Abstract
Objective
As poor medication adherence is common in bipolar disorder (BD), technology-assisted approaches may help to monitor and enhance adherence. This study evaluated preliminary feasibility, patient satisfaction and effects on adherence, BD knowledge, and BD symptoms associated with the use of a multicomponent technology-assisted adherence enhancement system.
Methods
This prospective study tested the system in five BD patients over a 15-day period. System components included: 1) an automated pill cap with remote monitoring sensor; 2) a multimedia adherence enhancement program; and 3) a treatment incentive program. This study evaluated system usability, patient satisfaction and effects on adherence (Morisky scale), knowledge (treatment knowledge test [TKT]), and symptoms (internal state scale [ISS]).
Results
Mean age of the sample was 62 years, 4/5 (80%) Caucasian, and 4/5 (80%) single/divorced or widowed. Most participants (4/5, 80%) were on a single BD medication. Participants had BD for an average of 21 years. Challenges included attaching the pill sensor to standard pharmacy bottles for individuals using very large pill containers or those with multiday pill boxes. Three of five (60%) individuals completed the full 15-day period. Usability scores were high overall. Mean Morisky scores improved. Means on all four subscales of the ISS were all in the direction of improvement. On the TKT, there was a 40% increase in mean scores.
Conclusion
A multicomponent technology-assisted BD adherence enhancement system is feasible. Challenges include accommodating multiple types of pill containers and monitoring multiple drugs simultaneously. The system can also generate adherence information that is potentially useful for treatment planning.
Introduction
Bipolar disorder (BD) is a chronic mental illness associated with reduced individual quality of life, devastating effects on families and children, and enormous costs to society.Citation1–Citation3 A cornerstone of treatment uniformly recommended by guidelines on illness management for BD patients is mood-stabilizing medication such as lithium, anticonvulsants, or atypical antipsychotic drugs.Citation1,Citation4–Citation6 Yet, approximately one in two individuals with BD are poorly adherent with prescribed medication treatments,Citation7–Citation10 resulting in a variety of negative consequences, including relapse, rehospitalization, suicide, and significantly increased costs of care.Citation3,Citation11–Citation17
Although evidence-based interventions to change health behavior exist, their high costs and resource-intensive nature make them impractical for many BD patients.Citation18–Citation20 Technology-assisted approaches are increasingly being studied and used to help people with mental illnesses, such as BD, better self-manage their health.Citation21–Citation23 These investigators have been developing a technology-enabled adherence enhancement system for patients with BD. This pilot study evaluated the preliminary feasibility of the system, patient satisfaction with the technology, and effects on medication adherence, knowledge of BD, and BD symptoms. Given the growing importance of technology in illness self-management, practical and patient-friendly approaches are needed.
Methods
Study overview
This was a prospective analysis of a multicomponent, technology-assisted adherence enhancement system in five patients with BD, who tested the system over a 15-day period. The DialogMeds-BD system () is designed to address the barriers to adherence in BD patients. The DialogMeds-BD components include: 1) an automated pill cap with remote monitoring sensor; 2) a multimedia adherence enhancement (MAE) program; and 3) a treatment incentive program (TIP) for motivating patients to remain adherent and improve treatment-related knowledge and skills. System data are intended for viewing by clinicians in real-time, web-based format, which can be used to help in treatment planning and medical decision-making. This preliminary study evaluated the feasibility of use, patient satisfaction with the system, and effects on BD adherence, knowledge, and symptoms.
Pill-cap monitoring sensor
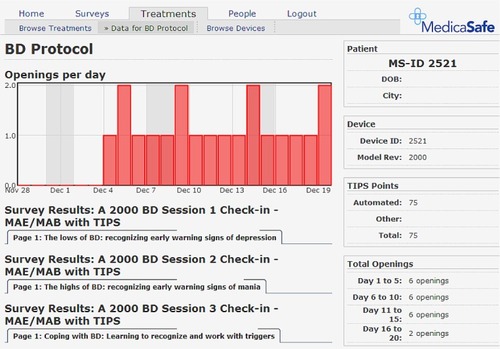
A prototype electronic pill-cap sensor was developed for this project. Designed to be attached to standard medication bottles dispensed by commercial pharmacies, the specialized sensor has an embedded computer chip and a two-line LCD screen. When removed from its bottle, the sensor records the instance of the bottle opening and stores it in memory, thereby making it possible to track patients’ presumed dosing episodes over the course of their treatment regimen. To help patients monitor their dosing for a given day, the LCD screen displays the hours since the last cap opening, as well as the number of total openings in the last 24 hours. A detailed adherence record that includes the number of openings per day for the previous 8-day period is also stored in the memory chip in the form of a “status code.” A 7–10 digit encrypted version of this status code is also displayed on the LCD screen. The encryption algorithm conceals the adherence data embedded in the status code by performing a logical XOR operation involving a rolling set of codes unique to each cap, resulting in what appears to be a random number. The encrypted code can then be entered by patients during weekly interactive voice response (IVR) check-ins, at which point it is decoded and included in the provider reports. illustrates a sample provider report.
MAE
The MAE program is designed to educate patients about illness knowledge and self-management skills empirically related to successful clinical outcomes. Materials for the program were adapted from an effective intervention designed to improve treatment adherence attitudes and behaviors among BD patients.Citation24,Citation25 Topics were condensed and presented in a series of brief 2–5 minute segments. A narrative script of a sample session is noted in .
TIP
The TIP is a contingency management approach for motivating patients to improve their treatment knowledge and remain adherent to their prescribed regimen. TIP points (TIPS), which can be redeemed for cash, are awarded to patients for completing MAE materials, checking-in as scheduled, and completing assessments.
Participants
Five (N=5) individuals with BD were recruited from the clinical and research practice of the psychiatrist investigator. Inclusion criteria required that patients be at least 18 years old and have a clinical diagnosis of BD type I or type II. For this initial assessment of the system, individuals who might be expected to have difficulty in following study procedures were excluded.
Specific procedures
Pill monitoring sensors were fitted onto an existing BD medication bottle. In the event that individuals were on more than one medication to manage their BD, the sensor was used on their most recently started maintenance BD medication. Upon in-person enrollment, BD patients registered by phone with the system. A research assistant (RA) walked the patient through the registration process and initial check-in, as well as reviewed the overall system usage. The registration and check-ins included listening to MAE material by phone and entering answers to adherence questions. Patients then checked in remotely on their own by phone on days 5, 10, and 15. TIP points were awarded for completing each of the four scheduled phone check-ins.
Usability and acceptance
Immediately after registering with the system, using a 5-point Likert scale, (one worst, five best) participants rated their satisfaction with the system and were asked to provide a brief explanation for their rating. At the conclusion of the study, participants completed an acceptance survey using a 7-point Likert scale (one worst, seven best) and were asked to provide responses to a series of open-ended questions regarding their perceptions of the system.
Clinical and adherence status measurements
In addition to collecting basic demographic and clinical data at baseline (age, sex, ethnicity, education level, and current medications), adherence, treatment knowledge, BD symptoms, and perceptions of system usability by phone were assessed at baseline and after the 15-day period of system use. Adherence was assessed with the self-reported Morisky questionnaire.Citation26 The internal state scale (ISS) measured BD symptoms.Citation27,Citation28 A 9-question treatment knowledge test (TKT) measured knowledge of concepts and practices known to be associated with improved treatment outcomes for BD patients and which were specifically covered in the MAE.
Data analysis
Given the primary feasibility focus of the study, the primary assessments were qualitative (eg, did patients accept and use the system?). Descriptive statistics were calculated for secondary outcomes (adherence, knowledge, and BD symptoms).
Results
Enrollment and system implementation
There were three male and two female participants, mean age 62 years (range 49–71 years), 4/5 (80%) Caucasian, and 4/5 (80%) single/divorced or widowed. Mean years of education was 13 years (range 12–14 years). The majority resided in private homes with other people. Most participants (4/5, 80%) were on a single medication for treatment of BD. On average, participants had BD for 21 years (range 5–43 years) and were clinically stable, treated BD patients. These pilot study participants were mostly euthymic or mildly depressed. Possible ratings on the ISS for depressive severity range from 0 to 200 with higher scores representing more severe depression. The study sample ISS scores were reflective of their overall clinical stability with a mean of 40 (SD 56).
There were some challenges with system implementation including medication containers that were too large to easily fit the pill sensor, child-proof pill caps that made it difficult to attach sensors, and some individuals who used multiday pill minders rather than pill bottles dispensed by the pharmacy. In these situations, patients used an empty pill bottle to store their medications for the 15-day study duration. Out of five participants, three (60%) were able to complete use of the system for the full 15-day period. Pill sensors came off of pill caps in two cases due to being dropped (N=1) or tape that affixed the sensor coming off (N=1). Individuals who had sensors come off their pill bottles continued to complete phone-in MAE and other assessments.
Usability and acceptability
Usability scores on the 5-point scale were high overall with means ranging from 4.8 to 5.0 on all usability questions. Endpoint acceptability scores on the 7-point scale were also high with means ranging from 4.8 to 6.3. When queried regarding what they liked best about the system, the most common response was the simplicity and ease of use, while the most common complaints or least liked components of the system were related to the difficulty with the temporary (tape) method used to affix the pill caps to the bottle. All patients completed all scheduled check-ins and thus earned the maximum eligible TIPS available.
Adherence, BD symptoms, BD knowledge
Mean scores on the Morisky scale improved from baseline to follow-up (3.20–3.60). Means on all four subscales of the ISS were all in the direction of improvement. Scores on perceived conflict (baseline 60, endpoint 48), activation (baseline 64, endpoint 12), and depression index (baseline 40, endpoint 10) all decreased, while scores on well-being (baseline 222, endpoint 258) increased. Finally, mean TKT scores increased from baseline to follow-up (14–19.6) with all five participants demonstrating improved treatment knowledge relative to the baseline test administration.
Clinician interface
As noted in , a graphic showing pill bottle openings per day and TIPS readily provided an overview of adherence behavior and BD education.
Discussion
Given the pervasive and negative effects of poor adherence on people with BD, approaches that improve adherence can improve outcomes and enhance the efficiency of care. Our preliminary experience with a technology-assisted multiple component adherence enhancement system suggests that the system is well accepted, feasible to implement, and has the potential to improve adherence and related health outcomes in people with BD. Somewhat similar to our experience in that it used a mobile device, focused on adherence in patients with BD, and was administered over a relatively short time-frame, Wenze et al evaluated the feasibility and acceptability of an ecological momentary intervention (EMI), delivered via personal digital assistants (PDAs), to improve treatment adherence.Citation21 Overall, participants in the Wenze et al study (N=14) reported satisfaction with the usefulness, timing and burden of sessions, as well as the method of delivery.Citation21 Negative aspects of the PDA-delivered approach were mainly in technical and logistic limitations. While our study also had a number of limitations including small sample size, short-term duration, and a temporary method of affixing pill sensors to pill bottles that was sub-optimal, findings may still be helpful in considering next steps for technology-assisted adherence enhancement methods in BD.
Patients with BD in our sample were overall very accepting of the system and considered it easy to use. However, a sensor that is designed to fit on top of a standard pharmacy pill bottle may not be generalizable for use in people who receive large mail-order pill containers (for example, 90-day supplies of medications that are taken multiple times per day) and for people who transfer their medications to multiday pill minders. Our sample of BD patients was mostly taking a single BD medication daily, and may not be broadly representative of most people with BD. Baldessarini et alCitation29 analyzed US national health plan claims data from 2000 to 2004 and found that approximately one-third of patients with BD are on medication polytherapy. Polytherapy rates might be expected to be even higher in public sector care where people with BD are more ill and more impaired.
While the symptom changes in our BD sample are too small to generalize, clinical outcomes (adherence, BD knowledge, BD symptoms) were possibly improved (and certainly not worse). It is possible that if the study had been continued for a longer period, these seeming improvements would not have persisted. Depp et alCitation23 very recently reported on a randomized single-blind controlled trial with 82 individuals with BD who completed a four-session psychoeducational intervention and were assigned to 10 weeks of either: 1) mobile device delivered interactive intervention linking patient-reported mood states with personalized self-management strategies, or 2) paper-and-pencil mood monitoring. Participants were assessed at baseline, 6, 12, and 24 weeks follow-up. Compared to the paper-and-pencil condition, participants in the augmented mobile intervention condition had greater reductions in depressive symptoms at 6 and 12 weeks although this was not maintained at 24 weeks.Citation23 It is possible that technology-assisted self-management support needs to be continued indefinitely to continue to provide support. On the positive side, technology approaches may be less invasive and expensive than the in-person methods that are used in typical clinical settings. Longer, more rigorous studies are needed to assess the effects of technology-assisted BD self-management on extended health outcomes.
The TIP approach to incentivize patients appears effective, although how this would be operationalized in real-world settings needs further consideration. Examples of widely used health care incentives include health insurance plans that give patients discounts or financial compensation for healthy behaviors like smoking cessation or blood pressure testing. In our multiple component adherence enhancement system, a clinician read-out provides adherence data that is potentially useful for care monitoring and planning. Based in a secure, web-based platform, adherence behavior information can help clinicians to make such key decisions as determination of reasons for sub-optimal medication response (poor adherence vs treatment refractory illness) and changes in drug formulation (consideration of once daily vs multiple times daily dosing vs long-acting or injectable long-acting drugs).
Conclusion
In conclusion, poor adherence is a major obstacle to good health outcomes for people with BD. Technology-assisted approaches need to be refined that can incentivize adherence, teach patients necessary information and skills to maximize adherence, and inform clinicians on patient adherence patterns.
Acknowledgments
This study was supported by an investigator-initiated grant from MedicaSafe, Inc., to the study PI (Sajatovic).
Disclosure
Martha Sajatovic is a consultant for Prophase, Otsuka, Sunovion, Pfizer, Amgen, and Bracket, and has received grant support from Pfizer, Merck, Ortho-McNeil Janssen, and Janssen. Michael Davis is employed by MedicaSafe Inc. Joseph Nestor is a former employee of MedicaSafe Inc. The other authors report no conflicts of interest in this work.
References
- American Psychiatric AssociationPractice guideline for the treatment of patients with bipolar disorder (revision)Am J Psychiatry20021594 suppl150
- MurrayCJLopezADGlobal mortality, disability, and the contribution of risk factors: global burden of disease studyLancet19973499063143614429164317
- BegleyCEAnnegersJFSwannACThe lifetime cost of bipolar disorder in the US: an estimate for new cases in 1998Pharmacoeconomics2001195 pt 148349511465308
- KeckPEJrPerlisRHOttoMWCarpenterDRossRDochertyJPThe Expert Consensus Guidelines: Treatment of Bipolar Disorder2004Postgraduate Medicine Report: Special Report1120
- GoodwinGMYoungAHThe British association for psychopharmacology guidelines for treatment of bipolar disorder: a summaryJ Psychopharmacol2003174 suppl3614964624
- YathamLNKennedySHO’DonovanCCanadian network for mood and anxiety treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversiesBipolar Disord20057suppl 356915952957
- SajatovicMValensteinMBlowFCGanoczyDIgnacioRVTreatment adherence with antipsychotic medications in bipolar disorderBipolar Disord20068323224116696824
- SajatovicMValensteinMBlowFGanoczyDIgnacioRVTreatment adherence with lithium and anticonvulsant medications among patients with bipolar disorderPsychiatr Serv200758685586317535948
- LingamRScottJTreatment non-adherence in affective disordersActa Psychiatr Scand2002105316417211939969
- PerlickDARosenheckRAKaczynskiRKozmaLMedication non-adherence in bipolar disorder: a patient-centered review of research findingsClin Approaches in Bipolar Disord2004325664
- VolppKGGurmankin LevyAAschDAA randomized controlled trial of financial incentives for smoking cessationCancer Epidemiol Biomarkers Prevent20061511218
- GriffithJDRowan-SzalGARoarkRRSimpsonDDContingency management in outpatient methadone treatment: a meta-analysisDrug Alcohol Depend200058556610669055
- GineXKarlanDZinmanJPut Your Money Where Your Butt is: A Commitment Savings Account for Smoking Cessation. Innovations for Poverty Action Internet2008 [cited March 1 2014]. Available from: http://documents.apec.umn.edu/DKarlanT&DSemSp08.pdf
- FornessSRKavaleKABlumIMLlyodJWMage-analysis of meta-analysis: what works in special education and related services?Teach Except Child19972949
- SchumacherJEMilbyJBWallaceDMeta-analysis of day treatment and contingency-management dismantling research: Birmingham homeless cocaine studies (1990–2006)J Consult Clin Psychol200775582382817907865
- LussierJPHeilSHMongeonJABadgerGJHigginsSTA meta-analysis of voucher-based reinforcement therapy for substance use disordersAddiction2006101219220316445548
- DutraLStathopoulouGBasdenSLLeyroTMPowersMBOttoMWA meta-analytic review of psychosocial interventions for substance use disordersAm J Psychiatry2008165217918718198270
- HullemanCSCordrayDSMoving from the lab to the field: the role of fidelity and achieved relative intervention strengthJ Res Educ Eff2009288110
- MiklowitzDJOttoMWFrankEPsychosocial treatments for bipolar depression: a 1-year randomized trial from the systematic treatment enhancement programArch Gen Psychiatry200764441942617404119
- SauroJKindlundEUsing a single usability metric (SUM) to compare the usability of competing productsPaper presented at: Human Computer Interaction International ConferenceJuly 22–27; 2005Las Vegas, USA
- WenzeSJArmeyMFMillerIWFeasibility and acceptability of a mobile intervention to improve treatment adherence in bipolar disorder: a pilot studyBehav Modif201438449751524402464
- BurnsMNBegaleMDuffecyJHarnessing context sensing to develop a mobile intervention for depressionJ Med Internet Res2011133e5521840837
- DeppCACeglowskiJWangVCAugmenting psychoeducation with a mobile intervention for bipolar disorder: a randomized controlled trialJ Affect Disord2015174233025479050
- SajatovicMLevinJTatsuokaCSix-month outcomes of customized adherence enhancement (CAE) therapy in bipolar disorderBipolar Disord201214329130022548902
- SajatovicMLevinJTatsuokaCCustomized adherence enhancement for individuals with bipolar disorder receiving antipsychotic therapyPsychiatr Serv201263217617822302337
- MoriskyDEGreenLWLevineDMConcurrent and predictive validity of a self-reported measure of medication adherenceMed Care19862467743945130
- BauerMSVojtaCKinosianBAltshulerLGlickHThe internal state scale: replication of its discriminating abilities in a multisite, public sector sampleBipolar Disord20002434034611252648
- BauerMSCrits-ChristophPBallWAIndependent assessment of manic and depressive symptoms by self-rating. Scale characteristics and implications for the study of maniaArch Gen Psychiatry19914898078121929771
- BaldessariniRHenkHSklarAChangJLeahyLPsychotropic medications for patients with bipolar disorder in the United States: polytherapy and adherencePsychiatr Serv200859101175118318832504