?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Persistence and adherence with subcutaneous growth hormone (GH; somatropin) therapy in children is widely acknowledged to be suboptimal. This study aimed to investigate how the use of a jet-delivery device, ZomaJet®, impacts on medication-taking behaviors compared to needle-based devices.
Materials and methods
A retrospective cohort study of children aged ≤18 years was conducted using a UK-based, nationwide database of GH home-delivery schedules. Data were evaluated for the period between January 2010 and December 2012 for 6,061 children receiving either Zomacton® (somatropin) via the ZomaJet jet-delivery device or one of six brands of GH all administered via needle-based devices. Persistence was analyzed for patients with appropriate data, measured as the time interval between first and last home deliveries. An analysis of adherence was conducted only for patients using ZomaJet who had appropriate data, measured by proportion of days covered. Brand switches were identified for all patients.
Results
Persistence with GH therapy was significantly longer in patients using ZomaJet compared to needle-based devices (599 days versus 535 days, respectively, n=4,093; P<0.001); this association was observed in both sexes and across age subgroups (≤10 and 11–16 years). The majority (58%) of patients using ZomaJet were classed as adherent (n=728). Only 297 patients (5%) switched GH brand (n=6,061), and patients tended to use ZomaJet for longer than other devices before switching.
Conclusion
It appears important that the choice of a jet-delivery device is offered to children prescribed daily GH therapy. These devices may represent a much-needed effective strategy for maintaining persistence with subcutaneous GH administration in children, potentially offering better clinical outcomes and greater cost-efficiency.
Introduction
In the UK, recombinant growth hormone (GH; somatropin) is recommended as a treatment option for children with growth failure associated with a variety of conditions including GH deficiency and Turner syndrome.Citation1 Long-term GH therapy can help achieve increments in adult height of 8–11 cm in children with GH deficiency.Citation1 Crucially, GH therapy involves daily subcutaneous injections of GH, which may lead to avoidance of therapy in many patients.Citation2 Indeed, a high proportion of children experience needle anxiety or injection pain;Citation3 therefore, alternative delivery options would be welcome.
It is widely acknowledged that many patients with long-term conditions do not take their medicines as prescribed.Citation4 For optimal treatment outcomes, long-term persistence and adherence with GH treatment is vital.Citation2 Although the effects may not be immediate, missing a large number of GH doses is likely to have a substantial long-term impact, including reduced adult height and cost inefficiencies for the health care system.Citation5–Citation7 Various factors may cause patients to miss GH doses, including a lack of understanding of the disease and the importance of regular GH administration, and inadequate contact with health care providers.Citation7,Citation8
The UK’s National Institute for Health and Care Excellence (NICE) recommends that patients should be given a free choice of GH product on an individual basis.Citation1 Factors influencing choice include ease of use and whether a needle is required.Citation9 Zomacton® (somatropin; Ferring Pharmaceuticals, London, UK) is the only GH therapy available in the UK that is delivered via a jet-delivery device – ZomaJet® (Ferring Pharmaceuticals).Citation10 The ZomaJet device is needle-free, and transjects GH through the skin of the user.Citation11 Bioequivalence between jet and needle administration has been demonstrated with similar GH-absorption volumes,Citation12 with no significant differences in serum IGF-1 levels.Citation13
Patients who receive GH treatment in the UK are offered a home-delivery service option through a number of home-care companies, one of which is called Healthcare at Home (HAH). The HAH service delivers the drug and ancillary items (such as the device and transjection heads), and also provides initial training in administration, as well as ongoing support from a dedicated nursing team.
This study investigated medication persistence, adherence, and switching in children prescribed GH therapy, using observational data from the HAH database of delivery schedules. The main objective was to investigate how the use of a jet-delivery device impacts on these medication-taking behaviors compared to needle-based devices.
Materials and methods
Study design and patients
This was a retrospective cohort study of patients prescribed once-daily subcutaneous doses of GH, which they received through the HAH service. Patients were either receiving Zomacton via the ZomaJet device (the only device available with Zomacton) or one of six brands of GH delivered via various needle-based devices: Genotropin® (Pfizer, Sandwich, UK), Humatrope® (Eli Lilly, Basingstoke, UK), Norditropin® (Novo Nordisk, Crawley, UK), NutropinAq® (Ipsen, Slough, UK), Omnitrope® (Sandoz, Camberley, UK), and Saizen® (Merck Serono, Feltham, UK).
The impact of a jet-delivery device on medication-taking behavior was evaluated in the context of other GH-delivery devices by assessing persistence and brand switching among patients receiving GH treatment. Persistence is defined as the duration of time from initiation to discontinuation of therapy.Citation14 An analysis of adherence was also conducted for the subset of patients using the ZomaJet device. Adherence is defined as the degree or extent of conformity to the recommendations about day-to-day treatment by the provider.Citation14 Analyses of the individual GH products that use needle-based devices were not conducted, as the required data was proprietary and thus unavailable for publication. Furthermore, needles are available to patients from sources other than HAH, which meant that adherence to individual needle-based devices could not be accurately analyzed using HAH data alone.
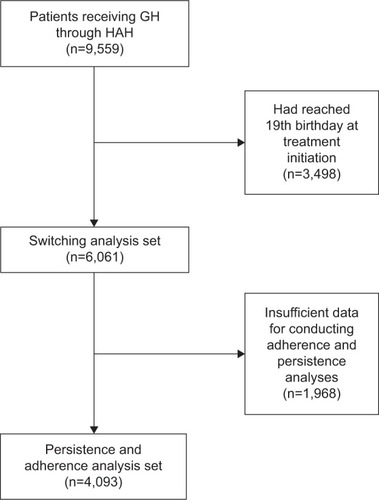
Patients who received deliveries of GH and/or ancillaries between January 2010 and December 2012 were identified from the HAH database of delivery schedules. Most patients (75%) received both their GH and ancillary items from HAH; a smaller number of “stores-only” patients (25%) received only their ancillary items from HAH and collected their GH from a community pharmacy. In the persistence and adherence analyses, patients were excluded if they had reached their 19th birthday by the date of their first prescription (ie, aged ≤18 years), if they did not have appropriate data for calculating a persistence or adherence value, or if they were listed as current at the end of the study period but had not received medication for the past 6 months, as these records were considered to reflect errors in the database or anomalous dispensing events (). Patients were excluded from the switch analysis if they had reached their 19th birthday (ie, aged ≤18 years) by the date of their first prescription ().
Study evaluations and measures
Persistence was quantified for each GH brand by the time interval between a patient’s first and last delivery of GH and/or ancillaries within the study period. Adherence was estimated using a validated and extensively studied measure known as proportion of days covered (PDC).Citation15 PDC was calculated as the ratio of the number of days a patient had access to viable ZomaJet heads (quantity of heads delivered × length of time each head should last) to the number of days they were prescribed GH treatment during the treatment period:
PDC was considered to provide a more conservative estimate of adherence compared to the medication-possession ratio (MPR),Citation16 and was more robust in this specific context, as it captured patient access to both heads and GH, whereas MPR would only capture one of these two factors.Citation17
A PDC score of >0.8 suggests that a patient is highly adherent.Citation15 A lower score suggests that a patient is using less drug or is reusing heads (contrary to recommendations). Higher scores can occur when prescriptions are refilled before the previous one is exhausted.Citation18 Ostensibly, excessive adherence scores typically arise through anomalies in dispensing practice,Citation19 eg, a patient may have been oversupplied with heads. Various thresholds and score ranges have been used to classify adherence in previous studies,Citation18,Citation19 but no particular range has been validated in this patient population. Therefore, to account for these points, an upper limit was defined within the range of previously used values, and patients with a PDC of 0.8–1.8 were classed as adherent. Switches between GH brands were identified on the basis of a change to an alternative brand during the study period.
Statistical analyses
For comparison of persistence between ZomaJet and needle-based devices, Mantel–Cox log-rank and χ2 tests were used to evaluate statistical significance. Independent-sample t-tests were used to evaluate differences in the mean patient age between treatment groups and differences in the percentage of adherent patients between sex and age subgroups. A significance level of P<0.01 was used in this study. Persistence was analyzed by linear regression, represented as Kaplan–Meier survival curves,Citation20,Citation21 with vertical tick marks denoting when individual patients desisted with treatment. All statistical analyses were undertaken using SPSS (IBM, Armonk, NY, USA).
Results
Patient disposition and demographics
A total of 9,559 patient records were extracted from the HAH database. Of these, 6,061 patients (63%) were aged ≤18 years at therapy initiation, of which 4,093 (68%) provided sufficient data for conducting adherence and persistence analyses (). In all, 728 of 4,093 (18%) were using ZomaJet; the remainder were using needle-based devices. The proportions of males and females in these two treatment groups were similar (). Patients using needle-based devices were significantly older, at 9.7 years, compared to patients using ZomaJet, at 8.4 years (P<0.001, ).
Table 1 Patient demographics
Persistence analysis
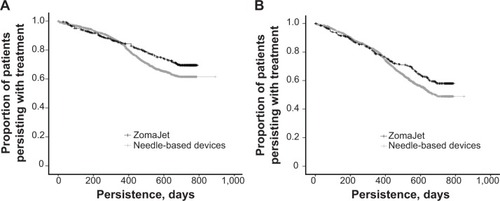
Mean persistence for patients using ZomaJet was significantly longer than for patients using needle-based devices (599 days versus 535 days, respectively; P<0.001; ). Survival analyses are shown in . Significantly longer persistence with ZomaJet compared to needle-based devices was observed in both males and females ().
Figure 2 Persistence with growth-hormone treatment in patients using ZomaJet or a needle-based delivery device. Vertical tick marks denote when individual patients desisted with treatment.
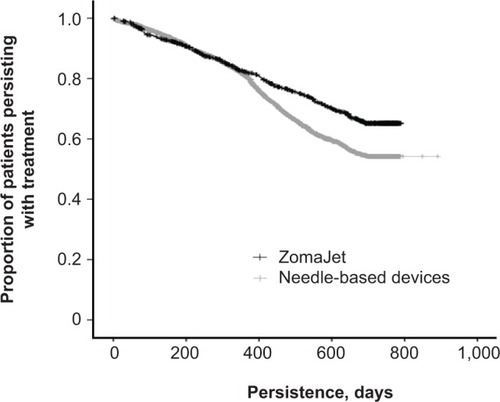
Table 2 Persistence with growth-hormone therapy
Persistence within the age-groups of ≤10 and 11–16 years was also examined to determine if age influences persistence to GH therapy. Previous studies have suggested that children aged <10 years rarely prepare their treatment themselves,Citation11 and that 10–11 years is the age range most commonly suggested by parents for children to begin self-administration.Citation3 In addition, Zomacton is licensed for treatment in children up until epiphyseal fusion,Citation22 which usually occurs at around 16 years in females and 18 years in males.Citation23 Therefore, a patient’s 17th birthday was considered an appropriate cutoff age for this analysis. Mean persistence with ZomaJet was significantly longer than with needle-based devices, particularly in patients aged ≤10 years and also in those aged 11–16 years (). Survival analyses are shown in . Among patients using ZomaJet, persistence was significantly longer in those aged ≤10 years compared to 11–16-year-olds (). This effect was not observed among patients using needle-based devices ().
Adherence analysis
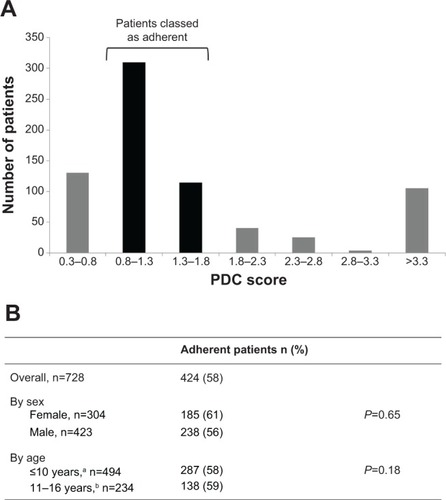
Overall, 424 of 728 patients (58%) using ZomaJet were classed as adherent (PDC 0.8–1.8, ). No correlation was observed between PDC score and service type (full HAH home delivery versus stores only) or dose (data not shown). No significant differences in adherence between males and females or the two age subgroups were observed ().
Figure 4 Adherence to growth-hormone treatment in patients using ZomaJet.
Abbreviation: PDC, proportion of days covered.
Switching analysis
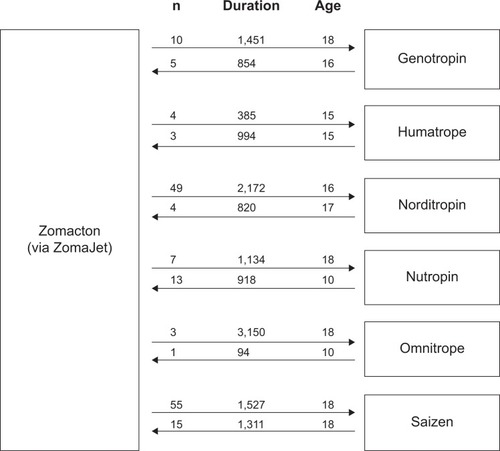
Of the 6,061 patients eligible for the switch analysis, only 297 (5%) switched GH brands during the study period. The mean age of patients switching from Zomacton to five of the six alternative brands was equal to or more than the mean age in the reverse switch (). In all but one case, patients used Zomacton for longer on average before switching to another brand, compared to the reverse switch ().
Discussion
This study examined medication-taking behaviors in children using the ZomaJet jet-delivery device to administer GH therapy, focusing on persistence and adherence with administration and switching between available products.
The results indicated that patients who chose ZomaJet persisted with treatment for significantly longer compared with those who chose needle-based devices (mean difference 64 days). This could be compared to the findings of an adherence analysis in a previous study in North American children by Desrosiers et al which reported that significantly more patients using a needle-based device had low adherence compared to a jet-delivery device.Citation24 It is important to note that children using ZomaJet in the current study were significantly younger than those using needle-based devices, potentially confounding the interpretation of the persistence analysis, as older patients may be more likely to discontinue treatment due to achieving their therapeutic end points (eg, target height gain or epiphyseal fusion). However, subgroup analysis revealed that ZomaJet was associated with improved persistence in both of the age-groups analyzed (≤10 years and 11–16 years). This supports the interpretation that ZomaJet is associated with improved persistence across age-groups in patients <17 years old.
NICE recommends that the choice of a GH delivery device should follow informed discussion between the responsible clinician and the patient/carer, taking into consideration the likelihood of adherence to treatment, among other factors.Citation1 In light of these current findings, it may be prudent for clinicians to highlight the growing evidence for an association between jet-delivery devices and improved persistence and adherence when discussing the choice of product with patients. This may be especially relevant in younger patients, who persisted with treatment for significantly longer than older patients when using ZomaJet (but not needle-based devices). When available options are carefully explored, needle-free devices are thought to be a popular choice for GH therapy, eg, 46% of pediatric patients chose a needle-free device over a needle-based device in a UK hospital clinic.Citation9 It is also important to recognize that devices, patient preferences, and published evidence are likely to change over the course of long-term treatment, so device choice should be reviewed regularly.Citation6
Although persistence with ZomaJet was significantly longer than with needle-based devices, persistence and adherence in general were markedly suboptimal. Patients persisted with treatment on average for less than 20 months over the course of the 3-year study period (599 and 535 days with ZomaJet and needle-based devices, respectively), and only 58% of patients were adherent in the ZomaJet-adherence analysis. This raises the specter of suboptimal treatment outcomes and cost inefficiencies, highlighting the need for effective strategies to help patients adhere to their treatment over the long term or identify patients who should discontinue treatment.
While persistence with and adherence to GH therapy have been repeatedly demonstrated to be suboptimal in previous studies,Citation6 small sample sizes and substantial differences in patient populations, health care settings, and methodology mean that estimates vary widely and direct comparisons are difficult. For instance, a recent review found large variations in estimated rates of nonadherence – from 5% to 82% – across the literature.Citation6 Furthermore, a recent France-based, prospective study of adherence to ZomaJet found that patients/carers administered 97% of prescribed GH therapy on average (n=87), suggesting substantially greater adherence than in the current study population.Citation25 This suggests that careful consideration of the specific setting is vital when evaluating interventions to improve medication-taking behavior. Results from the current study augment and further clarify our knowledge, being the first UK-based analysis of persistence and adherence to GH therapy in a large cohort of children.
The majority (58%) of patients using ZomaJet were classed as adherent in the current study, with no difference observed between sexes or age subgroups. While a comparison of adherence with needle-based devices was not undertaken (see Materials and methods), the findings of Desrosiers et al suggest improved adherence with jet- delivery devices in some patients.Citation24 Medication adherence is a complex area, affected by several factors that vary between patients and over time.Citation26 In GH therapy specifically, lack of choice of delivery device has been identified as a key barrier to adherence.Citation6 The choice of device is known to be strongly influenced by its ease of use,Citation6,Citation9 and research indicates that many children consider a jet-delivery device easier to use than a needle injection.Citation27 This may aid adherence by encouraging self-administration. An additional barrier to adherence that may be addressed through the use of a jet-delivery device is injection discomfort or anxiety. A Dutch study showed that children using a jet device found it less painful and less unpleasant for future use than patients using needle-based devices.Citation27 Adherence is also influenced by patient support (including injection training and contact with health care professionals).Citation6 The currently available products provide different support services, eg, patients using some GH brands, including ZomaJet, receive injection training and support from nurses for the duration of treatment. By meeting with nurses, a more comprehensive understanding of information from tertiary care specialists can be achieved.Citation3 This may also allow carers to feel more involved in treatment decisions,Citation3 which may aid adherence.
In all, 5% of children switched GH brands during the 3-year study period. Extrapolated over the course of long-term treatment, this could potentially represent a substantial proportion of patients. Patients in the current study generally showed a lengthy persistence with Zomacton before switching: in five out of six cases, patients spent a mean of >1,000 days using Zomacton before switching to an alternative product. Although not recorded by HAH, it is likely that switches occur for a variety of reasons. For instance, patient preferences for delivery device may change with age and time.Citation6 It should also be noted that the licensed indications differ between brands; in particular, Zomacton is licensed for use in children only up until epiphyseal fusion;Citation22 therefore, patients may be moved to another product thereafter. This is suggested by the large numbers of patients switching from Zomacton to Norditropin (n=49) or Saizen (n=55) at the age of 16 or 18 years, respectively (). Another reason for switching may be related to costs. The NICE guidelines specify that if no single product is preferred by the patient, the least costly should be chosen.Citation1 Therefore, a patient who has no preferred device may initially be prescribed a lower-priced brand by default. They may then opt to change device once therapy commences.
It is widely acknowledged that there are substantial economic repercussions when patients do not take their medication as prescribed. Indeed, it is estimated that across the UK, around £100 million is wasted each year on unused medicines alone.Citation28 GH treatment is expensive: costs are estimated by NICE at £6,000/cm gained for GH deficiency and up to £17,300/cm gained for Turner syndrome.Citation1 Therefore, medicine wastage through nonadherence and nonpersistence could potentially be very costly, and thereby outweigh the perceived advantage of using a low-cost product. Further costs may be incurred when these behaviors go undetected, as clinicians might employ additional diagnostic procedures and/or increase the dose of GH.Citation7 Therefore, strategies to improve persistence and adherence not only result in better treatment outcomes for patients but may also reduce costs by reducing medicine wastage and unnecessary effort by clinicians.Citation7 While further investigation of the financial impact is required, the improved persistence and adherenceCitation24 observed with jet-delivery devices in some children suggest these could potentially reduce health care costs.
The HAH database is a valuable data source, allowing meaningful insight into patient behaviors through evaluations of persistence, adherence, and switching. The principal advantages of a database analysis are its objectivity (not relying on subjective patient responses in a survey, for example), and the broad coverage of the patient population that can be achieved. However, the HAH database does not record complete diagnostic criteria or clinical outcomes, such as adult height attained, nor can it shed light on the origins or motivations underlying the observed medication-taking behaviors.
Further limitations of the results arise from certain aspects of the available data. A large number of patients were excluded from the persistence and adherence analyses for having insufficient data, which may indicate a high prevalence of missing or incomplete data in the database. While this represents a limitation of the results, it should be noted that the sample size remained high.
The nature of the available data meant PDC was chosen as a proxy for adherence, rather than MPR (see Materials and methods), which may be considered a more widely used and accepted measure.Citation17 In the persistence analysis, patient numbers were unequal between treatment groups, due to the inclusion and exclusion criteria of the study and the market landscape of the HAH dataset. This was a retrospective real-world study, and not conducted in a controlled study environment, where treatment group sizes can be predefined. However, utilizing a matched patient-pair analysis of this data set controlling for age, diagnosis, and sex would be an interesting approach for further research, and may strengthen the current results.
In the adherence analysis, a large proportion of patients (24%) were classed as overadherent (PDC >1.8), a phenomenon that has been reported in other database studies.Citation18,Citation19 Overadherence is possibly indicative of anomalies in dispensing practice, advocating a cautious interpretation of the results. Additionally, the adherence analysis was limited to patients using ZomaJet, which severely restricts the interpretation of the results. Exploring adherence and persistence with the various needle-based devices would be a promising avenue for future investigations.
It should be noted that in this study, it was not possible for a patient to use more than one brand at once through the HAH service, as patients are switched between devices rather than being permitted use of multiple devices concurrently. However, this analysis could not account for a patient requesting a different type of GH product from their primary care prescriber, in addition to their normal HAH delivery schedule. Further study would be required to elucidate the clinical consequences of changes in medicine-taking behaviors in GH therapy. It would also be interesting to evaluate the impact of home-delivery and support services compared to collection from a community pharmacy.
Conclusion
It has long been recognized that implementing strategies to improve persistence and adherence for parenteral treatment might be of particular clinical benefit to patients undergoing GH therapy.Citation6 This is the first large-scale UK-based analysis of persistence, adherence, and switching in children receiving GH therapy. In addition, it is the first persistence analysis comparing a jet-delivery device with needle-based devices.
Although younger on average, children using the ZomaJet device persisted with GH therapy for significantly longer compared with needle-based devices. As such, a treatment program incorporating a jet-delivery device may represent an effective strategy for maintaining regular long-term subcutaneous administration in selected patients. This could potentially improve treatment outcomes, and reduce costs through medicine wastage and unnecessary effort by clinicians,Citation7 and these are promising avenues for future investigation. Switching between different GH brands was recorded over this short time period, suggesting that preferences may change over time, perhaps due to patient age or differences between licensed indications. Taken together, the results indicate that the choice of a jet-delivery or needle-based device should be carefully explored and reviewed in discussions between the clinician and patient/carer, in accordance with NICE guidelines.Citation1
Acknowledgments
Editorial support was provided by Acumen Healthcare Communications Ltd., funded by Ferring Pharmaceuticals. The authors are grateful to Sue Whitten (Ferring Pharmaceuticals) and Matt Jones (formerly of Sciensus Ltd.) for their assistance with this study and manuscript.
Disclosure
This study was conducted by Sciensus (Brighton, UK), and initiated and funded by Ferring Pharmaceuticals (London, UK). Editorial support was provided by Acumen Healthcare Communications, funded by Ferring Pharmaceuticals. Helen Spoudeas has accepted fees for lecture invitations and received educational grants from Ferring Pharmaceuticals, Novo Nordisk, Pfizer, and Ipsen. Priti Bajaj is an employee of Ferring Pharmaceuticals. Nathan Sommerford is an employee of Sciensus.
References
- National Institute for Health and Care ExcellenceHuman Growth Hormone (somatropin) for the Treatment of Growth Failure in ChildrenLondonNICE2010
- YuenKCAminRDevelopments in administration of growth hormone treatment: focus on Norditropin® Flexpro®Patient Prefer Adherence2011511712421448295
- van DongenNKapteinAAParents’ views on growth hormone treatment for their children: psychosocial issuesPatient Prefer Adherence2012654755322927747
- National Institute for Health and Care ExcellenceMedicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting AdherenceLondonNICE2009
- CutfieldWLindbergAAlbertsson WiklandKChatelainPRankeMBWiltonPFinal height in idiopathic growth hormone deficiency: the KIGS experience. KIGS International BoardActa Paediatr Suppl199988428727510102057
- FisherBGAceriniCLUnderstanding the growth hormone therapy adherence paradigm: a systematic reviewHorm Res Paediatr201379418919623635797
- HaverkampFJohanssonLDumasHObservations of nonadherence to recombinant human growth hormone therapy in clinical practiceClin Ther200830230731618343269
- RosenfeldRGBakkerBCompliance and persistence in pediatric and adult patients receiving growth hormone therapyEndocr Pract200814214315418308651
- WickramasuriyaBPCaseyAAkhtarSFactors determining patient choice of device for GH therapyHorm Res2006651182216357486
- Haymarket Medical MediaMIMS MagMar-May2014
- KapteinAATransjecting growth hormone: continuous nightmare or controlled nuisance? Evaluation of a new needle-free devicePatient Prefer Adherence2013770370823926423
- VerhagenAEbelsJTDogteromAAJonkmanJHPharmacokinetics and pharmacodynamics of a single dose of recombinant human growth hormone after subcutaneous administration by jet-injection: comparison with conventional needle-injectionEur J Clin Pharmacol1995491–269728751024
- AgersøHMøller-PedersenJCappiSThomannPJesussekBSenderovitzTPharmacokinetics and pharmacodynamics of a new formulation of recombinant human growth hormone administered by ZomaJet 2 Vision, a new needle-free device, compared to subcutaneous administration using a conventional syringeJ Clin Pharmacol200242111262126812412826
- CramerJARoyABurrellAMedication compliance and persistence: terminology and definitionsValue Health2008111444718237359
- Pharmacy Quality AllianceProportion of days covered (PDC) as a preferred method of measuring medication adherence2014 Available from: http://pqaalliance.org/resources/adherence.aspAccessed March 18, 2014
- MartinBCWiley-ExleyEKRichardsSDominoMECareyTSSleathBLContrasting measures of adherence with simple drug use, medication switching, and therapeutic duplicationAnn Pharmacother2009431364419126828
- HalpernMTKhanZMSchmierJKRecommendations for evaluating compliance and persistence with hypertension therapy using retrospective dataHypertension20064761039104816651464
- YangMBarnerJCWorchelJFactors related to antipsychotic oversupply among Central Texas VeteransClin Ther20072961214122517692735
- RobinsonAHankinsMWisemanGJonesMMaintaining stable symptom control in inflammatory bowel disease: a retrospective analysis of adherence, medication switches and the risk of relapseAliment Pharmacol Ther201338553123823834298
- PetersonAMNauDPCramerJABennerJGwadry-SridharFNicholMA checklist for medication compliance and persistence studies using retrospective databasesValue Health200710131217261111
- KaplanELMeierPNonparametric Estimation from Incomplete ObservationsJ Am Stat Assoc195853282457481
- Ferring PharmaceuticalsZomacton: 10 mg injection [package insert]LondonFerring Pharmaceuticals2012
- FreemanJVColeTJChinnSJonesPRWhiteEMPreeceMACross sectional stature and weight reference curves for the UK, 1990Arch Dis Child199573117247639543
- DesrosiersPO’BrienFBlethenSPatient outcomes in the GHMonitor: the effect of delivery device on compliance and growthPediatr Endocrinol Rev20052Suppl 3S327S331
- WeillJNiezPAdherence to the treatment with Zomajet, a needle-free device transjecting growth hormone: results of French observational surveyPoster presented at: European Society for Paediatric Endocrinology Annual ConferenceSeptember 19–22, 2013Milan, Italy
- HorneRWeinmanJBarberNElliotRAMorganMConcordance, Adherence and Compliance in Medicine Taking: A Conceptual Map and Research PrioritiesLondonNational Institute for Health Research Service Delivery and Organisation R&D2006
- VerripsGHHirasingRAFekkesMVogelsTVerloove-VanhorickSPDelemarre-Van de WaalHAPsychological responses to the needle-free Medi-Jector or the multidose Disetronic injection pen in human growth hormone therapyActa Paediatr19988721541589512200
- Department of HealthPharmacy in England: Building on Strengths – Delivering the FutureLondonDepartment of Health2008