Abstract
Buprenorphine and buprenorphine–naloxone fixed combinations are effective for managing patients with opioid dependence, but constipation is one of the most common side effects. Evidence indicates that the rate of constipation is lower when patients are switched from sublingual buprenorphine–naloxone tablets or films to a bilayered bioerodible mucoadhesive buccal film formulation, and while the bilayered buccal film promotes unidirectional drug flow across the buccal mucosa, the mechanism for the reduced constipation is unclear. Pharmacokinetic simulations indicate that chronic dosing of sublingually administered buprenorphine may expose patients to higher concentrations of norbuprenorphine than buprenorphine, while chronic dosing of the buccal formulation results in higher buprenorphine concentrations than norbuprenorphine. Because norbuprenorphine is a potent full agonist at mu-opioid receptors, the differences in norbuprenorphine exposure may explain the observed differences in treatment-emergent constipation between the sublingual formulation and the buccal film formulation of buprenorphine–naloxone. To facilitate the understanding and management of opioid-dependent patients at risk of developing opioid-induced constipation, the clinical profiles of these formulations of buprenorphine and buprenorphine-naloxone are summarized, and the incidence of treatment-emergent constipation in clinical trials is reviewed. These data are used to propose a potential role for exposure to norbuprenorphine, an active metabolite of buprenorphine, in the pathophysiology of opioid-induced constipation.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Maintenance treatment of opioid-dependent patients typically involves a combination of psychosocial approaches (eg, counseling, prevention education, and recovery support services) and office-based pharmacological substitution therapy with an oral transmucosal agent. Options include buprenorphine or fixed combinations of buprenorphine and naloxone (BN) that are supplied in three formulations: sublingual tablets or single-layered sublingual films for sublingual or buccal use (sublingual buprenorphine–naloxone [SLBN], Suboxone,® Indivior Inc., Richmond, VA, USA) and bilayered bioerodible mucoadhesive buccal films (buccal buprenorphine–naloxone [BBN], Bunavail,® BioDelivery Sciences International, Inc., Raleigh, NC, USA).Citation1,Citation2 These BN agents have been shown to improve outcomes in opioid-dependent patients,Citation3–Citation5 and while they are generally safe and well tolerated, with predictable side-effect profiles, as with all opioids, constipation is among the most common side effects.Citation5
The mechanisms of opioid-induced constipation (OIC) are complex, involving mu-opioid-mediated effects on the enteric nervous system that result in decreased intestinal fluid secretion and increased fluid absorption, as well as decreased muscle contraction and motility of the small intestine and colon, resulting in increased colonic transit time.Citation6–Citation8 Endogenous opioids, including endorphins, enkephalins, and dynorphins, have been shown to reduce acetylcholine-mediated intestinal motor and secretory activities.Citation9–Citation11 Experimental data also indicate that mu-opioid receptors in the brain may also significantly delay intestinal transit.Citation12,Citation13 Local effects on mu-opioid receptors in the intestine may also impact intestinal functions. Thus, the intraluminal administration of opioid receptor antagonists (eg, naloxone, N-methylnaloxone), which are undetectable in the general circulation due to insufficient absorption from the intestinal lumen, may prevent intravascular morphine from depressing motility.Citation14 These effects, together with the increased resting anal sphincter tone and decreased reflex relaxation of the anal sphincter produced by exogenous opioids,Citation15 result in symptoms of OIC.
The significant clinical consequence of the development of OIC is such that patients may reduce or stop their opioid medication to achieve a positive impact on their quality of life,Citation3,Citation11,Citation16,Citation17 and OIC is one of the most common reasons patients avoid or abandon therapeutic opioid use.Citation18,Citation19 Although clinical experience suggests that most opioid-dependent patients experience mild or moderate symptoms that can be managed with over-the-counter laxatives, the potentially serious impact on quality of life, secondary symptoms, and complications of unmanaged constipation underscore its clinical importance ().Citation17,Citation20,Citation21
Table 1 Secondary symptoms and complications of unmanaged constipation
Methods
To facilitate the understanding of OIC and management of opioid-dependent patients at risk of developing OIC with the goal of preventing the problem, this paper briefly summarizes the clinical profiles of SLBN and BBN and reviews published evidence of the incidence of treatment-emergent constipation associated with these therapies. Separate searches were performed on PubMed for “sublingual buprenorphine naloxone” and “buccal buprenorphine naloxone”, with the filters set to include only clinical trials. Of the records related to SLBN (N=36) or BBN (N=3), only one clinical study with each formulation reported constipation rates.
Buprenorphine
Initially developed for the treatment of pain, buprenorphine is a semisynthetic partial agonist at mu-opioid receptor and an antagonist at kappa opioid receptor sites.Citation22,Citation23 Buprenorphine is widely used to treat opioid-dependent patients because the reward effects are milder than those of full mu-opioid agonistsCitation24, its binding to mu-opioid receptors is not easily displaced by other opioids, and it has a lower risk of abuse and dose-limited effects on respiratory depression.Citation25,Citation26 Oral dosing of buprenorphine is not feasible due to extensive first-pass liver metabolism, which markedly limits its bioavailability. In contrast, oral transmucosal administration is associated with bioavailability up to 50%.Citation27
Buprenorphine has been demonstrated to be safe and effective for use in induction, stabilization, and long-term maintenance of opioid-dependent patients, as measured by reduced consumption of illicit opioids.Citation28 A recent Cochrane review found it to be an effective medication in the maintenance treatment of heroin dependence, retaining people in treatment at any sublingual dose >2 mg and suppressing illicit opioid use when administered at sublingual doses ≥16 mg.Citation28 Compared with methadone, buprenorphine substitution treatment has been shown to decrease hospital admissions, morbidity, and mortality,Citation3,Citation4 with potentially less sedation.Citation29 It has a lower abuse potential, carries less stigma, and allows for greater flexibility in treatment than methadone.Citation30 In the gastrointestinal (GI) tract, buprenorphine inhibits acetylcholine-induced ileal muscle contraction, and subcutaneous injections in mice at doses ranging from 1.0 to 20.0 mg/kg can slow GI transit by <50%.Citation31 About 8% of subjects receiving buprenorphine reported an adverse event of constipation after 4 weeks of sublingual treatment with the medication, significantly more than placebo (~3%, P=0.03, ).Citation5
Table 2 Gastrointestinal adverse events (%) after 4 weeks of treatment with the sublingual tablet formulations of buprenorphine–naloxone (16/4 mg) or buprenorphine (16 mg)
Buprenorphine combination products
Sublingual buprenorphine–naloxone tablets
SLBN has been approved for the office-based management of patients with opioid dependence since 2002.Citation32 Its efficacy and safety in opioid-dependent patients have been documented in published studies.Citation5,Citation26,Citation30,Citation33 In a controlled clinical trial, it has significantly outperformed placebo for proportion of urine samples that were negative for opiates and the proportion of subjects with opiate craving (P<0.001 for both comparisons).Citation5 Despite an overall rate of adverse events that was roughly similar to placebo, as shown in , the incidence of constipation in SLBN-treated subjects was four times greater than placebo-treated subjects (P=0.03).Citation5
Buccal buprenorphine–naloxone films
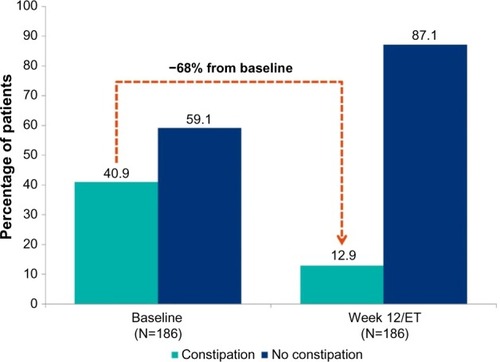
BBN is a novel transmucosal buprenorphine–naloxone combination product formulated as a small, thin, bilayered dissolvable film that adheres to the buccal mucosa and provides approximately twice the bioavailability of buprenorphine compared with SLBN tablets containing double the buprenorphine dose.Citation2,Citation34 Mechanistically, BBN can be distinguished from buccally administered SLBN by the presence of a backing layer, which promotes unidirectional flow across the buccal mucosa; SLBN for buccal administration is a single-layer film without a backing layer.Citation1,Citation2 In an open-label study in 249 adult opioid-dependent subjects stabilized on SLBN tablets or film at a dose of 16 mg/day and switched to a mean dose of 8 mg BBN for 12 weeks, 92% of subjects had urine samples that were negative for nonprescribed opioids. As shown in , of the 186 subjects who completed a checklist of common side effects (asking for presence or absence) of BN at baseline and day 84, 41% (76/186) reported constipation at the time of SLBN discontinuation, and before treatment with BBN, and 13% (24/186) reported constipation after 12 weeks of BBN treatment, a risk reduction of 68% (52/76; 95% confidence intervals 60%–77%).Citation34 Approximately 3% (7/249) of subjects on BBN reported treatment-emergent constipation over the course of the 12-week study.Citation34
Figure 1 Constipation* at baseline and at week 12 in patients converted from SLBN to BBN (N=186).
Abbreviations: BBN, buccal buprenorphine-naloxone film; ET, early termination; SLBN, sublingual buprenorphine-naloxone tablets or films.
The 2:1 buprenorphine dose conversion ratio from the mean baseline SLBN dose to the mean BBN dose at the end of the study aligns with results from a bioequivalence study in healthy volunteers. In that single-dose, crossover pharmacokinetic study, which compared the rate and extent of BN exposure from 4.2/0.7 mg BBN with 8/2 mg SLBN tablets in 80 healthy adults who had been given naltrexone, buprenorphine exposure from BBN was bioequivalent with SLBN (90% confidence intervals for maximum plasma concentration [Cmax], area under the plasma concentration-time curve from time 0 to the last measurable concentration [AUClast], and AUC extrapolated to infinity [AUCinf] ranged from 88% to 118%), while exposure to naloxone and norbuprenorphine were, respectively, 33% and 40% less than with SLBN ().Citation35
Table 3 Systemic exposure of 4.2/0.7 mg BBN film and 8/2 mg SLBN tablet (N=80)
Based on the single-dose data from the study comparing SLBN (8 mg) with BBN (4.2 mg) in healthy subjects, plasma buprenorphine and norbuprenorphine concentration exposures (AUC0–24) were modeled for steady-state conditions during daily dosing with SLBN 16 mg or BBN 8 mg. As shown in , projected norbuprenorphine exposure with SLBN was nearly double that of BBN, while the projected AUC0–24 exposures for buprenorphine were roughly equivalent with both formulations. Importantly, the projected steady-state plasma norbuprenorphine concentrations with SLBN exceeded the steady-state buprenorphine concentrations by ~30%, whereas projected plasma norbuprenorphine concentrations with BBN were nearly 30% lower than buprenorphine. The difference is presumed to be due to higher transmucosal bioavailability and lower amounts of buprenorphine exposed to first-pass liver metabolism.
Table 4 Predicted buprenorphine and norbuprenorphine exposure with SLBN tablets and BBN films: daily dosing and steady-state conditions
Potential role of norbuprenorphine in constipation
It is unclear why the rates of constipation decreased in subjects switched from SLBN to BBN. While subcutaneous naloxone (0.8 mg every 6 hours) has been found to accelerate transit in the colon of healthy human volunteers,Citation36 and evidence suggests it can reverse idiopathic chronic constipation, benefit patients with intestinal pseudo-obstruction and constipation-predominant irritable bowel syndrome,Citation37 and improve the symptoms of OICCitation38,Citation39 when given at high doses orally (eg, 10 mg bid in the constipation-predominant irritable bowel syndrome trial), it seems unlikely to have played a role in the different outcomes with respect to constipation in the study of opioid-dependent subjects stabilized on SLBN and switched to BBN. Not only do both study drugs contain naloxone, but also the rate and extent of naloxone exposure with BBN was one-third lower than with SLBN. Moreover, another orally administered, poorly bioavailable mu-opioid antagonist, naltrexone (50 mg) or subcutaneous methylnaltrexone (0.3 mg/kg), did not accelerate colonic transit in healthy volunteers.Citation40,Citation41 All of these data argue against a role for the naloxone in BBN as the reason for improved bowel function.
Rather, the steady-state plasma concentration projections observed in this study suggest that the lower incidence of constipation with BBN relative to SLBN may be due to reduced exposure of enteric mu-receptors to norbuprenorphine. In randomized studies in opioid-dependent subjects, mean steady-state plasma concentrations of this major peripherally active metabolite of buprenorphine have matched or surpassed the parent compound following sublingual administration.Citation42,Citation43 These data are therefore consistent with the hypothesis that, as a potent full agonist at mu-receptors,Citation44 norbuprenorphine contributes to the overall peripheral pharmacological effects of buprenorphine in the regulation of multiple GI processes in vivo. Its lack of penetration of the blood–brain barrier into the central nervous system suggests little central nervous system contribution of norbuprenorphine to the GI processes.Citation45
Implications for clinical practice
These data indicate that the lower rate of constipation observed with the BBN formulation may be the result of reduced exposure to norbuprenorphine. It therefore appears reasonable to query opioid-dependent patients who are at risk of or report OIC symptoms and possible effects on quality of life in order to enhance adherence to medication regimens. In addition, since there is no significant difference between SLBN tablets and single-layered films with regard to plasma levels of naloxone and norbuprenorphine,Citation46 and treatment-emergent constipation rates with SLBN films across 19 pharmacokinetic studies in healthy volunteers (11%)Citation46 are similar to the clinical study with SLBN tablets (12%),Citation5 if management of OIC is needed for patients who are being treated with SLBN, strategies to minimize exposure to norbuprenorphine may be considered. Evidence suggests that BBN provides adequate symptom control with respect to opioid dependence and is well tolerated, with low reported rates of constipation, good adherence, and favorable patient acceptance.Citation34
Conclusion
Buprenorphine is an effective intervention for the management of opioid dependence, but patients are at risk for a range of GI symptoms, including OIC. Pharmacokinetic simulations indicate that chronic dosing of sublingually administered agents may expose patients to higher concentrations of norbuprenorphine than buprenorphine due to swallowing of unabsorbed buprenorphine, whereas chronic dosing of the bilayered bioerodible mucoadhesive buccal formulation, which provides higher bioavailability and is efficiently absorbed across the buccal mucosa, results in higher buprenorphine concentrations than norbuprenorphine. These data support the hypothesis that exposure to norbuprenorphine, an active metabolite of buprenorphine, plays a role in the pathophysiology of OIC and that differences in norbuprenorphine exposure may explain the observed differences in constipation between SLBN and BBN.
Acknowledgments
The authors wish to thank Sarah DeRossett and Susan Kerls for their assistance in preparing the manuscript. Medical writing services were provided by Christopher Caiazza.
Disclosure
MC is supported by NIH RO1 DK92179. BioDelivery Sciences, Inc., developer of bioerodible mucoadhesive buprenorphine–naloxone buccal film, has provided research support to LRW and MC. AF is a retired employee of BioDelivery Sciences, Inc. The authors report no other conflicts of interests in this work.
References
- Suboxone [prescribing information] Available from: http://www.suboxone.com/content/pdfs/SuboxonePI.pdfAccessed November 17, 2015
- Bunavail [prescribing information] Available from: http://www.bdsi.com/siteres.aspx?resid=5a738443-a797-41cd-a39a-a8deb2a4a585Accessed November 17, 2015
- BellJTrinhLButlerBRandallDRubinGComparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatmentAddiction20091041193120019563562
- JonesESMooreBASindelarJLO’ConnorPGSchottenfeldRSFiellinDACost analysis of clinic and office-based treatment of opioid dependence: results with methadone and buprenorphine in clinically stable patientsDrug Alcohol Depend20099913214018804923
- FudalaPJBridgeTPHerbertSBuprenorphine/Naloxone Collaborative Study GroupOffice-based treatment of opiate addiction with a sublingual tablet formulation of buprenorphine and naloxoneN Engl J Med200334994995812954743
- BagnolDMansourAAkilHWatsonSJCellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tractNeuroscience1997815795919300443
- McKayJSLinakerBDTurnbergLAInfluence of opiates on ion transport across rabbit ileal mucosaGastroenterology1981802792846256253
- FickelJBagnolDWatsonSJAkilHOpioid receptor expression in the rat gastrointestinal tract: a quantitative study with comparison to the brainBrain Res Mol Brain Res199746189191072
- RangHPDaleMMRitterJMAnalgesic drugsPharmacology199913579603
- ThomasJOpioid-induced bowel dysfunctionJ Pain Symptom Manage20083510311317981003
- YuanCSFossJFO’ConnorMMossJRoizenMFGut motility and transit changes in patients receiving long-term methadone maintenanceJ Clin Pharmacol1998389319359807974
- ThörnSEWattwilMLindbergGSäweJSystemic and central effects of morphine on gastroduodenal motilityActa Anaesthesiol Scand1996401771868848916
- ManaraLBianchettiAThe central and peripheral influences of opioids on gastrointestinal propulsionAnnu Rev Pharmacol Toxicol1985252492733890704
- ReberPBrenneisenRFlogerziBBatistaCNetzerPScheurerUEffect of naloxone-3-glucuronide and N-methylnaloxone on the motility of the isolated rat colon after morphineDig Dis Sci20075250250717211696
- HolzerPOpioids and opioid receptors in the enteric nervous system: from a problem in opioid analgesia to a possible new prokinetic therapy in humansNeurosci Lett200436119219515135926
- ManciniIBrueraEConstipation in advanced cancer patientsSupport Care Cancer199863563649695203
- BenyaminRTrescotAMDattaSOpioid complications and side effectsPain Physician2008112 SupplS105S12018443635
- MeissnerWSchmidtUHartmannMKathRReinhartKOral naloxone reverses opioid-associated constipationPain20008410510910601678
- ThorpeDMManagement of opioid-induced constipationCurr Pain Headache Rep2001523724011309211
- KurzASesslerDIOpioid-induced bowel dysfunction: pathophysiology and potential new therapiesDrugs20036364967112656645
- HolzerPTreatment of opioid-induced gut dysfunctionExpert Opin Investig Drugs200716181194
- MelloNKMendelsonJBehavioral pharmacology of buprenorphineDrug Alcohol Depend1985142833033888577
- PergolizziJAloisiAMDahanACurrent knowledge of buprenorphine and its unique pharmacological profilePain Pract20101042845020492579
- LutfyKCowanABuprenorphine: a unique drug with complex pharmacologyCurr Neuropharmacol2004239540218997874
- MelloNKWalshSPrestonKStitzerMConeEBigelowGClinical pharmacology of buprenorphine: ceiling effects at high dosesClin Pharmacol Ther1994555695808181201
- StrainECPrestonKLiebsonIBigelowGBuprenorphine effects in methadone-maintained volunteers: effect at two hours after methadoneJ Pharmacol Exp Ther19952726286387853176
- YokellMAZallerNDGreenTCRichJDBuprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international reviewCurr Drug Abuse Rev20114284121466501
- JohnsonREJaffeJHFudalaPJA controlled trial of buprenorphine treatment for opioid dependenceJAMA1992267275027551578593
- MattickRPBreenCKimberJDavoliMBuprenorphine maintenance versus placebo or methadone maintenance for opioid dependenceCochrane Database Syst Rev20142CD00220724500948
- MaugerSFraserRGillKUtilizing buprenorphine-naloxone to treat illicit and prescription-opioid dependenceNeuropsychiatr Dis Treat20141058759824741316
- ZhouPLLiYLYanLDEffect of thienorphine on intestinal transit and isolated guinea-pig ileum contractionWorld J Gastroenterol2013191444145023539497
- U.S. Food and Drug AdministrationSuboxone Approval History Available from: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/20732,20733ltr.pdf
- FinchJWKamienJBAmassLTwo-year experience with buprenorphine-naloxone (Suboxone) for maintenance treatment of opioid dependence within a private practice settingJ Addict Med2007110411021768942
- SullivanJGWebsterLNovel buccal film formulation of buprenorphine-naloxone for the maintenance treatment of opioid dependence: a 12-week conversion studyClin Ther2015371064107525823919
- VasishtNStarkJBaiSAFinnABuprenorphine/naloxone buccal film has a relative buprenorphine bioavailability approximately twice that of buprenorphine/naloxone sublingual tablet2014 ASAM 45th Annual Medical-Scientific Conference, Poster #10April 10–13, 2014Orlando, FL
- KaufmanPNKrevskyBMalmudLSRole of opiate receptors in the regulation of colonic transitGastroenterology198894135113562834257
- DeHaven-HudkinsDLDeHavenRNLittlePJTechnerLMThe involvement of the µ-opioid receptor in gastrointestinal pathophysiology: therapeutic opportunities for antagonism at this receptorPharmacol Ther200811716218718022696
- DePriestAZMillerKOxycodone/naloxone: role in chronic pain management, opioid-induced constipation, and abuse deterrencePain Ther2014311525135384
- SmithKHoppMMundinGNaloxone as part of a prolonged release oxycodone/naloxone combination reduces oxycodone-induced slowing of gastrointestinal transit in healthy volunteersExpert Opin Investig Drugs201120427439
- Foxx-OrensteinAECamilleriMSzarkaLADoes co-administration of a non-selective opiate antagonist enhance acceleration of transit by a 5-HT4 agonist in constipation-predominant irritable bowel syndrome? A randomized controlled trialNeurogastroenterol Motil20071982183017539894
- WongBSRaoASCamilleriMThe effects of methylnaltrexone alone and in combination with acutely administered codeine on gastrointestinal and colonic transit in healthAliment Pharmacol Ther20103288489320839388
- KuhlmanJJJrLevineBJohnsonREFudalaPJConeEJRelationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphineAddiction1998935495599684393
- BrownSMHoltzmanMKimTKharaschEDBuprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically activeAnesthesiology20111151251126022037640
- YassenAKanJOlofsenESuidgeestEDahanADanhofMPharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in ratsJ Pharmacol Exp Ther200732159860717283225
- HuangPKehnerGBCowanALiu-ChenLYComparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonistJ Pharmacol Exp Ther200129768869511303059
- Commonwealth of AustraliaAustralian Public Assessment Report for Buprenorphine/Naloxone Available from: https://www.tga.gov.au/auspar/auspar-buprenorphine-naloxoneAccessed March 9, 2016
