Abstract
Purpose
To describe the process used to develop an evidence-based patient decision aid (PDA) that facilitates shared decision-making for treatment intensification in inadequately controlled type 2 diabetes mellitus (T2DM) consistent with International Patient Decision Aids Standards.
Methods
A PDA was developed by a multidisciplinary steering committee of clinicians, patient advocate, nurse, certified diabetes educators, and decision scientist, using a systematic development process. The process included defining the PDA scope and purpose, outlining the framework, content creation, and designing for integration into clinical practice. This was accomplished through a review of the literature and publically available educational materials and input from practicing clinicians and patients during development and iteratively refining content based on input. Patients with poorly controlled T2DM on metformin considering additional medication assessed the PDA during a pilot.
Results
Testing identified six preference-sensitive domains important for choosing T2DM treatment: degree of glycemic response, avoiding weight gain, hypoglycemia risk and other adverse events, avoiding injections, convenience of dose administration, blood glucose monitoring, and cost of therapy. Patient feedback guided content revision. Treatment options were offered after presenting medication class risk–benefit information and eliciting patient values, goals, and preferences. The PDA received the highest International Patient Decision Aids Standards global score to date, 88/100, with 100% of criteria fully met for the following dimensions: development process, disclosures, evaluation process, evidence quality, guidance for users, information quality, language/readability, testing, and eliciting patient values.
Conclusion
A PDA was developed to help T2DM patients make decisions regarding medication choice. This approach may be applicable to other chronic conditions.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Metformin is the recommended initial antihyperglycemic agent for patients with type 2 diabetes mellitus (T2DM).Citation1 As diabetes progresses, additional antihyperglycemic agents are necessary to maintain and/or achieve glycemic control.Citation1,Citation2 Commonly used agents combined with metformin include dipeptidyl peptidase-4 inhibitors, glucagon-like peptides (mimetics), sodium-glucose co-transporter 2 inhibitors, thiazolidinediones, sulfonylureas, and insulin.Citation1 Treatments vary in effectiveness, dosing, administration convenience, risk of adverse events, and cost. Decisions about which additional treatment to choose are difficult because of the many treatments that are available, decisional domains that must be considered, and the trade-offs that must be made among these factors. Patient’s inadequate, incomplete, or incorrect knowledge of the benefits and risks of these options impedes informed decision-making. Often, patients are not even aware that they have a choice among treatments.Citation3 This can result in patients delaying or making ineffective decisions such as starting on a medication that may not align with individual’s circumstances, preferences and values, and evidence of decisional conflict.Citation4
In its most recent Standards of Medical Care in Diabetes, the American Diabetes Association (ADA) emphasized a patient-centered approach to determine treatment selection when choosing medications added to metformin.Citation2 The ADA recommends physicians use an approach that ensures that patients understand the aspects of each class of medications and that patient preferences for treatment are considered in treatment decisions. Considerations include efficacy, cost, side effects, impact on weight, comorbidities, hypoglycemia risk, and patient preferences for these outcomes. Optimal T2DM management requires a systematic patient-centered approach that involves health care professionals partnering with patients to choose the best treatment within this context.
Helping patients make decisions aligned with their personal values is integral to patient-centered care, an Institute of Medicine mandate.Citation5,Citation6 The Agency for Healthcare Research and Quality in its Consumer Assessment of Healthcare Providers and Systems (CAHPS) program further supports a patient-centered approach to care by measuring aspects of patient–provider interactions as it relates to health care decisions through its measurement system. Related to decision-making, CAHPS measures how often patients report their providers explain things clearly, listen carefully, show respect, and provide easy to understand instructions. Patients are queried via CAHPS regarding whether providers discuss reasons why patient might or might not want to take medication.
Shared decision-making (SDM) is a patient-centered approach consistent with Institute of Medicine and CAHPS that helps patients make better health care decisions based on their informed preferences in collaboration with their health care provider.Citation6–Citation9 Early attempts demonstrate that SDM has been successfully applied in diabetes and other chronic conditions to facilitate patient engagement and decision-making, including decisions about long-term medications. Publicly available patient decision aids (PDAs) in diabetes to support SDM are available.Citation10,Citation11
PDAs are evidence-based SDM tools that are designed to help people make informed decisions about their treatment options.Citation12 They provide balanced, neutral information about treatment alternatives and their relative benefits and disadvantages.Citation4,Citation9,Citation13 A Cochrane review of 118 trials found PDAs consistently improved knowledge of options and outcomes, led to more realistic expectations, helped patients match their values to their choices, and reduced decisional conflict and passivity in decision-making, with no negative emotional effects.Citation9 PDAs improved patient–provider communication, promoted discussion of treatments with their provider,Citation14,Citation15 and promoted SDM.Citation11,Citation16–Citation18 Some PDAs appear to reduce health care utilization and costsCitation19 in patients with diabetes, while some may promote better adherence and outcomes, and this is the subject of ongoing research.Citation20–Citation22
The International Patient Decision Aids Standards (IPDAS) Collaboration established in 2003, has developed criteria for evaluating the quality of PDAs, using an international panel of collaborators via a Delphi process.Citation23,Citation24 The IPDAS checklist guides developers to include suggested components in PDAs and facilitates determination of whether PDAs underwent rigorous development and evaluation.
In collaboration with a multidisciplinary team of clinicians with expertise in managing diabetes, SDM, and patient education, we developed a PDA to support the choice of antihyperglycemic agent when metformin use alone does not achieve glycemic control.
Methods
The purpose of the PDA is to help patients with T2DM treated with metformin make decisions about adding additional therapy. Target users of the PDA were adults with T2DM currently taking metformin and in whom their physician considered additional medicine was needed to achieve adequate glycemic control. The PDA was designed to prepare patients for decision-making by providing information on medication options, eliciting and communicating their preferences to clinicians, and promoting SDM during a physician consultation. We describe a practical approach for developing the PDA that can be applied to other health conditions.
Framework, formative work, and content development
Based on our review of current clinical guidelines for T2DMCitation1,Citation2 risk–benefit profiles of available medications, formative interviews with patients and clinicians, and expert opinion from the steering committee, we identified six domains as being important for choosing an additional treatment. These domains are (1) degree of glycemic response, (2) avoiding weight gain, (3) risk of hypoglycemia and other adverse events, (4) avoidance of injections, (5) convenience of dose administration and blood glucose monitoring, and (6) cost of therapy. These six domains were translated into multimedia, interactive modules by a team of medical writers, literacy experts, medical artists, animation specialists, decision scientist, and a voice talent (Emmi Solutions, LLC). This process involved sequentially developing an outline, drafting the script and graphics suitable for a middle school-level audience, recording the script, and developing animated graphics to produce an online prototype. All development occurred between January and July 2013. Each iteration was reviewed and edited by a multidisciplinary steering committee until consensus was achieved. The committee consisted of two endocrinologists, one a member of the clinical practice committee of the American Diabetes Association (Fonseca), and one a founder of a not-for-profit patient advocacy organization that provides teaching and motivation support for patients in diabetes self-care (Edelman). Also included were certified diabetes educator, one of which is a nurse (Funnell, Polonsky), and an expert in decision science and SDM who serves on the Steering Committee for the IPDAS collaboration (Col). The content included in each domain was guided by IPDAS standards,Citation23,Citation24 and the development process is outlined in .
Patient and clinician evaluation and input, iterative development
Following development of a prototype designed to be delivered via video content, two patient focus groups each with seven participants reviewed the prototype and provided structured input. Focus group participants included patients with T2DM representing a wide range of educational and socioeconomic backgrounds. During the focus groups, the prototype PDA was viewed and patients were given the opportunity to make suggestions, were given opinions about the clarity and value of the information presented, and the factors that are important in decision-making were discussed. Following focus group input, a revised “beta” version of the PDA content was prepared.
Three practicing primary care clinicians who were not involved in developing the PDA participated in the assessment of the revised beta PDA. Each was asked to invite at least two patients from the target audience (ie, adults, T2DM, not adequately controlled on metformin who were advised to consider medication intensification) to view the PDA at home prior to a planned in-office consultation for medication management. Physicians and patients were given opportunities to comment on the content and their satisfaction with the PDA to inform medication decision-making and promoting SDM.
IPDAS assessment
The PDA underwent a preliminary internal review to determine congruence with the IPDAS instrument,Citation24 which provides a framework to rate PDAs using 23 standards for quality of content and 20 standards for the development process. The final PDA was submitted to IPDAS (Cardiff University, Wales) for independent evaluation.
Results
An online, interactive multimedia PDA was developed that requires approximately 25 minutes to view.
The PDA is subdivided into a general introduction to the purpose of the aid and online “chapters” containing information and questionnaires to guide the decision-making process. The content categories of the PDA are presented in .
After setting the context for the decision, general information on the role of the pancreas in producing insulin to control blood glucose and pathology in disease progression is discussed. The six preference domains () are introduced and then the different medication classes are discussed (sulfonylureas, dipeptidyl peptidase-4 inhibitors, thiazolidinediones, sodium-glucose co-transporter 2 inhibitors, glucagon-like peptide, and insulin). The PDA compares treatment options, explains the choice that has to be made regarding additional medication, and discusses the options available to patients. Evidence about the risks and benefits of different treatments are presented as they relate to the key preference domains.
To promote values clarification, the PDA includes simple questions that assess patient values, preferences, treatment goals, and treatment predisposition. Goals for therapy are expressed in terms of the magnitude of blood glucose control and weight impact desired. Values are assessed as the relative importance of avoiding hypoglycemia, avoiding injections, taking medication only once a day, minimizing blood glucose monitoring, and avoiding weight gain. Treatment predisposition is assessed by asking patients what they would choose if they had to make a decision on medication today. Because content is delivered via Health Insurance Portability and Accountability Act-compliant online format, functionality is included to electronically provide clinicians with the results of patient questionnaires. Throughout the PDA, patients are regularly prompted to jot down questions that can be printed and shared with their clinician and/or family members.
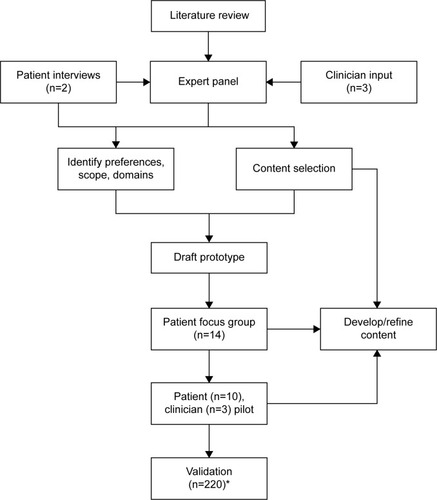
A final summary section shows patients a “fact sheet” that compares the risks and benefits of each class of medication verbally and graphically. Risks and potential benefits are illustrated by using plain language, color-coded pictographs, and summaries, in a balanced, unbiased manner such as shown in .
Figure 3 Printable summary information for patients.*
Abbreviations: DPP-4, dipeptidyl peptidase-4 inhibitor; GLP-1, glucagon-like peptide-1; SGLT2s, sodium-glucose co-transporter 2 inhibitors; TZD, thiazolidinedione.
Patient input on content development
Feedback received from two focus groups, one consisting of individuals with grade school and at least some high school education (n=7), one consisting of college-educated individuals (n=7) who viewed the draft version of the PDA was generally very positive. Their feedback was used to finalize the “beta” version of the PDA. Three physicians not involved in developing the PDA reviewed the beta version with a total of ten of their patients, with each patient assessing and providing input on the PDA content. The majority of patients who viewed the PDA when invited by their physician were positive in their responses. Nine of ten (90%) patients reported that the PDA helped them to think about the pros and cons of their medication options, prepared them to make a better decision, helped them recognize that the decision depends on what matters most to them, helped them organize their thoughts about the decision, and felt they were better prepared to have a follow-up conversation with their doctor.
Clinicians (100%) using the PDA with patients reported that it helped patients a great deal or quite a bit to: be involved in the decision-making process, make a more informed decision, understand the issues that are important to patients, and facilitated follow-up consultation. Two of the three clinicians stated that the PDA was a reasonable length at 25 minutes to complete; all stated that the amount of information was “just right”.
External evaluation: IPDAS assessment
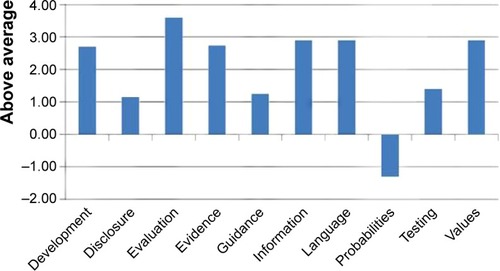
Upon completion of the PDA production, the development team submitted the PDA to IPDAS instrument for formal evaluation. IPDAS communicated that the PDA received the highest global score issued by IPDAS to date, 88 out of 100, with above-average scores benchmarked against all other submissions as of the date reviewed as of May 25, 2014 (), with 100% of criteria fully met for the following dimensions: development process, disclosures, evaluation process, evidence quality, guidance for users, information quality, language/readability, testing, and patient values establishment. The only below-average score was for presenting the risks of adverse events using “probabilities”.
Figure 4 International Patient Decision Aids Standards z-scored benchmark report (z-scores represent standard deviations compared to other submissions).
The evaluation process is not yet complete as the validation study is in progress.
Discussion
SDM is now nearly universally endorsed in the United States by policy makers, and PDAs are being developed for a growing number of conditions and health decisions. Criteria for rating the quality of PDAs have been developed, and it is known that PDAs that scored high on having a systematic development process achieved high scores on other IPDAS criteria.Citation25 Yet what constitutes a systematic development process is not well defined.
Many PDAs that have been developed and evaluated do not publish or clearly describe how their tools were developed, and there are only a few frameworks for developing PDAs that have been published. These include the Ottawa framework,Citation26 Cardiff University,Citation23 and Dutch Institute for HealthCare Improvement,Citation27 and these frameworks do not always give specific guidance on approaches to implement some of the suggested steps. Furthermore, many currently published PDAs were funded by large research grants and required several years to develop and test, a timeline that is costly and impractical for developing future PDAs, especially where the knowledge base and number of treatment options is rapidly evolving.
This manuscript provides insight into the systematic process used to develop a PDA, including input of an interdisciplinary group of content experts, health care professionals, patients, and clinicians. Our PDA development process incorporated input from a wide range of stakeholders throughout all stages of development. The PDA is evidence based and the content is consistent with current recommendations and clinical guidelines. The presentation of risks were based upon data from clinical trials and systematic reviews and the need for presenting structured information to patients was confirmed, as patients indicated that clear and relevant information is currently hard to find. The PDA itself offers the opportunity to fill gaps in information for SDM in T2DM. Many of the approaches used to communicate risk and introduce complex topics can be applied to other PDAs.
Patient response was positive and feedback from clinicians confirmed that both they and their patients with T2DM value the use of the PDA for decision-making. Based on this feedback, it is likely that this PDA supports the T2DM medication decision-making process. The results on the effectiveness are being further explored through a validation study. The development process outlined can be used to develop other PDAs.
Strengths and limitations
The development process outlined in this paper is pragmatic, replicable, and can be done within a short time frame. The decision addressed by the PDA is potentially generalizable to a number of health conditions as it involves the consideration of many different medication classes, each with different benefits and risks. One of the challenges faced in designing the PDA was deciding what information to include and how to frame it so that it is understandable to a wide range of patients while avoiding information overload. We do not provide guidance about what specific content to include in the PDA, but rather we present a process for determining the type of information and vetting it by affected patients and providers. The strengths of the development process include a systematic approach, quality control through critical input and review of an expert panel, obtaining opinions of patients throughout the development process, feedback loop from questionnaires regarding patient values, eliciting preferences and communicating these to clinicians in real time, and including feedback from clinicians not involved in the development process. Our testing involved a small number of patients and clinicians, but they were carefully selected to reflect the target audience of future users. The main shortcoming of our PDA, as noted by IPDAS instrument, was not including the probabilities of specific adverse events of therapies. However, because this PDA was designed to discuss major drug classes, and not specific agents (of which there are many per class), not presenting detailed probabilities of events was intentional on the part of developers. Reliance on the Internet for content delivery reduces access for those lacking Internet access or impaired users. However, it expands access for the vast majority of patients who have Internet access and simplifies future updating of the content. The PDA is currently available only in English, though a Spanish adaptation is in development. The impact of the PDA on medication choice, patient–provider communication, and patient outcomes is unknown at this time, as is its adoption and use in clinical practice. A prospective trial in 20 primary care sites in the United States is currently underway. The high-quality rating from IPDAS suggests that the PDA would be likely to be successful in clinical practice, but the association between IPDAS rating and impact in clinical setting is unknown at this time. However, a highly rated PDA is more likely to gain clinician confidence, which should increase their likeliness to use it with their patients.Citation28
Implications for clinicians
This article provides insights into the systematic development of a PDA for patients with T2DM on metformin who need to intensify their medication regimen, including input of an interdisciplinary group of content experts, health care professionals, patients, and clinicians. Our PDA development process incorporated input from a wide range of stakeholders throughout all stages of development. The PDA is evidence based and the content is consistent with current recommendations and clinical guidelines. It is now available for use by “clinicians” at www.diabetesdecisionaid.com
Acknowledgments
The authors thank the Development Steering Committee members: Nananda Col, MD, MPP, MPH, FACP (University of New England, Shared Decision Making Resources); Steve Edelman, MD (University of California at San Diego); Martha Funnell, RN, CD (University of Michigan); Vivian Fonseca, MD, FRCP (Tulane University); and William Polonsky, PhD, CDE (University of California, San Diego). The authors wish to acknowledge the contributions of Anne Farley (EPI-Q) in collecting data during the feasibility assessment; Chris Kabir (EPI-Q) for his assistance in tabulation and displaying feasibility assessment results; Jacquelyn Waugh and Dianna Dillinger (Emmi Solutions) for project management and writing during the development of the PDA; and Natalie Joseph-Williams (IPDAS) for facilitation throughout the submission and review process.
Disclosure
This research and report were developed with funding from Janssen Scientific Affairs, LLC. The authors report no other conflicts of interest in this work.
References
- GarberAJAbrahamsonMJBarzilayJIAmerican Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement – executive summaryEndocr Pract201319353655723816937
- American Diabetes AssociationStandards of Medical care in Diabetes. 2015201538suppl 1S1S80
- SepuchaKRFagerlinACouperMPLevinCASingerEZikmund-FisherBJHow does feeling informed relate to being informed? The DECISIONS surveyMed Decis Making2010305 suppl77S84S20881156
- LeBlancAKennyDAO’ConnorAMLegareFDecisional conflict in patients and their physicians: a dyadic approach to shared decision makingMed Decis Making2009291616819196706
- Institute of MedicineCrossing the Quality Chasm: A New Health System for the 21st CenturyWashington, DCNational Academies Press2001
- BarryMJEdgman-LevitanSShared decision making – pinnacle of patient-centered careN Engl J Med2012366978078122375967
- BraddockCH3rdEdwardsKAHasenbergNMLaidleyTLLevinsonWInformed decision making in outpatient practice: time to get back to basicsJAMA1999282242313232010612318
- LegareFWittemanHOShared decision making: examining key elements and barriers to adoption into routine clinical practiceHealth Aff (Millwood)201332227628423381520
- StaceyDLegareFColNFDecision aids for people facing health treatment or screening decisionsCochrane Database Syst Rev20141CD00143124470076
- MontoriVMGafniACharlesCA shared treatment decision-making approach between patients with chronic conditions and their clinicians: the case of diabetesHealth Expect200691253616436159
- WeymillerAJMontoriVMJonesLAHelping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trialArch Intern Med2007167101076108217533211
- BekkerHLWinterbottomAEButowPDo personal stories make patient decision aids more effective? A critical review of theory and evidenceBMC Med Inform Decis Mak201313suppl 2S924625283
- O’ConnorAUsing patient decision aids to promote evidence-based decision makingACP J Club20011351A11A1211471526
- HansonLCCareyTSCaprioAJImproving decision-making for feeding options in advanced dementia: a randomized, controlled trialJ Am Geriatr Soc201159112009201622091750
- SheridanSLShadleJSimpsonRJJrPignoneMPThe impact of a decision aid about heart disease prevention on patients’ discussions with their doctor and their plans for prevention: a pilot randomized trialBMC Health Serv Res2006612117005051
- HessEPKnoedlerMAShahNDThe chest pain choice decision aid: a randomized trialCirc Cardiovasc Qual Outcomes20125325125922496116
- MontoriVMShahNDPencilleLJUse of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trialAm J Med2011124654955621605732
- MullanRJMontoriVMShahNDThe diabetes mellitus medication choice decision aid: a randomized trialArch Intern Med2009169171560156819786674
- ArterburnDWellmanRWestbrookEIntroducing decision aids at group health was linked to sharply lower hip and knee surgery rates and costsHealth Aff201231920942104
- JoostenEADeFuentes-MerillasLde WeertGHSenskyTvan der StaakCPde JongCASystematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health statusPsychother Psychosom200877421922618418028
- ParchmanMLZeberJEPalmerRFParticipatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet studyAnn Fam Med20108541041720843882
- WilsonSRStrubPBuistASAsthma Treatment (BOAT) Study GroupShared treatment decision making improves adherence and outcomes in poorly controlled asthmaAm J Respir Crit Care Med2010181656657720019345
- ElwynGO’ConnorAStaceyDInternational Patient Decision Aids Standards (IPDAS) CollaborationDeveloping a quality criteria framework for patient decision aids: online international Delphi consensus processBMJ2006333756541716908462
- ElwynGO’ConnorAMBennettCAssessing the quality of decision support technologies using the International Patient Decision Aid Standards Instrument (IPDASi)PLoS One200943e470519259269
- Joseph-WilliamsNNewcombeRPolitiMToward minimum standards for certifying patient decision aids: a modified Delphi consensus processMed Decis Making201334669971023963501
- LegareFO’ConnorAMGrahamIDWellsGATremblaySImpact of the Ottawa decision support framework on the agreement and the difference between patients’ and physicians’ decisional conflictMed Decis Making200626437339016855126
- RaatsCJvan VeenendaalHVersluijsMMBurgersJSA generic tool for development of decision aids based on clinical practice guidelinesPatient Educ Couns200873341341718768285
- CoulterAStilwellDKryworuchkoJMullenPDNgCJvan der WeijdenTA systematic development process for patient decision aidsBMC Med Inform Decis Mak201313suppl 2S224625093