Abstract
Background
Extracellular matrix degradation may play an important role in the etiology of urethral stricture. MMP1 and TIMP1 are involved in extracellular matrix degradation. The aim of this study was to investigate the roles of MMP1, TIMP1, and MMP1:TIMP1 ratio at the remodeling phase of urethral stricture in an animal model.
Methods
This research was carried out in collaboration between the Bogor Institute of Agriculture, Universitas Indonesia, and the Eijkman Institute Indonesia. This was an experimental in vivo study in adult male New Zealand rabbits, divided into two groups: a urethral stricture group and a control group. Euthanasia was performed in four rabbits of each group on days 7, 14, 21, 28, and 56. Urethral stricture was confirmed with an 8 F urethral catheter. Several laboratory examinations were done, including H&E and Masson trichrome staining, immunohistochemistry, and ELISA, to determine levels of MMP1 and TIMP1. Percentages of total collagen and collagen type 1 were counted with ImageJ 1.46q software. A general linear model was used for statistic analysis.
Results
We found that the level of MMP1 was lower, TIMP1 higher, and MMP1:TIMP1 ratio lower in the urethral stricture group than the control group. There was a correlation between MMP1 level with total collagen percentage (r=0.561, P=0.010) and no correlation between TIMP1 and total collagen (r=0.307, P=0.188).
Conclusion
Imbalance in extracellular matrix degradation was marked by decreased MMP1 level and MMP1:TIMP1 ratio and increased TIMP1 level. This results showed that urethral stricture is not only caused by collagen decomposition, but also by the imbalance of extracellular matrix degradation.
Keywords:
Introduction
Urethral stricture is an abnormality in which there is scarring process that involves the epithelium and corpus spongiosum, causing narrowing of the urethra.Citation1 In general, the incidence of urethral stricture is estimated to be 0.6% of the population.Citation2 Incidence varies. In the United States, the Veterans Hospital reported that 193 of all 100,000 outpatients suffered from urethral stricture in 2003.Citation3 In Indonesia, 128 urethral stricture patients underwent surgery in 6 years in Cipto Mangunkusumo Hospital, 316 patients were recorded with urethral stricture in 4 years in Soetomo Hospital, and 221 patients with urethral stricture during 2007–2013 in Hasan Sadikin Hospital. The incidence of urethral stricture is quite high in Indonesia, a developing country.
Urethral stricture may be caused by inflammation, trauma or iatrogenesis. Trauma has been reported as the most common etiology (up to 88.6% of cases), with trauma due to fall from a height being the most common (44.3%).Citation4 Palmienteri et al reported that 38.6% were iatrogenic, of which 16.3% were due to catheter placement, 12.2% a result of hypospadias surgery, and 9.1% a result of transurethral surgery.Citation5 Management of urethral stricture consists of dilatation of the urethra, internal urethrotomy, urethral stent placement, and reconstruction surgery. These procedures have not given a satisfactorily result, as urethral strictures may recur. The incidence of recurrent urethral stricture is up to 47.6% in patients with dilatation and having received internal urethrotomy.Citation6
The pathophysiology of urethral stricture is not entirely clear. Fibrosis is often associated with excessive deposition of extracellular matrix components, including collagen. Disrupted degradation of the extracellular matrix resulting in matrix deposition in the remodeling phase. MMP plays a major role in tissue remodeling. The role of MMP and TIMP is also evident in other organs, such as the heart, bone, and skin.Citation7–Citation9 The aim of this study was to investigate the roles of MMP1, TIMP1, and MMP1:TIMP1 ratio at the remodeling phase of urethral stricture and their correlation with collagen formation.
Methods
The study was an experimental animal study performed in the Surgery and Radiology Division of the Clinical Reproduction and Pathology Department, Faculty of Veterinary Medicine, Bogor Institute of Agriculture, Bogor, Indonesia. Laboratory examination was performed in Department of Pathological Anatomy, Faculty of Medicine, Universitas Indonesia, Jakarta and the Eijkman Institute for Molecular Biology, Jakarta. Animal models were New Zealand male rabbits weighing 3–4 kg, and research was carried out in accordance with the Animal Ethics Committee of Bogor Institute of Agriculture (02-2012 IPB).
Rabbits were divided randomly into two groups. Group 1 consisted of 20 rabbits as a urethral stricture model group, and group 2 consisted of 20 rabbits as a control group. Initially, rabbits were housed in standard cages (60×50×40 cm) for 7 days, where they were fed 100 g twice a day and given drink ad libitum. On day 5, rabbits were given an antibiotic (enrofloxacin 5 mg/kg), anthelmintic (ivermectin 0.02 mg/kg), and antiparasitic (albendazole 5 mg/kg). Rabbits were fasted on the evening prior to surgery. Anesthesia comprised ketamine (Ilium; Troy Laboratories, Sydney, Australia) 35 mg/kg, and xylazine (Troy Laboratories) 10 mg/kg intra-muscularly. The urogenital area was cleaned, shaved, and disinfected with povidone iodine.
In group 1, procedures were carried out in accordance with the methods of Scott and Foote, in which a sagittal incision as long as 1 cm was made on the urethra (two-thirds diameter of urethra) up to the mucosa.Citation10 By using an electro-coagulation device, coagulation was performed on the entire circumference of the urethra to the mucosa. Then, the area was sutured into the corpus spongiosum with a polydioxanone suture (PDS II 6.0). The coagulated side of the urethra was marked with 3.0 silk. Then, the skin was sutured with 3.0 silk. In group 2 (control group), Scott and Foote procedures were followed, but the urethra remained intact. Then, the corpus spongiosum was sutured with the PDS. The incised urethral side was marked with silk 3.0. The skin was closed with silk 3.0.
Four rabbits from each group were euthanized on days 7, 14, 21, 28, and 56. Part of the urethra tissue were taken by penectomy that was performed 1 cm proximally from the intervention area. Part of this was taken as a specimen. Urethral specimens were put in 10% formalin for 24 hours and then made into paraffin blocks. Some samples were put in Eppendorf tubes and then placed in a box containing dry ice for the frozen section. Specimens were then transferred to closet storage at −80°C and stored until they were examined using ELISA. Urethra tissue or specimens were examined by the Indonesian Eijkman Institute. Specific ELISA examinations for rabbits were carried out by laboratory staff under the supervision of researchers at the Eijkman Institute.
Protein extractions were performed and subsequent examinations of enzyme levels of MMP1 and TIMP1 using ELISA specific for rabbit done. Urethral stricture was assessed during urethral catheter insertion. Histopathological examination with H&E and total collagen examination with Masson trichrome were performed. Calculation of total collagen was done with ImageJ 1.46q. General linear model analysis was carried out to distinguish observation-time trends (days 7, 14, 21, 28, and 56) in the control and urethral stricture group.
Results
The average weight of all rabbits was 3.2 kg. Average rabbit weight in group 1 (urethral stricture group) was 3.22 kg, while that in group 2 (control group) was 3.24 kg. All rabbits lived until the end of each observation day group (days 7, 14, 21, 28, and 56). Euthanasia were done at the end of study.
H&E examinations
Urethral mucosa epithelium
On day 7, epithelialization of the control group and the urethral stricture group was completed. The difference between the two groups was that the control group had normal epithelia with pseudostratified columns, while the urethral stricture group had epithelial hyperplasia with pseudostratified columns. This situation was evident until day 56.
Subepithelium
On day 7, the control and urethral stricture groups both had chronic inflammatory cells, longitudinal muscle, and vascular congestion, but in the urethral stricture group, acute inflammatory cells were found. On days 14 and 21, neither the control nor the urethral stricture group had acute inflammatory cells, but there was evidence of chronic inflammatory cells, longitudinal muscle, and fibrosis. Differences in density were noted on day 21, where the urethral stricture group had denser fibrosis and vascular congestion. On days 28 and 56, neither the control nor urethral stricture group had acute inflammatory cells or vascular congestion, but there were chronic inflammatory cells and longitudinal muscle. The urethral stricture group had denser fibrosis on day 56.
Masson trichrome examination
On day 7, the control group appeared to have normal epithelia, and in subepithelia there was collagen connective tissue. On the next day, we observed density in collagen changes up to day 56. In the urethral stricture group, epithelialization appeared to be complete. Collagen connective tissue was found under the epithelium from day 7. Collagen was a major component of connective tissue below the epithelium on the next day. Density increased with time until day 56. Collagen also appeared to infiltrate the muscle. The percentage of total collagen was calculated using the program ImageJ 1.46q. In the control group, from day 7 to day 28, total collagen percentage ranged 51%–54% and up to 61% at day 56 (). In the urethral stricture group, from day 7 to day 56 there was an increase in total collagen percentage of 31%–61.5% (). The percentage of total collagen in the urethral stricture group was lower than in the control group on days 7–21. The percentage of total collagen in the urethral stricture group was higher than the control group on day 56. To assess whether there were differences in total collagen levels between groups, statistical tests were performed using general linear model techniques, and P=0.035 was obtained. Based on these results, because the data were not normally distributed, they are given as medians with ranges.
Table 1 Proportions of total collagen in control and urethral stricture groups by observation time
MMP1-examination results using ELISA
The level of MMP1 was examined using a rabbit-specific ELISA. In the control group, MMP1 in urethral tissue varied, with a tendency to stabilize (). In the urethral stricture group, MMP1 in the urethral tissue tended to decrease from day 7 to day 21. On the next day, the levels of MMP1 in urethral tissue tended to increase (). MMP1 in urethral tissue in the urethral stricture group was higher than the control group on days 7 and 14. while on days 21–28 MMP1 levels in urethra tissue in the urethral stricture group were lower than in the control group (). To assess whether there was any difference in urethra-tissue MMP1 levels between groups, a test was conducted using general linear model techniques, and P=0.035 was obtained.
Figure 1 MMP1 levels in the urethral stricture group were lower than the control group on days 21 and 28.
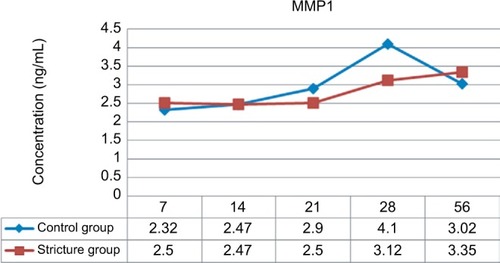
Table 2 MMP1 levels in urethra stricture and control groups by observation time
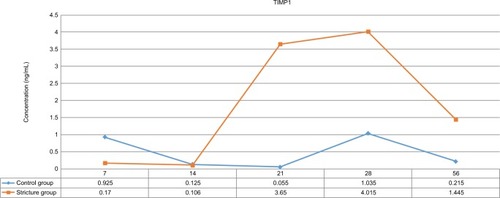
TIMP1-examination results using ELISA
TIMP1 levels were examined using rabbit-specific ELISA. In the control group, TIMP1 levels in urethra tissue were varied. In the urethral stricture group, TIMP1 levels in urethra tissue were likely to increase from day 7 to day 28. On the next day, TIMP1 levels in urethra tissue tended to decrease ().
Table 3 TIMP1 levels in urethral stricture and control groups by observation time
TIMP1 levels in urethra tissue in the urethral stricture group were lower than the control group on days 7 and 14. while on days 21, 28, and 56 TIMP1 levels in urethral stricture group tissue were higher than the control group (). To assess the difference in TIMP1 in urethra tissue between groups, a test was conducted using general linear model techniques, and P=0.037 was obtained.
Correlation of total collagen with MMP1 and TIMP1 levels
Assessment of the correlation between the percentage of total collagen by MMP1 and TIMP1 levels was done using the Spearman test, with the following results. There was a weak correlation between MMP1 and total collagen (r=0.561, P=0.1) and no correlation between TIMP1 and total collagen (r=0.307, P=0.188).
Discussion
Our study showed that the epithelialization process was complete in urethral stricture tissues and the epithelium became hyperplastic or metaplastic. For urethral stricture that occurs in humans due to trauma, the epithelium changes from simple stratified to stratified squamous.Citation4,Citation11 Stratified epithelium or metaplasia is very fragile and can be easily stretched during micturition, such that it can cause a fistula or even ulcers on the mucosa of the urethra. When the fistula or ulcers are formed, they will create fibrosis in the subepithelial layers.Citation4 Proximal epithelia found in urethral stricture will change to metaplastic through chronic pressure during micturition.Citation4,Citation12,Citation13
In subepithelial tissue in urethral stricture, there was an ongoing inflammatory process that extended to acute inflammatory cells encountered. In urethral stricture patients that do not receive urination diversion as treatment, there is also settled inflammation in urethral tissue.Citation14,Citation15 Fibrosis density also looked more solid, and conversely increased with total collagen percentage until the 56th day. Collagen quantification in this experiment was assessed using ImageJ program for Masson trichrome coloration. In other experiments, collagen quantification was done by calculation of hydroxyproline percentage. Da Silva et al found that tissue in urethral stricture had collagen levels up to 32.3%, higher than in normal urethral tissue.Citation7 Baskin et al found that there was no significant difference between collagen level in urethral stricture tissue and normal urethral tissue. However, they did not use an adequate control group in his study, there was a difference in composition between the two groups.Citation16 Difference in type I collagen:type III collagen ratio have also been found in some studiesCitation16 on urethral stricture tissue and normal urethral tissue.
There were differences in percentage of total collagen in the control group. The percentage of type I Collagen decreased until day 28, illustrated by changes in collagen composition. The percentage of type I collagen increased again on the 56th day. This showed that there was a change in extracellular matrix collagen. This finding was different from other studies, because the remodeling phase was ongoing. Other research has shown increases in type I collagen:type III collagen ratio of 1.9:1–4.8:1.Citation17 This then causes changes in urethral lumen diameter, obstruction, patient complaints, and urodynamic flow.Citation7 This study found no difference in percentage of type I collagen between the two groups.
MMPs are very important enzymes, due to their function in the development of healthy tissue, especially in remodeling the extracellular matrix. The main physiological function in the remodeling phase is to degrade the extracellular matrix. In this study, low levels of MMP1 were found. Day 7 showed an acute phase of trauma that was characterized by higher levels of MMP1. MMP1 was lower in the treated group than the control group, and decreased until day 21. Macromolecular structures found in MMP are needed during the developmental and morphogenesis phases, and also play a role in the architecture and homeostasis of tissue. This enzyme can degrade components of the entire extracellular matrix.Citation18 In this study, there was a difference in MMP1 levels between the treated and control groups. Decreased MMP1 activity was also found in pathological processes in other organs, such as the liver, lungs, and even coronary blood vessels. In this study, it was found that MMP1 decreased to low levels such that it almost could not be detected. This was similar to findings in other studies on fibroproliferative disease, such as hypertrophic cardiomyopathy.Citation19,Citation20 Maximum MMP1 levels were different from one another, depending on the organs that were involved. In this study, we found that the maximum MMP1 levels were reached on days 7 and 28. The maximum level that was found on day 7 showed that inflammatory processes were still happening, and the maximum level found on day 28 showed that inflammatory processes had finished. MMP1 increases were accompanied by increased TIMP1 levels.
Reparation and formation of connective tissue occurs when there is a balance between the degradation and replacement of extracellular matrix that is controlled by MMP1 and its inhibitors – TIMPs.Citation19,Citation21 TIMPs have a role in MMP1 function and also in the remodeling phase of the extracellular matrix. TIMPs also have antiapoptotic effects. TIMP1 serves as a potent inhibitor of MMP1 that organizes regulation of the extracellular matrix. Levels of TIMP1 to day 14 were lower, and increased until the end of the study. This showed that the formation of TIMP1 was slower. However the level was higher in the urethral stricture group on following days. This showed imbalanced extracellular matrix degradation. TIMP1 levels in the urethral stricture group were increased on day 21. This happened as a reaction to the increased level of MMP1 activity, similar to what has been found in other fibroproliferative disease. TIMP controls the activity of MMP by inhibiting its active form and also by inhibiting the activation of MMP. This inhibition is reversible. There are four types of TIMP: TIMP1, TIMP2, TIMP3, and TIMP4. TIMPs are produced by many cells in various conditions, though generally TIMPs can inhibit any type of MMP, but with various levels of inhibition. TIMP also has a role in cell development. There were differences in TIMP1 levels in the control group.
Connective tissue turnover was not only affected by expression levels or levels of MMP and TIMP but also by the ratio between MMP and TIMP.Citation20 Extracellular matrix deposition depends on the balance of activity of MMP and TIMP enzymes. The ratio between MMP1 and TIMP1 represents the balance of the extracellular matrix. MMP1:TIMP1 ratios decreased on days 21–56. This ratio is an important addition to MMP1 and TIMP1 levels. Further research is needed to determine the ideal ratio for the balance of the extracellular matrix of the urethra. Ulrich et al found that a change in balance in MMP and TIMP levels occurred without actual changes in either enzyme’s level.Citation22 Peters et al found that imbalanced levels between MMP and TIMP will cause an accumulation of extracellular matrix and thus decrease tissue compliance.Citation22 Fibrosis occurs as a result of the imbalanced levels of MMP and TIMP. Collagen synthesis that is faster than its degradation will cause an increased level of collagen. The fibrosis that happened in this study was relatively hypocellular. This may have happened because of incomplete degradation of extracellular matrix, and recently many studies have suggested inhibiting fibrotic changes by changing the levels of MMP.Citation23,Citation24 TIMP has a unique role, as it inhibits only MMP, but also activates MMP.
There was a correlation in subepithelial variables. A weak positive correlation between MMP1 levels and total collagen percentage was found. The decreased level of MMP1 will cause an increase in collagen levels and thus fibrosis. Fibrosis is not only caused by an increase level of collagen deposition but also by a decreased level of collagen degradation. No correlation was found between TIMP1 levels and percentage of total collagen, and nor was there any correlation between MMP1 and TIMP1 levels with percentage of collagen type I. The weak or no correlations found in this study do not mean that there are conflicts in results with previous research. This was an in vivo study, which distinguishes it from other studies. In in vivo study, there is interaction among many variables and growth factors that play a role in the healing process of the urethra, so the correlation is not linear. If correlation tests are performed, results may not be statistically significant. Animal studies have limitations on the number of samples. This is stipulated in regulations on animal welfare. The lower number of samples tends to provide statistically insignificant results.
Conclusion
Urethral stricture is not only caused by collagen decomposition but also by an imbalance in extracellular matrix degradation, which is marked by decreased MMP1 levels and MMP1:TIMP1 ratio and increased TIMP1 levels. Our study also provides an overview of the involvement of MMP1 and TIMP1 in urethral stricture. It is expected that numerous studies will aim to determine a variable whose role is to prevent the occurrence of urethral stricture, and hopefully be inspired by our study. Future research can be directed toward modulating MMP1 and MMP1:TIMP1 balance to reduce or prevent urethral stricture.
Ethics statement
Guidelines followed for the welfare of the animals were those of the eighth edition of the Guide for the Care and Use of Laboratory Animals (NRC 2011).
Acknowledgments
We would like to thank Professor Eef Hogervorst, Lough-borough University for her general support in this article.
Disclosure
The authors report no conflicts of interest in this work.
References
- JordanGHSchlossergSMSurgery of the penis and urethraCampbell-Walsh Urology9th edWeinAJKavoussiLRNovickACPartinAWPetersCAPhiladelphiaElsevier Inc200710541056
- TonkinJBJordanGHManagement of distal anterior urethral stricturesNat Rev Urol200961053353819736550
- SantucciRAJoyceGFWiseMMale urethral stricture diseaseJ Urol200717751667167417437780
- MundyARAndrichDEReview Article urethral stricturesBJU Int201062620553475
- PalminteriEBerdondiniEVerzePde NunzioCVitarelliACarmignaniLContemporary urethral stricture characteristics in the developed worldUrology201381119119723153951
- ChhetriRKShresthaGKJoshiHNShresthaRKManagement of urethral strictures and their outcomeNepal Med Coll J20091115819769228
- da-SilvaEASampaioFJDornasMCDamiaoRCardosoLEExtracellular matrix changes in urethral stricture diseaseJ Urol2002168280580712131371
- LovelockJDBakerAHGaoFHeterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblastsAm J Physiol Heart Circ Physiol20052882H461H46815650153
- DasuMRBarrowRESpiesMHerndonDNMatrix metalloproteinase expression in cytokine stimulated human dermal fibroblastsBurns200329652753112927975
- HinzBPhanSHThannickalVJGalliAPiallatMLBGabbianiGBiological perspectives the miofibroblast one function, multiple originsAm J Pathology200717018071816
- ScottTMFooteJEarly events in stricture formation in the guinea pig urethraUrol Int19803553343397423678
- WitteMBBarbulAGeneral principles of wound healingSurg Clin North Am19977735095289194878
- DelaiFZimingWTieCDevelopment and characterization of urethral stricture model in rabbitsJournal of Medical Colleges of PLA2010256351358
- CavalcantiAGCostaWSBaskinLSMcaninchJASampaioFJA morphometric analysis of bulbar urethral stricturesBJU Int2007100239740217617144
- SievertKDSelent-StierCWiedemannJIntroducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic studyJ Urol201218731101110922266012
- BaskinLSConstantinescuSCHowardPSBiochemical characterization and quantitation of the collagenous components of urethral stricture tissueJ Urol19931502 Pt 26426478326613
- FaydacıGTarhanFTuncerMComparison of two experimental models for urethral stricture in the anterior urethra of the male rabbitUrology2012801225.e722225
- LopesJFSchnedAEllsworthPICendronMHistological analysis of urethral healing after tubularized incised plate urethroplastyJ Urol200116631014101711490287
- WoessnerJFMatrix metalloproteinases and their inhibitors in connective tissue remodelingFaseb J199158214521541850705
- CambraneroFMarinFRoldanVRomeroDHValdesMLipGHBiomarker of patophysiology in cardiomiopathy: implication for clinical management and prognosisEur Health J200930139151
- LanHYDiverse roles of TGF-β/Smads in renal fibrosis and inflammationInt J Biol Sci2011771056106721927575
- UlrichDLichteneggerFEblenkampMRepperDPalluaNMatrix metalloproteinases, tissue inhibitors of metalloproteinases, aminoterminal propeptide of procollagen type III, and hyaluronan in sera and tissue of patients with capsular contracture after augmentation with Trilucent breast implantsPlast Reconstr Surg2004114122923615220598
- IlleperumaRPRyuMHKimKYTilakaratneWMKimJRelationship of fibrosis and the expression of TGF-β1, MMP-1, and TIMP-1 with epithelial dysplasia in oral submucous fibrosisOral Medicine & Pathology20101512128
- YangLLiuRWangXHeDImbalance between matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinase-1 (TIMP-1) contributes to bladder compliance changes in rabbits with partial bladder outlet obstruction (PBOO)BJU Int20131124E391E39723305285