol Chem200227724214532145711967257
- CohenJAChunJMechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosisAnn Neurol201169575977721520239
- MehlingMJohnsonTAAntelJKapposLBar-OrAClinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosisNeurology2011768 suppl 3S20S2721339487
- ChoiJWGardellSEHerrDRFTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor (S1P1) modulationProc Natl Acad Sci U S A2011108275175621177428
- GrovesAKiharaYChunJFingolimod: direct CNS effects on sphingosine 1-phosphate (S1) receptor modulation and implications in multiple sclerosis therapyJ Neurol Sci20133281–291823518370
- MullershausenFZecriFCetinCBillichAGueriniDSeuwenKPersistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptorsNat Chem Biol20095642843419430484
- Van DoornRVan HorssenJVerzijlDSphingosine 1-phosphate receptors 1 and 3 are upregulated in multiple sclerosis lesionsGlia201058121465147620648639
- ScottLJFingolimod: a review of its use in the management of relapsing-remitting multiple sclerosisCNS Drugs2011867369821790210
- DevonshireVHavrdovaERadueEWFREEDOMS study groupRelapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomized, placebo-controlled FREEDOMS studyLancet Neurol20121142042822494956
- GasperiniCRuggieriSDevelopment of oral agent in the treatment of multiple sclerosis: how the first available oral therapy, fingolimod will change therapeutic paradigm approachDrug Des Devel Ther20126175186
- GasperiniCRuggieriSMancinelliCRPozzilliCAdvances in the treatment of relapsing-remitting multiple sclerosis – critical appraisal of fingolimodTher Clin Risk Manag20139738523483794
- MehlingMKapposLDerfussTFingolimod for multiple sclerosis: mechanism of action, clinical outcomes, and future directionsCurr Neurol Neurosci Rep20111149249721789537
- RommerPSZettlUKKieseierBRequirement for safety monitoring for approved multiple sclerosis therapies: an overviewClin Exp Immunol2013175339740724102425
- WardMJonesDEGoldmanMDOverview and safety of fingolimod hydrochloride use in patients with multiple sclerosisExpert Opin Drug Saf201413798999824935480
- KapposLCohenJCollinsWFingolimod in relapsing multiple sclerosis: an integrated analysis of safety findingsMultiple Sclerosis and Related Disorders20143449450425877062
- BeranRGHegaziYSchwartzRSCordatoDJRebound exacerbation multiple sclerosis following cessation of oral treatmentMult Scler Relat Disord20132325225525877732
- AlroughaniRAlroughaniRAlmullaALamdhadeSThussuASevere reactivation of multiple sclerosis after discontinuation of fingolimod: an IRIS-associated phenomenonMult Scler Relat Disord201436748
- HavlaJBPellkoferHLMeinlIGerdesLAHohfeldRKumpfelTRebound of disease activity after withdrawal of fingolimod (FTY720) treatmentArch Neurol201269226226422332194
- GhezziARoccaMABaronciniDDisease reactivation after fingolimod discontinuation in two multiple sclerosis patientsJ Neurol2013260132732923161460
- RommerPSZettlUKKieseierBRequirement for safety monitoring for approved multiple sclerosis therapies: an overviewClin Exp Immunol2014175339740724102425
- SingerBAInitiating oral fingolimod treatment in patients with multiple sclerosisTher Adv Neurol Disord20136426927523858329
- OntanedaDHara-CleaverCRudickRACohenJABermelRAEarly tolerability and safety of fingolimod in clinical practiceJ Neurol Sci20123231–216717223040960
- ThomasKZiemssenTManagement of fingolimod in clinical practiceClin Neurol Neurosurg2013115suppl 1S60S6424321158
- ThöneJEllrichmannGOral available agents in the treatment of relapsing remitting multiple sclerosis: an overview of merits and culpritsDrug Healthc Patient Saf20135374723459383
- BergerJRVaricella vaccination after fingolimod: a case reportMult Scler Relat Disord20132439139425877852
- JainNBhattiMTFingolimod-associated macular edema: incidence, detection, and managementNeurology201278967268022371414
- CoppesOGutierrezIRederATKsiazekSBernardJSevere early bilateral macular edema following fingolimod therapyMult Scler Relat Disord20132325625825877733
- GoldRComiGPalaceJFIRST Study InvestigatorsAssessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label studyJ Neurol2014261226727624221641
- CammJHlaTBakshiRBrinkmannVCardiac and vascular effects of fingolimod: mechanistic basis and clinical implicationsAm Heart J2014168563264425440790
- HorgaACastrilloJMontalbanXFingolimod for relapsing multiple sclerosis; an updateExpert Opin Pharmacother2010111183119620367536
- FDARevised Drug MonitoringUS Food and Drug Administration2014 Available from: http://www.fda.gov/Drugs/DrugSafety/ucm303192.htmAccessed December 8, 2014
- FDAFirst Reported Death on Gilenya: 24hrs Post FDOUS Food and Drug Administration2014 Available from: http://www.fda.gov/Drugs/DrugSafety/ucm284240.htmAccessed December 8, 2014
- ThomasKSchrötterHHalankMZiemssenTFingolimod in a patient with heart failure on the background of pulmonary arterial hypertension and coronary artery diseaseBMC Neurol20141412624906818
- LaroniABrogiDMorraVBEAP InvestigatorsSafety of the first dose of fingolimod for multiple sclerosis: results of an open-label clinical trialBMC Neurol2014146524690227
- FaberHFischerH-JWeberFProlonged and symptomatic bradycardia following a single dose of fingolimodMult Scler201319112612822729989
- SingerBRossAPTobiasKOral fingolimod for the treatment of patients with relapsing forms of multiple sclerosisInt J Clin Pract201165888789521679286
- CalabresiPARadueEWGoodinDSafety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomized, placebo-controlled, phase 3 trialLancet Neurol201413654555624685276
- JohnsonTAShamesIKeezerMReconstitution of circulating lymphocyte counts in FTY720-treated MS patientsClin Immunol20101371152020599429
- RosenstielPVGottschalkRCappielloLZhangYSaidMKapposLLong-term safety of fingolimod: interim evaluation of data from the longterms trialMult Scler Relat Disord20143675225891590
- HughesBCascioneMFreedmanMSFirst-dose effects of fingolimod after switching from injectable therapies in the randomized, open-label, multicenter, evaluate patient outcomes (EPOC) study in relapsing multiple sclerosisMult Scler Relat Disord201435620628
- ConzettKBKolmIJelcicIMelanoma occurring during treatment with fingolimod for multiple sclerosis: a case reportArch Dermatol2011147899199221844470
- LorvikKBBogenBCorthayAFingolimod blocks immunosurveillance of myeloma and B-cell lymphoma resulting in cancer development in miceBlood201211992176217722383793
- KarlssonGFrancisGKorenGPregnancy outcomes in the clinical development program of fingolimod in multiple sclerosisNeurology201482867468024463630
- JonesBMultiple sclerosis: study reinforces need for contraception in women taking fingolimodNat Rev Neurol201410312524514867
- AlwanSChambersCDArmentiVTDessa-SadovnickAThe need for disease-specific prospective pregnancy registry for multiple sclerosis (MS)Mult Scler Relat Disord20154161725787048
- HoutchensMKKolbCMMultiple sclerosis and pregnancy: therapeutic considerationsJ Neurol201326051202121422926165
- HellmannMALevNLotanITumefactive demyelination and a malignant course in an MS patient during and following fingolimod therapyJ Neurol Sci20143441–219319725001515
- PilzGHarrerAWipflerPTumefactive MS lesions under fingolimod: a case report and literature reviewNeurology201381191654165824097813
- CastropFKowarikMCAlbrechtHSevere multiple sclerosis relapse under fingolimod therapy: incident or coincidence?Neurology2012781292893022402856
- JanderSTurowskiBKieseierBCHartungH-PEmerging tumefactive multiple sclerosis after switching therapy from natalizumab to fingolimodMult Scler2012181650165223100527
- DaelmanLMaitrotAMaaroufAChaunuMPPapeixCTourbahASevere multiple sclerosis reactivation under fingolimod 3 months after natalizumab withdrawalMult Scler2012111647164922907938
- HakikiBPortaccioEGianniniMRazzoliniLPastoLAmatoMPWithdrawal of fingolimod treatment for relapsing-remitting multiple sclerosis: report of six casesMult Scler201218111636163922829326
- CentonzeDRossiSRinaldiFGalloPSevere relapses under fingolimod treatment prescribed after natalizumabNeurology201279192004200523035063
- AlroughaniRAhmedSAl-HashelJThe risk of short-term relapse in patients switching from natalizumab to fingolimodMult Scler Relat Disord201436748749
- FDABrain Infection Case. US Food and Drug Administration2014 Available from: http://www.fda.gov/Drugs/DrugSafety/ucm366529.htmAccessed December 8, 2014
- CalicZCappelen-SmithCHodgkinsonSJMcDougallACuganesanRBrewBJTreatment of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome with intravenous immunoglobulin in a patient with multiple sclerosis treated with fin-golimod after discontinuation of natalizumabJ Clin Neurosci201422359860025523125
- KhatriBOPelletierJKapposLEffect of prior treatment status and reasons for discontinuation on the efficacy and safety of fingolimod vs interferon B-1a intramuscular: subgroup analyses of the trial assessing injectable interferon vs. fingolimod oral in relapsing-remitting multiple sclerosis (TRANSFORMS)Mult Scler Relat Disord20133335536325876473
- KhatriBBarkhofFComiGTRANSFORMS Study GroupComparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomized extension of the TRANSFORMS studyLancet Neurol201110652052921571593
- RadueEWO’ConnorPPolmanCHFTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) Study GroupImpact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosisArch Neurol201269101259126922751847
- YamoutBKhourySZeineddineMHouranyREfficacy and safety of fingolimod treatment in multiple sclerosis: the clinical experience of the AUBMC Multiple Sclerosis Center in LebanonMult Scler Relat Disord20143674925891584
- ZiemmsenTDiaz-LorenteMAbdelkaderMCornelissenCPAN-GAEA: post-authorization Noninterventional German safety Study of Gilenya in relapsing-remitting multiple sclerosis (RRMS) patients: a 24-month interim analysis of a German five-year fingolimod registry studyMult Scler Relat Disord201436751
- FazekasFBajenaruOBergerTHow does fingolimod (gilenya(®)) fit in the treatment algorithm for highly active relapsing-remitting multiple sclerosis?Front Neurol201341023641231
- HansonKAAgashivalaNWyrwichKWRaimundoKKimEBrandesDWTreatment selection and experience in multiple sclerosis: survey of neurologistsPatient Prefer Adherence2014841542224729689
- OntanedaDCohnSFoxRJRisk stratification and mitigation in multiple sclerosisMult Scler Relat Disord20143563964925221744
- FoxEEdwardsKBurchGOutcomes of switching directly to oral fingolimod from injectable therapies: results of the randomized, open-label, multicenter, evaluate patient outcomes (EPOC) study in relapsing multiple sclerosisMult Scler Relat Disord201435607619
- HavlaJTackenbergBHellwigKFingolimod reduces recurrence of disease activity after natalizumab withdrawal in multiple sclerosisJ Neurol201326051382138723266894
- de SezeJOngagnaJCCollonguesNReduction of the washout time between natalizumab and fingolimodMult Scler2013199124823722322
- HalpernRAgarwalSBortonLOneacreKLopez-BresnahanMVAdherence and persistence among multiple sclerosis patients after one immunomodulatory therapy failure: retrospective claims analysisAdv Ther201128976177521870169
- AgashivalaNWuNAbouzaidSCompliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort studyBMC Neurol20131313824093542
- TanHCaiQAgarwalSStephensonJJKamatSImpact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosisAdv Ther2011281516121153000
- MenzinJCaonCNicholsCWhiteLAFriedmanMPillMWNarrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosisJ Manag Care Pharm2013191 suppl AS24S4023383731
- Therapeutic decision making in multiple sclerosis: best practice algorithms for the MS care clinicianInt J MS Care201416suppl 613624688349
