Abstract
Background
Patients with osteoarthritis (OA), rheumatoid arthritis (RA), or ankylosing spondylitis (AS) are commonly treated with nonsteroidal anti-inflammatory drugs (NSAIDs), sometimes with a concomitant gastroprotective proton pump inhibitor (PPI). The present study examines real-life patient adherence to PPIs when coprescribed with NSAIDs.
Methods
This retrospective medical record survey identified patients diagnosed with OA, RA, or AS who had PPIs coprescribed with NSAIDs for prevention of NSAID-associated gastrointestinal ulcers. Actual NSAID and PPI intake was retrospectively recorded using a self-reported questionnaire. Adherence to PPI treatment was assessed using descriptive statistics.
Results
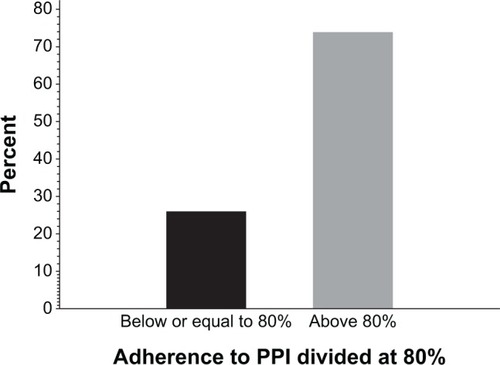
In total, 96 patients (69% female, mean age 67 years, 72% OA, 16% RA, 12% AS) were included. The mean patient-reported adherence to coprescribed PPIs was 73%–81%. The percentage of patients with a self-reported adherence of ≤80% was 26%. No predictive factors for low adherence could be identified.
Conclusion
Despite doctors’ instructions to use PPIs concomitantly with NSAIDs, the mean patient-reported adherence to coprescribed PPIs in this population indicates a risk of a “gastroprotective treatment gap”. The patients’ adherence to gastroprotective PPIs for the prevention of NSAID-associated upper gastrointestinal ulcers can be improved.
Introduction
Patient adherence to medication is necessary if clinical treatment regimens are to be successful and associated with positive patient outcomes.Citation1–Citation3 However, poor patient adherence to prescribed treatments is a common issue, seen almost independently of the therapeutic area. In patients with arthritis, adherence ranges from 55% to over 80% depending on the drug studied.Citation4
Nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin and selective cyclooxygenase-2 inhibitors, are a widely used treatment in arthritis.Citation5,Citation6 Adverse events, for example, gastrointestinal events like peptic ulcers, are the main concern of NSAID treatment.Citation7–Citation11 These may also lead to disruption of NSAID treatment, potentially reducing both positive clinical outcomes and elevating health care costs.Citation12
There is strong evidence that the risk of ulcers and bleeding in the upper, but not lower, gastrointestinal tract may be decreased by concomitant therapy with proton pump inhibitors (PPIs).Citation13 Concomitant gastroprotective treatment with a PPI is also recommended in guidelines as a therapy to lower the risk of NSAID-induced gastrointestinal side effects.Citation14,Citation15 Adherence to PPI therapy is important in NSAID-treated patients, and the “gastroprotection gap”, such as low utilization of gastroprotective strategies and low adherence to gastroprotection among users of NSAIDs at high risk of adverse gastrointestinal events,Citation16,Citation17 increases the risk of gastrointestinal events, death, and health care costs.Citation12,Citation18–Citation20 Knowledge of real-life patient adherence to PPIs in NSAID-treated patients is lacking.
This study specifically focused on measuring self-reported adherence to PPIs over a 7-day period in patients with osteoarthritis (OA), rheumatoid arthritis (RA), or ankylosing spondylitis (AS) linked to their intake of coprescribed NSAID treatment.
Patients and methods
Study design and objectives
This was a retrospective, cross-sectional, observational study to assess patient-reported adherence to PPI treatment when coprescribed NSAID treatment (Anatomical Therapeutic Chemical Classification M01A, except M01AH and M01AX) for the prevention of upper gastrointestinal side effects associated with NSAID treatment in patients with OA, RA, or AS in Sweden. Patients should have been instructed by their physician to take a PPI on every day of NSAID intake. The study was approved by the regional ethical review board of Stockholm (DNR 2011/2118-31/3) and registered at ClinicalTrials.gov (NCT01519375). The study was conducted in accordance with the principles stated in the Declaration of Helsinki.
Patient population
Male and female patients, ≥18 years of age, with a diagnosis of OA, RA, or AS were consecutively identified from medical records. The patients were required to have current prescriptions of oral NSAID treatment and PPIs for the prevention of NSAID-associated gastrointestinal ulcers, with a doctor’s instruction to use the drugs on the same day. Patients were excluded if they were participating in any other trial involving a PPI or an NSAID, had been prescribed a PPI as an acute treatment for gastrointestinal events or symptoms (eg, gastrointestinal ulcer, dyspepsia, gastritis, or gastroesophageal reflux disease) within the last 8 weeks, if they reported taking NSAIDs on fewer than three of the reported days, or if they were unable to complete a study-specific patient self-reported questionnaire (SRQ). Seven primary care centers and one rheumatology center participated in the study. Diagnosis of OA, RA, or AS was according to the clinical practice at each participating center. Data were collected between March and May 2012.
Study conduct
Patients who fulfilled the inclusion criteria submitted a signed informed consent form and a completed SRQ to the investigators. Data on PPIs and NSAIDs were recorded in separate sections of the SRQ. The first question in each section asked patients about their general use of the drug. Patients were then asked to retrospectively specify their NSAID and PPI intake during the previous 7 days using “yes”, “no”, or “do not recall” for each specific day. The data were entered into a web-based case report form together with complementary information from patients’ medical records on disease characteristics and prescribed medications.
Assessing adherence
The level of adherence to PPIs was assessed retrospectively over a 7-day period using the SRQ. The objective was to assess patient-reported adherence to PPI treatment on actual days of NSAID treatment and to assess the proportion of patients with reported adherence ≤80%. For the primary variable, adherence to PPI treatment was defined as the proportion of NSAID treatment days on which the patient also indicated taking a PPI.
Adherence to the PPI was then calculated as the mean percentage of adherence in the total study population, assessed for all patients using two different methods. The first was a more conservative approach, where adherence was calculated using only the answers concerning PPI intake for the days where a definite “yes” or “no” for adherence was available. In the second and less conservative (sensitivity) approach, a day with non-reported PPI intake data or where the answer for PPI intake was “do not recall” was considered to be a day of PPI nonadherence, if NSAID intake on the same day was “yes”.
Statistical analysis
All data were analyzed using descriptive statistics. Factors predictive of low adherence were tested using logistic regression. Data are presented using summary statistics.
Results
Patient demographics
In total, 74% (134/180) of the patients who received a questionnaire completed it. Of these, 96 patients (69% females, mean age 67 years) fulfilled all inclusion criteria and were included in the final analyses. The majority of the excluded 38 patients only reported taking NSAIDs less than 3 days per week. Seventy-two percent of the patients had a diagnosis of OA, 16% of RA, and 12% of AS; 39% and 22% had medical record histories of dyspepsia and gastroesophageal reflux disease, respectively.
Drugs prescribed
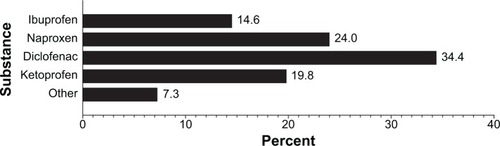
The three NSAIDs most commonly used by patients were diclofenac (34%), naproxen (24%), and ketoprofen (20%, ). The most common PPI was omeprazole, used by 94% of patients.
Patient-reported adherence
Overall patient-reported adherence to coprescribed PPIs when taking NSAIDs (calculated as a mean percentage of all patients) was 81.1% () and 73.4% () using the conservative and less conservative approach, respectively. The holistic interpretation of adherence data from six patients had an effect on the mean overall adherence in the less conservative (sensitivity) approach (), resulting in a marked and lowered adherence for the total population.
Figure 2 Distribution of patient-reported mean adherence to coprescribed PPIs when taking NSAIDs using (A) the conservative, and (B) the less conservative (sensitivity) approach, respectively.
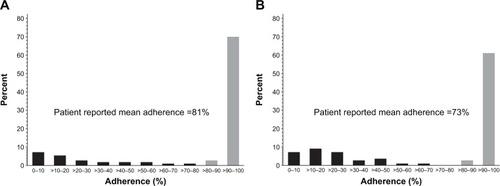
Table 1 Patient-reported adherence for seven patients, including general questions on PPI intake over a longer period of time and PPI intake on actual days of NSAID treatment over a 7-day period
Overall, six patients reported “yes” on one day of PPI intake and then had missing data for the remaining 6 days. All responded “never take PPI” or “I refrain from taking PPI on at least 3 days a week” to the general question on PPI intake over a longer period of time. One patient reported a “no” on one day of PPI intake and then had missing data for the remaining 6 days, but the adherence did not change when analyzed using the less conservative approach.
Twenty-six percent of the patients had a self-reported adherence of ≤80%, calculated using the conservative approach (). Adherence differences between high-dose and low-dose NSAIDs, type of NSAID drug, sex, and diagnosis of OA, RA, or AS were tested, but no significant differences were detected. No factors predictive of low adherence could be identified.
Discussion
Few studies have assessed patient adherence to medication for the chronic treatment of nonmalignant pain.Citation21–Citation23 Here a patient SRQ method was used to directly determine patient-reported adherence to PPI treatment when coprescribed with NSAIDs to prevent upper gastrointestinal ulcers in patients with OA, RA, or AS. The patients all required treatment with gastroprotective agents based on physicians’ clinical judgment and were instructed to always coadminister PPI with their NSAID treatment. Nevertheless, the patient-reported adherence in this study was between 73% (less conservative approach) and 81% (conservative approach). This corresponds to PPI adherence rates previously reported in real life registry studies.Citation24–Citation27 An adherence below 80% indeed indicates that there is a “gastroprotection gap” in approximately 20%–30% of NSAID-treated OA, RA, and AS patients at risk of adverse upper gastrointestinal events, despite a physician’s instruction to coadminister the drugs.
Adherence is measured commonly as a percentage over a period of time using one or a combination of methods, and can be measured either directly or indirectly. Direct methods measure serum drug/drug metabolite levels, which reflect actual drug intake, but are costly and provide no feedback to the point of care.Citation1,Citation28 Indirect methods include pill counts, pharmacy dispensing records, refill adherence, medication event monitoring system, and patient self-reported data, such as SRQs.Citation1,Citation29 While indirect methods are more common and easier to use, they risk overestimation of adherence and do not necessarily measure drug intake. All methods have their limitations and there is no “gold standard”.Citation1
SRQs are easy to use, cheap, measure adherence at source, and provide direct feedback. One disadvantage of the SRQ method is that it only provides an overall estimate of adherence over the specified time period.Citation29 It may also be subject to “answering bias”, where only a selection of patients actually respond to the SRQ, and the adherence may appear higher than when measured directly in the full study population.Citation29,Citation30 Further, adherence rates also tend to increase when patients know that they are being monitored, ie, so-called “pleasing bias”.Citation31
The retrospective SRQ method was used in this study because it may provide a more accurate indication of true patient level adherence, since patients’ answers concern actual, real-life drug intake and reduce the bias of patients being reminded to take medication merely by participating in the study.
Patients in this study received the SRQs from and returned them to their treating physician, which may have increased adherence. This pleasing bias may also have made nonadherent patients less willing to participate in the study, thereby also influencing the patient-reported adherence rate that corresponds with rates seen previously.Citation4,Citation24–Citation27
The potential risk of overestimating patient adherence with this method was analyzed by taking both a conservative and a less conservative (sensitivity) approach to the data in this study. The conservative analysis may have overestimated mean patient adherence because it excluded data where PPI intake on an NSAID day was uncertain. On the other hand, the less conservative approach may have underestimated mean patient adherence. However, the less conservative approach is supported by the patients’ responses regarding long-term PPI intake patterns.
Although patients in this study were asked in the SRQ to state their drug intake for the previous 7 days, they still may have incorrectly recalled the drugs that they took over this short period. Further, the low number of patients in this study makes generalization of the results difficult because even a few patients may have had a large impact on overall adherence rates. Nevertheless, the adherence rates reported here are very similar to previous studies in general and within the same field.Citation24–Citation27
The results indicate that there is a “gastroprotection gap” in approximately 20%–30% of NSAID-treated patients with OA, RA, or AS who are at risk of adverse upper gastrointestinal events. Estimates of the elevated risk of upper gastrointestinal events range from 1.8-fold to 4.0-fold in patients with inadequate gastroprotective agent protection or poor PPI adherence.Citation20,Citation24,Citation27,Citation32 Moreover, for every 10% decrease in adherence to PPI, the risk of upper gastrointestinal bleeding/ulcers and upper gastrointestinal bleeding alone increases by 9% and 6%, respectively.Citation27 Similar results were shown in other studies.Citation19,Citation26 Since the risk of gastrointestinal events and death in nonadherent patients is increased and also associated with a societal economic burden,Citation12,Citation18–Citation20 further studies on how to alleviate the problem of poor adherence to coprescribed PPI gastroprotective therapy in this vulnerable population of patients are needed.
Conclusion
In this study, the mean patient-reported adherence to coprescribed PPI in patients with OA, RA, or AS who were instructed to take PPIs on the same day as taking NSAIDs for gastroprotection was estimated to be 73%–81%. The level of patient adherence to PPI therapy in this group corresponds to that seen previously in registry studies, and indicates that there is still room for improvement in patient adherence to PPIs when used for the prevention of NSAID-associated upper gastrointestinal ulcers.
Acknowledgments
The study investigators were Kenneth Henriksson, Reuma City, Stockholm (coordinating investigator); Olle Beneus, Kyrktorgets Vårdcentral, Partille; Lars-Bertil Olsson, Vårdcentralen, Kristinehamn; Lars Haglund, Capio Citykliniken, Kristianstad; Tomas Bergholtz, Näsets Läkargrupp, Skanör; Bo Sundqvist, Vännäs Vårdcentral, Vännäs; Hans Åke Söderberg, Vårdcentralen, Höga Kusten, Ullånger; and Ellika Mann, Carema Hälsocentral, Limhamn.
Disclosure
KH is a consultant for AstraZeneca and AbbVie AB. JF and GS are full-time employees at AstraZeneca. This study was funded by AstraZeneca. Dr Grażyna Söderbom, Klipspringer AB, provided medical writing support funded by AstraZeneca. This manuscript was prepared in line with the guidelines established by the International Committee of Medical Journal Editors.
References
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005448749716079372
- SimpsonSHEurichDTMajumdarSRA meta-analysis of the association between adherence to drug therapy and mortalityBMJ20063331516790458
- World Health OrganizationAdherence to long-term therapies2003 Available from: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdfAccessed April 29, 2014
- DeyoRAInuiTSSullivanBNoncompliance with arthritis drugs: magnitude, correlates, and clinical implicationsJ Rheumatol198189319367328568
- BlondellRDAzadfardMWisniewskiAMPharmacologic therapy for acute painAm Fam Physician20138776677223939498
- LeeYCEffect and treatment of chronic pain in inflammatory arthritisCurr Rheumatol Rep20131530023292816
- Rodriguez-MonguioROteroMJRoviraJAssessing the economic impact of adverse drug effectsPharmacoeconomics20032162365012807365
- HenrikssonAESvenssonJOUpper gastrointestinal bleeding. With special reference to blood transfusionEur J Surg19911571931961678629
- OhmannCThonKHengelsKJImhofMIncidence and pattern of peptic ulcer bleeding in a defined geographical area. DUSUK Study GroupScand J Gastroenterol1992275715811641583
- RockallTALoganRFDevlinHBNorthfieldTCIncidence of and mortality from acute upper gastrointestinal haemorrhage in the United KingdomBMJ19953112222267627034
- VreeburgEMSnelPde BruijneJWBartelsmanJFRauwsEATytgatGNAcute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcomeAm J Gastroenterol1997922362439040198
- VonkemanHEKlokRMPostmaMJBrouwersJRvan de LaarMADirect medical costs of serious gastrointestinal ulcers among users of NSAIDsDrugs Aging2007468169017702536
- GoldsteinJLHochbergMCFortJGZhangYHwangCSostekMClinical trial: the incidence of NSAID-associated endoscopic gastric ulcers in patients treated with PN 400 (naproxen plus esomeprazole magnesium) vs enteric-coated naproxen aloneAliment Pharmacol Ther20103240141320497139
- Swedish National Board of Health and WelfareNationella riktlinjer för rörelseorganens sjukdomar. Osteoporos, artros, inflammatorisk ryggsjukdom och ankyloserande spondylit, psoriasisartrit och reumatoid artrit [National guidelines for diseases in musculoskeletal organs. Osteoporosis, arthrosis, inflammatory back disease and ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis]2012 Available from: http://www.socialstyrelsen.se/nationellariktlinjerforrorelseorganenssjukdomar/Documents/nr-rorelseorganen-vetenskapligtunderlag.pdfAccessed April 29, 2014 Swedish
- National Institute for Health and Care ExcellenceThe care and management of osteoarthritis in adults. Clinical guidelines CG59 issued February 2008, replaced by CG177 Osteoarthritis Available from: http://www.nice.org.uk/guidance/CG177Accessed April 29, 2014
- de JongHJKorevaarJCvan DijkLVoogdEvan DijkCEvan OijenMGSuboptimal prescribing of proton-pump inhibitors in low-dose aspirin users: a cohort study in primary careBMJ Open201337
- Van der LindenMWGaugrisSKuipersEJVan den BemtBJvan Herk-SukelMPHeringsRMGastroprotection among new chronic users of non-steroidal anti-inflammatory drugs: a study of utilization and adherence in The NetherlandsCurr Med Res Opin20092519520419210152
- AbrahamNSCastilloDLHartmanCNational mortality following upper gastrointestinal or cardiovascular events in older veterans with recent non-steroidal anti-inflammatory drug useAliment Pharmacol Ther2008289710618397385
- JonassonCHatlebakkJGLundellLKouriJPAndersenMGranathFAssociation between adherence to concomitant proton pump inhibitor therapy in current NSAID users and upper gastrointestinal complicationsEur J Gastroenterol Hepatol20132553153823269097
- van SoestEMSturkenboomMCDielemanJPVerhammeKMSiersemaPDKuipersEJAdherence to gastroprotection and the risk of NSAID-related upper gastrointestinal ulcers and haemorrhageAliment Pharmacol Ther20072626527517593072
- BroekmansSDobbelsFMilisenKMorlionBVanderschuerenSMedication adherence in patients with chronic non-malignant pain: is there a problem?Eur J Pain200913211512318467138
- IngersollKSCohenJThe impact of medication regimen factors on adherence to chronic treatment: a review of literatureJ Behav Med200831321322418202907
- SrivastavaKAroraAKatariaACappelleriJCSadoskyAPetersonAMImpact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysisPatient Prefer Adherence2013741943423737662
- GoldsteinJLHowardKBWaltonSMMcLaughlinTPKruzikasDTImpact of adherence to concomitant gastroprotective therapy on non-steroidal-related gastroduodenal ulcer complicationsClin Gastroenterol Hepatol200641337134517088110
- LanasAPolo-TomásMRoncalesPGonzalezMAZapardielJPrescription of and adherence to non-steroidal anti-inflammatory drugs and gastroprotective agents in at-risk gastrointestinal patientsAm J Gastroenterol201210770771422334248
- ValkhoffVEvan SoestEMMazzagliaGAdherence to gastro-protection during cyclooxygenase 2 inhibitor treatment and the risk of upper gastrointestinal tract events: a population-based studyArthritis Rheum2012642792280222508379
- van SoestEMValkhoffVEMazzagliaGSuboptimal gastroprotective coverage of NSAID use and the risk of upper gastrointestinal bleeding and ulcers: an observational study using three European databasesGut2011601650165921636644
- VoilsCIHoyleRHThorpeCTMaciejewskiMLYancyWSJrImproving the measurement of self-reported medication nonadherenceJ Clin Epidemiol20116425025421194887
- de AchavalSSuarez-AlmazorMETreatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosusInt J Clin Rheumtol2010531332620676388
- ShiLLiuJKolevaYFonsecaVKalsekarAPawaskarMConcordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devicesPharmacoeconomics2010281097110721080735
- ColemanCILimoneBSobierajDMDosing frequency and medication adherence in chronic diseaseJ Manag Care Pharm20121852753922971206
- SturkenboomMCBurkeTATangelderMJDielemanJPWaltonSGoldsteinJLAdherence to proton pump inhibitors or H2-receptor antagonists during the use of non-steroidal anti-inflammatory drugsAliment Pharmacol Ther2003181137114714653834