Abstract
Addiction is a chronic disorder in which consumption of a substance or a habitual behavior becomes compulsive and often recurrent, despite adverse consequences. Substance p (SP) is an undecapeptide and was the first neuropeptide of the neurokinin family to be discovered. The subsequent decades of research after its discovery implicated SP and its neurokinin relatives as neurotransmitters involved in the modulation of the reward pathway. Here, we review the neurokinin literature, giving a brief historical perspective of neurokinin pharmacology, localization in various brain regions involved in addictive behaviors, and the functional aspects of neurokinin pharmacology in relation to reward in preclinical models of addiction that have shaped the rational drug design of neurokinin antagonists that could translate into human research. Finally, we will cover the clinical investigations using neurokinin antagonists and discuss their potential as a therapy for drug abuse.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Drugs of abuse such as opioids, cocaine, amphetamines, alcohol, and nicotine affect the reward pathway in unique ways, leading to the potential of addiction. In the United States, the cost of substance abuse to society is more than $700 billion per year, necessitating new strategies in the management of addiction.Citation1 Particularly alarming is the rate of deaths due to heroin overdose, which has skyrocketed since 2010. The National Institute on Drug Abuse (NIDA) and Centers for Disease Control and Prevention attribute this increase in heroin usage and mortality to an inadvertent consequence of reducing the availability of prescription painkillers.Citation2 While abstinence from drugs of abuse seems like the most logical strategy, this has proven to be only an illusory goal. Therefore, the FDA and NIDA have planned to change the requirements for new therapies designed as deterrents for drugs of abuse; a reduction in the use of drugs of abuse over the long term may be the more appropriate requirement for FDA approval. Neurokinins are a family of peptide transmitters involved in the reward pathway for each of the drugs of abuse, giving researchers a target to design new medications aimed at reducing the addictive profile of said drugs of abuse.
Substance p (SP) was the first member of the neurokinin family of peptides to be isolated, initially from equine intestine and brain in 1931, and shown to act as a vasodepressor.Citation3 The subsequent decades of research implicated SP and its neurokinin cousins in numerous central nervous system (CNS) disorders including anxiety, depression, migraine, schizophrenia, and addiction. Here, we review the basic and clinical science of the last 80 years that have helped shape our current understanding of how neurokinins specifically alter the neuronal pathway involved in addiction. We will then introduce potential neurokinin-directed therapies that may have efficacy in clinical practice relating to addiction.
Historical overview of neurokinins
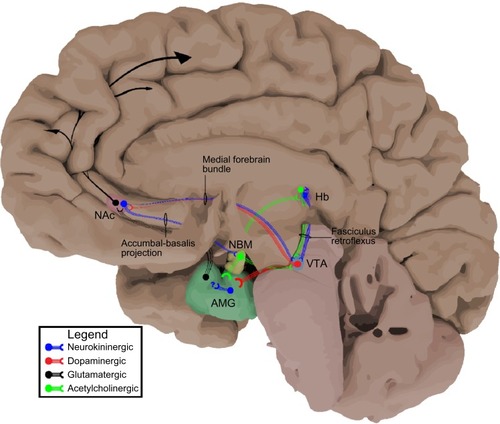
In 1931, Ulf Von Euler and John Gaddum were on an expedition of sorts, in search of the distribution of acetylcholine in various equine organs. They came across a previously unidentified substance that they were able to concentrate in a powdered form, thus naming it “substance p”.Citation4 By the 1950s, SP was well accepted as a polypeptide located in the CNS, particularly concentrated in the thalamus, hypothalamus, basal ganglia, and tegmentum in addition to the dorsal root ganglia of the peripheral nervous system, mediating nociceptive transmission from the primary afferent.Citation5,Citation6 However, it was not until 1970 when Chang and Leeman were able to isolate, characterize, and sequence SP as an 11-amino-acid peptide that the neurokinin field really evolved.Citation7 With the newly available antibodies to SP, immunohistochemical techniques allowed more precise characterization of SPergic neurons in the CNS. Even with the rather rudimentary techniques available in the 1970s (ie, no optogenetics), SPergic projections were specifically found traveling from habenula (Hb) to the interpeduncular nucleus (IPN) and ventral tegmental area (VTA) via the fasciculus retroflexus,Citation8 the striatum to the substantia nigra via the striato-nigral pathway,Citation9 and the nucleus accumbens (NAc) to the ventral pallidum (specifically the nucleus basalis magnocellularis),Citation10 with further evidence supporting SP as a neurotransmitter with vesicular release ().Citation11 At roughly the same time, the importance of dopamine in the mesolimbic and mesocortical pathways on drug seeking behavior was coming to fruition.Citation12,Citation13 Most importantly, it seemed that dopaminergic projections from the VTA to the NAc and other regions facilitated reward.Citation14 With the basic topography of SP signaling in place, the next step was determining its functionality in the mesolimbic system. Indeed, it was shown that SP could directly activate dopaminergic neurons in the VTA,Citation15 but how was SP signaling to the postsynaptic neuron and were there other ligands of the same family in humans?
Figure 1 Neurokininergic projections in the reward pathway.
Abbreviations: Hb, habenula; VTA, ventral tegmental area; IPN, interpeduncular nucleus; NAc, nucleus accumbens; NBM, nucleus basalis magnocellularis or nucleus basalis of Meynert; AMG, amygdala; SP, substance p.
Basic neurokinin pharmacology
The ensuing era of neurokinin research revolved around the characterization of two more human neurokinins and their receptors. In 1983, neurokinin A and neurokinin B (NKA and NKB, respectively) were discovered and characterized, putting them in the same family as SP (the tachykinin familyCitation16) based on similar −CO2 terminal sequences.Citation17 By 1984, all three neurokinin receptors had been proposed,Citation18 followed by the permanent nomenclature: neurokinin-1 receptor (NK1R), neurokinin-2 receptor (NK2R), and neu-rokinin-3 receptor (NK3R) in 1986.Citation19 Each ligand can bind and activate each receptor; however, they all have their preference owing to a graded affinity: SP preferentially activates NK1R, NKA preferentially activates NK2R, and NKB preferentially activates NK3R ().Citation20 Cellular and molecular experiments linked neurokinin receptor activation to inositol phospholipid hydrolysisCitation21 (later referred to as Gq coupling). Following receptor activation, the NK1R is rapidly internalized, leading to visual NK1R+ endosomal varicosities in the dendrites and somata of neurons, disappearing an hour later.Citation22 This discovery made future cellular investigations into neurokinin pharmacology easier to trace.
Table 1 IC50 values of the three primary endogenous neurokinin ligands for the three primary neurokinin receptors
Receptor localization is important in determining how the pharmacology affects a local neuronal circuit. Unfortunately for neuroscientists, the reward circuitry and accompanying pharmacology is quite complex. Further complicating the picture, G-protein-coupled receptors like the neurokinin family of receptors can act in both rapid (Ca2+ or Na+ induced cell activation) and delayed (transcriptional) ways. Validating this notion, the activation of the neurokinin family of receptors will ultimately lead to an increase in intracellular Ca2+, thus potentiating neuronal mechanisms of firing an action potential, in addition to activating the nuclear translocation of certain transcription factors including NF-κB.Citation23 Additionally, recent evidence implicates swift activation of a Na+ leak channel, NALCN, as well as the closure of G-protein-linked inwardly rectifying K+ channels in the rapid SP-induced activation of neuronal action potentials.Citation24,Citation25
For years, neurokinin antagonist studies were made very difficult by the lack of penetration of the available ligands (ie, only peptidergic antagonists were available).Citation26 The breakthrough came in 1991 when scientists at Pfizer discovered the first nonpeptide molecule with classical competitive antagonism at the NK1R.Citation27 The identification of this compound led to the discovery of even more selective, structurally diverse, nonpeptidic NK1R antagonists at other pharmaceuticals, namely, Merck’s MK-869, which later became known in the clinic as the antiemetic aprepitant (EMEND®, Merck & Co, NJ, USA).Citation28 While considerable time and money went into the possibility of aprepitant working as a standalone analgesic and/or antidepressant (without much success),Citation29 the prospect of an NK1R antagonist for the treatment of addiction still remains viable. The rest of this review will cover the specifics of neurokinin pharmacology in addiction.
Neurokinins in the reward pathway
While SPergic cell bodies have been found in a number of CNS foci including the septal complex, nucleus tractus diagonalis (diagonal band of Broca), NAc, and Hb,Citation30 with projections to various regions including the nucleus basalis magnocellularis, VTA, and IPN, each constituent seems to have a unique function in the limbic loop. A recent report links NK1R activation to µ-opioid receptor recycling, offering direct evidence for a neurokinin-mediated opioid resensitization.Citation31 In fact, the neurokinin system is consistently found colocalized or in other ways affecting endogenous opioid, dopamine, and serotonergic signaling, thereby exerting its effect on affective and drug-seeking behavior.Citation32,Citation33
Ventral tegmental area
Important to the reward pathway and the study thereof, the VTA most notably sends dopaminergic projections to the NAc where dopamine release triggers euphoria or positive reinforcement.Citation34 Critically, intra-VTA injections of both an NK1R agonist and NK3R agonist facilitate dopamine release in the NAc.Citation35 Indeed, autoradiographic studies using the radiolabeled NK3R agonist [3H]senktide confirmed the presence of NK3Rs in the VTA in addition to the IPN and Hb.Citation36 This complemented the previously studied NK1R localization in the VTA among other CNS locations.Citation37 While in vitro electrophysiologic studies in the VTA demonstrated NK3Rs may mediate more of the excitatory effects of dopaminergic neurons while leaving a role for SP out,Citation38 in vivo electrophysiologic recordings in the VTA confirmed that systemic administration of an NK1R antagonist was sufficient to block dopamine cell firing.Citation39 In addition, studies demonstrated an increased firing rate of VTA dopaminergic neurons due to the application of SP.Citation40 These apparent discrepancies in dopaminergic activity and neurokinin pharmacology may be in part due to the marked receptor heterogeneity of the VTA.Citation41
The expression of various neurokinin receptor subtypes is rather diverse. For example, while there is somatodendritic expression of the NK1R in the cell membrane of dopaminergic and nondopaminergic neurons of the VTA,Citation42 NK3Rs are often found in the cytoplasm of dopaminergic and nondopaminergic neurons of the VTA.Citation43 Additionally, the NK3Rs found in the plasma membrane are frequently extrasynaptic. Furthermore, VTA glia exhibit substantially more NK3Rs than NK1Rs, suggesting the importance of the immune cells in the reward pathway (see “Involvement of the immune system in addiction” for more details). Overall, the expression of NK3Rs in the VTA is twice that of NK1Rs.Citation44 Interestingly, NK3Rs, but not NK1R or NK2Rs, were found within the nuclear envelope of projection neurons of the VTA. This suggests the possibility of direct NK3R involvement in gene transcription. Indeed, ligand-dependent and -independent nuclear translocation of the NK3R in the VTA has been observed.Citation45,Citation46 The significance of these nuclear events in the reward pathway has not been fully elucidated.
The literature on the NK2R in the VTA is sparse; however, it has been shown that intravenous infusion of the selective NK2R antagonist SR-48968 did not alter basal dopaminergic firing rate in rats.Citation47 Peculiarly, the acute administration of SR-48968 intraperitoneal (ip) increased the number of spontaneously active VTA DA neurons; however, this may be due to dosing differences or possible pharmacologically active metabolites. The intracellular interaction between neurokinin receptors has also been studied. When expressed by the same cell, NK1R activation sequesters β-arrestins in endosomes, impeding ligand-dependent NK3R endocytosis.Citation48 The paucity of information regarding how heterologous interactions between neurokinin receptors affects the reward pathway indicates the necessity of future research in addiction.
Some of the earliest investigations into neurokinin’s ability to functionally impel the reward pathway came in 1985 when Staubli and HustonCitation49 showed that injection of SP into the medial forebrain bundle, the neuronal tract that connects the VTA to the NAc, resulted in positive conditioned place preference (CPP). With regard to specific drugs of abuse, microinjection of the SP analog DiMe-C7 induced reinstatement of cocaine-seeking behavior, which could be significantly reduced by the D1 receptor antagonist SCH23390.Citation50
Nucleus accumbens
The NAc is often regarded as the limbic–motor interface receiving inputs from the VTA and amygdala, among other regions, and sending projections to the cortex, ventral pallidum, globus pallidus, and reciprocal projections to the VTA and amygdala.Citation51 One study provided evidence that the NAc required input from both the VTA and the basolateral amygdala for excitation of NAc efferents.Citation52
SP injected into the NAc by itself increases concentrations of extracellular DA but does not induce positive CPP.Citation53 An SP antibody injected into the NAc prevents amphetamine-induced increase of extracellular DA in the NAc.Citation54 Likewise, NAc administration of the NK1R antagonist L-733,060 significantly diminishes cocaine-induced DA release.Citation55
Nucleus basalis magnocellularis-substantia innominata
Evidence for SPergic fibers projecting to the nucleus basalis magnocellularis (nucleus basalis of Meynert) comes from simple light microscopic images and immunohistochemical staining.Citation56 Accordingly, the injection of SP or the C-terminal fragment of SP into the nucleus basalis magnocellularis resulted in a positive CPP, which the authors attributed to the positive reinforcing effects of the specific C-terminal sequence of SP.Citation57 Of course, the C-terminal fragment is shared among all tachykinin ligands, so resolving which receptor subtype responsible was an obvious next step. With the use of selective agonists, they went on to show that this effect was mediated by both NK1R and NK3R activation.Citation58 Furthermore, SP injection into the nucleus basalis magnocellularis increased extracellular dopamine content in the NAc,Citation59 a barometer of positive reinforcement. The fact that the injection of the NK3R agonist amino-senktide into the nucleus basalis magnocellularis inhibits alcohol intake at first glance contradicts the aforementioned positive reinforcement.Citation60 The authors speculated that alcohol may actually be mediating its effects on the reward pathway via NK3R, thereby rendering an NK3R agonist a substitute for the rewarding properties of alcohol.Citation61 Whether or not alcohol engages the NK3R system in the nucleus basalis magnocellularis remains to be investigated.
NKB fibers have been traced from the dorsal AND ventral striatum to the substantia innominata.Citation62 Pre-protachykinin-B, the mRNA for NKB, has also been found heavily concentrated in fibers from the lateral stripe of the striatum, a region just lateral to the shell of the NAc, to the nucleus basalis magnocellularis.Citation63 The importance of these projections in reward is not well understood; however, it should be remembered that NKB is the most efficacious endogenous ligand for the NK3R in mammals.
Habenula
The Hb is an understudied brain region, let alone the role neurokinins play in its function. What is known is that the Hb white matter tracts tie it extensively to other regions of the limbic pathway, including the ventral pallidum and v entral midbrain. Moreover, SPergic and NKBergic cell bodies are indeed found in the medial Hb with axons projecting to the VTA and the adjacent IPN.Citation64,Citation65 The role of the VTA in reward is well described; the IPN also seems to contribute to positive reinforcement.Citation66 The Hb has long been known to be involved in nicotine dependence and, more recently, in cocaine and morphine dependence as well.Citation67 No studies to our knowledge have examined the direct role of habenular neurokinin antagonists on addictive behavior in animals; however, NK1R and NK3Rs were found to be involved in nicotine-induced excitation of habenular neurons.Citation68
Amygdala
Neurokinin receptors have been found in relatively high concentrations in the amygdala of primates.Citation69 Accordingly, SP microinjection into the central amygdala enhanced passive avoidance learning behaviorsCitation70 and generated CPP.Citation71 While efferent projections from the amygdala are abundant and promiscuous, specific projections to regions of the limbic loop will be discussed here.
Regarding neurokinins in addiction, the smoking gun of sorts came in the seminal 2000 Nature paper by Murtra et alCitation72 when they documented NK1R−/− exhibited a lack of morphine CPP. Knockouts additionally eliminated CPP to amphetamines but surprisingly retained a positive CPP to cocaine and food, indicating distinct mechanisms mediating reward for each of these natural and unnatural rewards.Citation73 The role of NK1R in opioid reward was further corroborated using the self-administration paradigm in NK1R−/− mice.Citation74 To better understand which neuroanatomical location may play the principle role in neurokinin-mediated opioid reward, SP-saporin (a ribosome inactivating toxin) was used to ablate NK1R expressing neurons in the amygdala. Indeed, mice with ablation of NK1R+ neurons in the amygdala demonstrated similar CPP scores for both morphine and saline.Citation75 These observations support the role of the neurokinin system in facilitating opioid reward via amygdaloid processes, although it does not rule out the importance of other limbic regions. Importantly, the neurokinin knockout data are supported by the fact that intracerebral ventricular administration of an NK1R antagonist has no effect on cocaine self-administration,Citation76 ruling out possible developmental confounders to the knockout mice. Although cocaine CPP was not altered in the NK1R−/− mice, reinstatement of cocaine is in fact supplemented by administration of the SP analog [SarCitation9 Met(O2)Citation11]-SP.Citation77 This, at the very least, implicates the neurokinin system in cocaine reinstatement, albeit endogenous SP may not play a role as two separate NK1R antagonists were unable to inhibit cocaine reinstatement.
Alcohol reward may be mediated in the amygdala as well. SP levels were lower in the central amygdala of Indiana alcohol-preferring rats than nonpreferring rats as measured by SP mRNA.Citation78 For this reason, the investigators microinfused SP into the central amygdala of alcohol-preferring rats, rendering the animals indifferent to alcohol consumption while sucrose seeking remained the same. The apparent paradox in neurokinin signaling and alcohol reward has been noted twice: SP injection in the nucleus basalis magnocellularis reduces alcohol consumption in Sardinian alcohol-preferring rats and SP injection in the central amygdala facilitates a similar effect in Indiana alcohol-preferring rats. The concern with these studies lies in the fact that the alcohol-preferring animals are selectively bred and do not represent the typical rodent or primate condition.Citation79 These studies contradict the vast majority of studies in rodents and humans that indicate a neurokinin antagonist reduces alcohol preference (see “Neurokinin in the clinic” for more details).Citation80
Clear evidence exists linking stress to alcoholic relapse.Citation81 Mild and severe emotional stressors are sufficient to release SP in the medial amygdala.Citation82 Naturally, linking stress-induced SP release to stress-induced alcohol reinstatement was studied. Expectedly, the NK1R antagonist, L822429, was adequate to prevent alcohol reinstatement in an alcohol self-administration paradigm in wild-type Wistar rats.Citation83 A related study demonstrated efficacy of the NK1R antagonist in suppressing alcohol seeking in wild-type mice at baseline and in preventing escalation of voluntary alcohol intake.Citation84 Corroborating this data, NK1R silencing with a microRNA directed at the receptors’ transcript reduced alcohol consumption in mice.Citation85 The study noted attenuated NK1R expression in the hippocampus, the only subcortical area they examined for proof of action. To the contrary, ezlopitant, the NK1R antagonist developed for chemotherapy induced nausea and vomiting, exhibited little to no efficacy in reducing operant self-administration of alcohol in Long–Evans rats.Citation86 The aforementioned inconsistency raises a valid point about nonconserved regions of the neurokinin receptors between humans and rodents, giving rise to potential obstacles in extrapolating preclinical models to the human condition.Citation87
While stress has been shown to increase extracellular SP in the amygdala, it should be mentioned that SP and NKA have been found colocalized in neurons of the infundibulum of the CNS and myenteric plexus of enteric nervous system, a possibility that has not been specifically investigated in neurons of the amygdala.Citation88,Citation89 In fact, NK2Rs do appear in a significant concentration in the amygdala,Citation90 and the NK2R antagonist SR48968 was sufficient to block stress-induced behaviors in mice and central neuronal markers of stress in rats.Citation91
An analogous pathway observed in the alcohol reward system in relation to neurokinin pharmacology is that observed with corticotrophin-releasing factor (CRF).Citation92 There is extensive research into the effects of CRF and other neuropeptides on addiction that are out of the scope of this review. In general, it is accepted in the addiction field that the stress response is mediated by several neurotransmitter systems including CRF and SP, thus precipitating undesirable outcomes such as relapse.
Frontal cortex
The literature on neurokinins in the cortex is more scant than other brain regions; nevertheless, cortical neurokinins seem to play an important role in the limbic system. As one of the terminal sites of mesencephalic dopaminergic projections, the frontal cortex has been shown to have increased dopamine metabolites (DOPAC) in response to stress (ie, foot-shock).Citation93 This increase in cortical dopamine is correlated to periods of intoxication and craving, particularly with cocaine abuse.Citation94 Pretreatment with the selective NK1R antagonist (S)-GR205171 ip was sufficient to prevent footshock-induced dopamine release in the cortex.Citation95 In addition to stress, morphine injections ip increased SP levels in the cortex that subsequently significantly decreased due to the administration of the opioid antagonist, naloxone.Citation96 The relative importance of the frontal cortex in neurokinin-mediated addiction is not well understood and warrants further exploration.
Involvement of the immune system in addiction
The role of the resident immune cells in the brain, the glia (astrocytes, microglia, and oligodendrocytes), has been emerging in the last 20 years as critical for normal neuronal signaling.Citation97 Importantly, microglia and astrocytes have recently been implicated in addictive processes as activated microglia release “proinflammatory” cytokines that act at the neuronal synapse, strengthening the signal.Citation98 Expanding on this notion, alcohol, cocaine, morphine, and amphetamines have all been indicted for their role in microglial activation with microglial activation proven to be critical to the maintenance of addictive behaviors.Citation99 Critically, NK1Rs are located on microglia and are inducible by IL-1β in astrocytes.Citation100,Citation101 SP has been shown to activate NF-κB in microglia, which has a strong, yet neglected, role in the progression of addiction.Citation102 In astrocytes, SP application induces a complex depolarization by modulating Cl− and K+ currents.Citation103 Astrocytes are probably most notorious for their role in glutamate homeostasis, and so there is a high likelihood of the neurokinin system modulating extracellular glutamate in brain regions, including those of the limbic system. There is substantial information on both neurokinins and glia in the reward pathway, yet a dearth of information on the interaction between the two. It may end up representing one of the more promising avenues in addiction research.
Neurokinin in the clinic
We have outlined the neural and pharmacological basis for the use of neurokinin antagonists in addiction. To summarize, SP appears to be overexpressed after chronic administration of drugs of abuse and mediates some of the negative effects such as CPP and reinstatement. Here, the focus will be on the use of neurokinin antagonists specifically in humans and the potential success as a therapeutic. While SP has long been infamous as one of the primary pronociceptive neurotransmitters, an NK1R antagonist did not achieve appreciable analgesia as a standalone medication in patients suffering from pain.Citation29 However, one of the first investigations into neurokinins in human disease with positive results demonstrated elevated cerebrospinal fluid levels of SP in psychiatric patients with depression or schizophrenia.Citation104 With the development of radiolabeled substance p antagonists (SPAs), imaging of receptor localization and saturation in humans became possible with positron emission tomography.Citation105 [18F]-SPA-RQ was taken up in the brain of healthy male volunteers in the regions already described that are involved in reward including the VTA, amygdala, Hb, and ventral striatum.Citation106 The most notable differences from rats were the high density of NK1Rs in the cortex of humans and a greater NK1R/NK3R ratio in the VTA of humans.Citation107,Citation108
Functionally, the NK1R antagonist aprepitant has an effect on positive incentive in humans. In an experiment enlisting healthy volunteers of both sexes, monetary incentive delay was the paradigm used to determine if the NK1R antagonist could prevent NAc activation typical of incentive anticipation. Indeed, when subjects expected a monetary reward for completing a task in the study, aprepitant reduced NAc blood oxygenation-level-dependent (BOLD) contrast compared to control as seen on fMRI, indicating the attenuation of NAc activation.Citation109
An association between various NK1R gene (TACR1) single nucleotide polymorphisms (SNPs) and alcoholism may exist. In a large sample of heavy drinkers (7 drinks per day on average), 5 SNPs of the TACR1 gene were predictive of BOLD activation as assessed by fMRI in response to alcohol cues.Citation110 In a separate study, 1 SNP and 2 haplotypes (a specific combination of alleles on the same chromosome) of the TACR1 gene were associated with alcohol dependence.Citation111 The significance of these studies is not well understood; however, they point to a link between a specific neurokinin genotype and alcohol-dependent phenotype that may have potential as a drug target. Of course, the NK1R is not the only SNP found to dysregulate the reward pathway as OPRM1 (µ-opioid receptor) has also been highlighted as a troublesome gene of interest.Citation112 Much like carriers of certain OPRM1, SNPs are more sensitive to the effects of naltrexone on reducing alcohol cravings, so too should NK1R antagonists on specific TACR1 SNP-related addictions.Citation113
Unexpectedly, in a clinical study examining the role of aprepitant on oxycodone abuse liability, the authors found the NK1R antagonist actually increased the abuse potential of oxycodone in patients who were already opioid drug abusers.Citation114 Several explanations for the unanticipated outcomes were proposed, including the pharmacokinetic interaction between the two drugs. That is, aprepitant and oxycodone compete for metabolism by the enzyme CYP3A4, rendering higher concentrations of serum oxycodone than expected. The unfortunate pharmacokinetic profiles of many drugs have hindered their success in the past, despite promising pharmacodynamic actions on the biology of the system. Future studies on opioid dependence may require a novel neurokinin antagonist that is not involved in CYP3A4 metabolism, a requirement that will surely prove challenging though not impossible.
In alcohol-dependent humans who were recently detoxified, LY686017, a brain penetrant NK1R antagonist with high bioavailability was efficacious in suppressing spontaneous alcohol cravings as assessed by the Alcohol Urge Questionaire.Citation115 When the alcohol-dependent subjects were then provoked with a combined stress test and alcohol-cue challenge, the treatment group still had reduced cravings for alcohol compared to controls. To the contrary, psychiatric patients with comorbid posttraumatic stress disorder (PTSD) and alcoholism experienced no reduction in symptoms of alcohol craving after administration of an NK1R antagonist.Citation116 This may point to the fact that comorbidity with PTSD complicates the syndrome by adding another “stress”-related illness.
Neurokinin prospects for therapy
The neurokinin field indeed does seem poised to produce significant contributions to addiction research and therapy. We have outlined the role neurokinins play in the reward pathway, particularly via NK1Rs and NK3Rs. Accordingly, GlaxoSmithKline has a dual NK1R/NK3R antagonist, GSK1144814, in the pipeline for future clinical trials for psychiatric disorders.Citation117 Vanda Pharmaceuticals acquired worldwide licensing for LY686017 (now called VLY-686) from Eli Lilly after the proof of concept studies in alcohol cravings mentioned earlier. Vanda is now attempting to commercialize and develop this compound “for all human conditions”, including an indication for substance abuse. Our pharmacology/chemistry group has created several opioid agonist/NK1R antagonist compounds that have efficacy in antinociception and do not produce CPP or increase extracellular dopamine content in the NAc.Citation118–Citation120
In addition to the new compounds in the pipeline, the original gold standard NK1R antagonist is still under investigation for its effects on substance abuse potential since it already has FDA approval for the clinic. A brief ClinicalTrials.gov search reveals that aprepitant is currently undergoing clinical trials for the evaluation of its effects on cannabis cravings in cannabis-dependent outpatients, comorbid alcoholic and cannabis-dependent patients, and in opioid-dependent patients. More compounds that selectively block the neurokinin system will undoubtedly materialize in the drug pipeline as preclinical and clinical studies further identify the role of the neurokinin system in drug addiction.
Disclosure
The authors report no conflicts of interest in this report.
References
- Trends and Statistics Available from http://www.drugabuse.gov/related-topics/trends-statistics#costsAccessed July 28, 2015
- VolkowNWhat is the Federal Government doing to Combat the Opioid Abuse Epidemic?Bethesda, MDNIDA: NIH2015
- V EulerUSGaddumJHAn unidentified depressor substance in certain tissue extractsJ Physiol1931721748716994201
- GaddumJHSchildHDepressor substances in extracts of intestineJ Physiol193483111416994608
- PernowBDistribution of substance P in the central and peripheral nervous systemNature1953171435674613054699
- YakshTLJessellTMGamseRMudgeAWLeemanSEIntrathecal morphine inhibits substance P release from mammalian spinal cord in vivoNature198028657691551576157098
- ChangMMLeemanSEIsolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance PJ Biol Chem197024518478447905456150
- MrozEDBrownsteinMJLeemanSEEvidence for substance P in the habenulo-interpeduncular tractBrain Res19761133597599953755
- KanazawaIEmsonPCCuelloACEvidence for the existence of substance P-containing fibres in striato-nigral and pallido-nigral pathways in rat brainBrain Res19771192447453830395
- NapierTCMitrovicIChurchillLKlitenickMALuXYKalivasPWSubstance P in the ventral pallidum: projection from the ventral striatum, and electrophysiological and behavioral consequences of pallidal substance PNeuroscience199569159708637633
- CuelloACJessellTMKanazawaIIversenLLSubstance P: localization in synaptic vesicles in rat central nervous systemJ Neurochem1977294747751591950
- KellyPHIversenSDSelective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in ratsEur J Pharmacol197640145561033072
- SpyrakiCFibigerHCPhillipsAGAttenuation of heroin reward in rats by disruption of the mesolimbic dopamine systemPsychophamacology1983792–3278283
- BaikJHDopamine signaling in reward-related behaviorsFront Neural Circuits2013715224130517
- StinusLKelleyAEIversenSDIncreased spontaneous activity following substance P infusion into A10 dopaminergic areaNature19782765688616618214711
- ErspamerVAnastasiAPolypeptides active on plain muscle in the amphibian skinPaper presented at: Proceedings of the International Symposium: Hypotensive PeptidesOctober 25–29, 19651966Florence, Italy
- KimuraSOkadaMSugitaYKanazawaIMunekataENovel neuropeptides, neurokinin-alpha and neurokinin-beta isolated from porcine spinal-cordP Jpn Acad B Phys1983594101104
- BuckSHBurcherEShultsCWLovenbergWO’DonohueTLNovel pharmacology of substance K-binding sites: a third type of tachykinin receptorScience198422646779879896095447
- HenryJLSubstance P and neurokininsPaper presented at: Proceedings of “Substance P and Neurokinins-Montreal ’86.” A Satellite Symposium of the XXX International Congress of The International Union of Physiological Sciences1986Montreal, Canada
- RegoliDDrapeauGDionSD’Orleans-JustePPharmacological receptors for substance P and neurokininsLife Sci19874021091172432376
- MantyhPWPinnockRDDownesCPGoedertMHuntSPCorrelation between inositol phospholipid hydrolysis and substance P receptors in rat CNSNature198430959717957976204206
- MantyhPWAllenCJGhilardiJRRapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivoProc Natl Acad Sci U S A1995927262226267535928
- SteinhoffMSvon MentzerBGeppettiPPothoulakisCBunnettNWTachykinins and their receptors: contributions to physiological control and the mechanisms of diseasePhysiol Rev201494126530124382888
- LuBSuYDasSPeptide neurotransmitters activate a cation channel complex of NALCN and UNC-80Nature2009457723074174419092807
- Koike-TaniMCollinsJMKawanoTSignal transduction pathway for the substance P-induced inhibition of rat Kir3 (GIRK) channelJ Physiol2005564Pt 248950015731196
- IversenLIversen LeslieSquireLRThe History of Neuroscience in Autobiography6OxfordOxford University Press2009190225
- SniderRMConstantineJWLoweJAIIIA potent nonpeptide antagonist of the substance P (NK1) receptorScience199125149924354371703323
- KramerMSCutlerNFeighnerJDistinct mechanism for antidepressant activity by blockade of central substance P receptorsScience19982815383164016459733503
- HillRNK1 (substance P) receptor antagonists – why are they not analgesic in humans?Trends Pharmacol Sci200021724424610871891
- LjungdahlAHokfeltTNilssonGDistribution of substance P-like immunoreactivity in the central nervous system of the rat – I. Cell bodies and nerve terminalsNeuroscience1978310861943366451
- BowmanSLSoohooALShiwarskiDJSchulzSPradhanAAPuthenveeduMACell-Autonomous Regulation of Mu-Opioid Receptor Recycling by Substance PCell Rep201510111925193625801029
- SantarelliLGobbiGBlierPHenRBehavioral and physiologic effects of genetic or pharmacologic inactivation of the substance P receptor (NK1)J Clin Psychiatry200263Suppl 11111712562138
- MantyhPWNeurobiology of substance P and the NK1 receptorJ Clin Psychiatry200263Suppl 1161012562137
- WiseRAKoobGFThe development and maintenance of drug addictionNeuropsychopharmacology201439225426224121188
- ElliottPJMasonGSStephens-SmithMHaganRMBehavioural and biochemical responses following activation of midbrain dopamine pathways by receptor selective neurokinin agonistsNeuropeptides19911921191261719444
- DamTVEscherEQuirionRVisualization of neurokinin-3 receptor sites in rat brain using the highly selective ligand [3H]senktideBrain Res199050611751791689199
- MantyhPWGatesTMantyhCRMaggioJEAutoradiographic localization and characterization of tachykinin receptor binding sites in the rat brain and peripheral tissuesJ Neurosci1989912582792536418
- SeabrookGRBoweryBJHillRGPharmacology of tachykinin receptors on neurones in the ventral tegmental area of rat brain slicesEur J Pharmacol19952731–21131197537676
- MinabeYEmoriKToorAStutzmannGEAshbyCRJrThe effect of the acute and chronic administration of CP 96,345, a selective neu-rokinin1 receptor antagonist, on midbrain dopamine neurons in the rat: a single unit, extracellular recording studySynapse199622135458822476
- KorotkovaTMBrownRESergeevaOAPonomarenkoAAHaasHLEffects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the ratEur J Neurosci200623102677268516817870
- Sanchez-CatalanMJKauflingJGeorgesFVeinantePBarrotMThe antero-posterior heterogeneity of the ventral tegmental areaNeuroscience2014282C19821625241061
- LessardAPickelVMSubcellular distribution and plasticity of neurokinin-1 receptors in the rat substantia nigra and ventral tegmental areaNeuroscience200513541309132316165296
- LessardAGradyEFBunnettNWPickelVMPredominant surface distribution of neurokinin-3 receptors in non-dopaminergic dendrites in the rat substantia nigra and ventral tegmental areaNeuroscience200714441393140817197098
- LessardASavardMGobeilFJrPierceJPPickelVMThe neurokinin-3 (NK3) and the neurokinin-1 (NK1) receptors are differentially targeted to mesocortical and mesolimbic projection neurons and to neuronal nuclei in the rat ventral tegmental areaSynapse200963648450119224600
- HetherSMisonoKLessardAThe neurokinin-3 receptor (NK3R) antagonist SB222200 prevents the apomorphine-evoked surface but not nuclear NK3R redistribution in dopaminergic neurons of the rat ventral tegmental areaNeuroscience2013247122423673279
- SladekCDStevensWLevinsonSRSongZJensenDDFlynnFWCharacterization of nuclear neurokinin 3 receptor expression in rat brainNeuroscience2011196354821939739
- MinabeYAshbyCRJrEffect of the acute and chronic administration of the selective neurokinin2 receptor antagonist SR 48968 on midbrain dopamine neurons in the rat: an in vivo extracellular single cell studySynapse19972521962049021900
- SchmidlinFDeryOBunnettNWGradyEFHeterologous regulation of trafficking and signaling of G protein-coupled receptors: beta-arrestin-dependent interactions between neurokinin receptorsProc Natl Acad Sci U S A20029953324332911880656
- StaubliUHustonJPCentral action of substance P: possible role in rewardBehav Neural Biol19854311001082581538
- PlacenzaFMFletcherPJRotzingerSVaccarinoFJInfusion of the substance P analogue, DiMe-C7, into the ventral tegmental area induces reinstatement of cocaine-seeking behaviour in ratsPsychopharmacology20041771–211112015167979
- MogensonGJWuMJonesDLLocomotor activity elicited by injections of picrotoxin into the ventral tegmental area is attenuated by injections of GABA into the globus pallidusBrain Res198019125695717378773
- AmbroggiFIshikawaAFieldsHLNicolaSMBasolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neuronsNeuron200859464866118760700
- SchildeinSAgmoAHustonJPSchwartingRKIntraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activityBrain Res19987901–21851949593886
- ElliottPJNemeroffCBKiltsCDEvidence for a tonic facilitatory influence of substance P on dopamine release in the nucleus accumbensBrain Res198638523793822430671
- LoonamTMNoaillesPAYuJZhuJPAnguloJASubstance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatumLife Sci200373672773912801594
- BeachTGTagoHMcGeerEGLight microscopic evidence for a substance P-containing innervation of the human nucleus basalis of MeynertBrain Res19874081–22512572439166
- HasenohrlRUGerhardtPHustonJPPositively reinforcing effects of the neurokinin substance P in the basal forebrain: mediation by its C-terminal sequenceExp Neurol199211522822911370940
- NikolausSHustonJPHasenohrlRUReinforcing effects of neurokinin substance P in the ventral pallidum: mediation by the tachykinin NK1 receptorEur J Pharmacol19993702939910323256
- BoixFSandorPNogueiraPJHustonJPSchwartingRKRelationship between dopamine release in nucleus accumbens and place preference induced by substance P injected into the nucleus basalis magnocellularis regionNeuroscience1995644104510557538637
- CiccocioppoRPanockaIPolidoriCDe CaroGRegoliDMassiMStimulation of tachykinin NK-3 receptors in the nucleus basalis magnocellularis reduces alcohol intake in ratsPeptides1997189134913559392836
- CiccocioppoRPanockaIPolidoriCFroldiRAngelettiSMassiMMechanism of action for reduction of ethanol intake in rats by the tachykinin NK-3 receptor agonist aminosenktidePharmacol Biochem Behav19986144594649802842
- FurutaTMoriTLeeTKanekoTThird group of neostriatofugal neurons: neurokinin B-producing neurons that send axons predominantly to the substantia innominataJ Comp Neurol2000426227929610982469
- ZhouLFurutaTKanekoTNeurokinin B-producing projection neurons in the lateral stripe of the striatum and cell clusters of the accumbens nucleus in the ratJ Comp Neurol2004480214316115514933
- VelasquezKMMolfeseDLSalasRThe role of the habenula in drug addictionFront Hum Neurosci2014817424734015
- BurgunderJMYoungWSIIINeurokinin B and substance P genes are co-expressed in a subset of neurons in the rat habenulaNeuropeptides19891331651692469031
- Antolin-FontesBAblesJLGorlichAIbanez-TallonIThe habenulo-interpeduncular pathway in nicotine aversion and withdrawalNeuropharmacology201596Pt B21322225476971
- GlickSDRamirezRLLiviJMMaisonneuveIM18-Methoxycoonaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self-administration in ratsEur J Pharmacol20065371–3949816626688
- DaoDQPerezEETengYDaniJADe BiasiMNicotine enhances excitability of medial habenular neurons via facilitation of neurokinin signalingJ Neurosci201434124273428424647947
- NaganoMSaitowFHanedaEKonishiSHayashiMSuzukiHDistribution and pharmacological characterization of primate NK-1 and NK-3 tachykinin receptors in the central nervous system of the rhesus monkeyBr J Pharmacol2006147331632316331282
- KertesELaszloKBertaBLenardLEffects of substance P microinjections into the globus pallidus and central nucleus of amygdala on passive avoidance learning in ratsBehav Brain Res2009198239740319071162
- KertesELaszloKBertaBLenardLPositive reinforcing effects of substance P in the rat central nucleus of amygdalaBehav Brain Res2009205130731019555724
- MurtraPSheasbyAMHuntSPDe FelipeCRewarding effects of opiates are absent in mice lacking the receptor for substance PNature2000405678318018310821273
- MurtraPSheasbyAHuntSPDe FelipeCLoss of Rewarding Effects of Morphine and Amphetamine, But Not Cocaine, in Mice with Disruption of the Substance P Receptor (NK1) GeneNew Orleans, LASociety for Neuroscience2000
- RipleyTLack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptorsNeuropharmacology20024381258126812527475
- GaddCAMurtraPDe FelipeCHuntSPNeurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouseJ Neurosc2003232382718280
- PlacenzaFMFletcherPJVaccarinoFJErbSEffects of central neurokinin-1 receptor antagonism on cocaine- and opiate-induced locomotor activity and self-administration behaviour in ratsPharmacol Biochem Behav20068419410116757018
- PlacenzaFMVaccarinoFJFletcherPJErbSActivation of central neurokinin-1 receptors induces reinstatement of cocaine-seeking behaviorNeurosci Lett20053901424716125318
- YangARYiHSMamczarzJJuneHLJrHwangBHJuneHLSrDeficits in substance P mRNA levels in the CeA are inversely associated with alcohol-motivated respondingSynapse2009631197298119593822
- LumengLHawkinsTDLiTKNew strains of rats with alcohol preference and nonpreferenceRonaldGThurmanJRWDrottHenryChanceBrittonAlcohol and Aldehyde Metabolizing Systems: Intermediary Metabolism and Neurochemistry3New YorkAcademic Press Inc1977537544
- HigleyAEKoobGFMasonBJTreatment of alcohol dependence with drug antagonists of the stress responseAlcohol Res201234451652123584117
- SinhaRHow does stress lead to risk of alcohol relapse?Alcohol Res201234443244023584109
- EbnerKRupniakNMSariaASingewaldNSubstance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in ratsProc Natl Acad Sci U S A2004101124280428515024126
- SchankJRPickensCLRoweKEStress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429Psychopharmacology2011218111111921340476
- ThorsellASchankJRSingleyEHuntSPHeiligMNeurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in micePsychopharmacology2010209110311120112009
- BaekMNJungKHHalderDArtificial microRNA-based neurokinin-1 receptor gene silencing reduces alcohol consumption in miceNeurosci Lett2010475312412820347940
- SteenslandPSimmsJANielsenCKHolgateJBito-OnonJJBartlettSEThe neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanolPLoS One201059e1252720824145
- JensenCJGerardNPSchwartzTWGetherUThe species selectivity of chemically distinct tachykinin nonpeptide antagonists is dependent on common divergent residues of the rat and human neurokinin-1 receptorsMol Pharmacol19944522942997509441
- HrabovszkyEBorsayBARaczKSubstance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular regionPloS One201388e7236923977290
- DeaconCFAgostonDVNauRConlonJMConversion of neuropeptide K to neurokinin A and vesicular colocalization of neurokinin A and substance P in neurons of the guinea pig small intestineJ Neurochem19874811411462432172
- NaganoMOishiTSuzukiHDistribution and pharmacological characterization of primate NK-2 tachykinin receptor in the central nervous system of the rhesus monkeyNeurosci Lett20115031232621855604
- SteinbergRAlonsoRGriebelGSelective blockade of neurokinin-2 receptors produces antidepressant-like effects associated with reduced corticotropin-releasing factor functionJ Pharmacology Exp Ther20012992449458
- LeggioLCardoneSFerrulliATurning the clock ahead: potential preclinical and clinical neuropharmacological targets for alcohol dependenceCurr Pharm Des201016192159211820482506
- HermanJPGuillonneauDDantzerRScattonBSemerdjian-RouquierLLe MoalMDifferential effects of inescapable footshocks and of stimuli previously paired with inescapable footshocks on dopamine turnover in cortical and limbic areas of the ratLife Sci19823025220722146180276
- GoldsteinRZVolkowNDDrug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortexAm J Psychiatry2002159101642165212359667
- HutsonPHPatelSJayMTBartonCLStress-induced increase of cortical dopamine metabolism: attenuation by a tachykinin NK1 receptor antagonistEur J Pharmacol20044841576414729382
- MorleyJEYamadaTWalshJHMorphine addiction and withdrawal alters brain peptide concentrationsLife Sci19802626223922446157071
- PfriegerFWBarresBASynaptic efficacy enhanced by glial cells in vitroScience19972775332168416879287225
- KovacsKJMicroglia and drug-induced plasticity in reward-related neuronal circuitsFront Mol Neurosci201257422707932
- CrewsFTZouJQinLInduction of innate immune genes in brain create the neurobiology of addictionBrain Behav Immun201125Suppl 1S4S1221402143
- GuoCJDouglasSDGaoZInterleukin-1beta upregulates functional expression of neurokinin-1 receptor (NK-1R) via NF-kappaB in astrocytesGlia200448325926615390113
- RasleyABostKLOlsonJKMillerSDMarriottIExpression of functional NK-1 receptors in murine microgliaGlia200237325826711857684
- YangJYuJJiaXInhibition of nuclear factor-kappaB impairs reconsolidation of morphine reward memory in ratsBehav Brain Res2011216259259620816896
- BackusKHBergerTKettenmannHActivation of neurokinin receptors modulates K+ and Cl− channel activity in cultured astrocytes from rat cortexBrain Res199154111031091709385
- RimonRLe GrevesPNybergFHeikkilaLSalmelaLTereniusLElevation of substance P-like peptides in the CSF of psychiatric patientsBiol Psychiatry19841945095166203562
- HargreavesRImaging substance P receptors (NK1) in the living human brain using positron emission tomographyJ Clin Psychiatry200263182412562139
- HietalaJNymanMJEskolaOVisualization and quantification of neurokinin-1 (NK1) receptors in the human brainMol Imaging Biol20057426227216155744
- WhittyCJPaulMABannonMJNeurokinin receptor mRNA localization in human midbrain dopamine neuronsJ Comp Neurol199738233944009183701
- OkumuraMArakawaRItoHQuantitative analysis of NK1 receptor in the human brain using PET with 18F-FE-SPA-RQJ Nucl Med200849111749175518927336
- SajiKIkedaYKimWAcute NK(1) receptor antagonist administration affects reward incentive anticipation processing in healthy volunteersInt J Neuropsychopharmacol20131671461147123406545
- BlaineSClausEHarlaarNHutchisonKTACR1 genotypes predict fMRI response to alcohol cues and level of alcohol dependenceAlcohol Clin Exp Res201337Suppl 1E125E13023078527
- SeneviratneCAit-DaoudNMaJZChenGJohnsonBALiMDSusceptibility locus in neurokinin-1 receptor gene associated with alcohol dependenceNeuropsychopharmacology200934112442244919553914
- HeiligMGoldmanDBerrettiniWO’BrienCPPharmacogenetic approaches to the treatment of alcohol addictionNat Rev Neurosci2011121167068422011682
- AntonRFOrosziGO’MalleySAn evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) studyArch Gen Psychiatry200865213514418250251
- WalshSLHeiligMNuzzoPAHendersonPLofwallMREffects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusersAddict Biol201318233234322260216
- GeorgeDTGilmanJHershJNeurokinin 1 receptor antagonism as a possible therapy for alcoholismScience200831958691536153918276852
- KwakoLEGeorgeDTSchwandtMLThe neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental studyPsychopharmacology2015232129530425030801
- RidlerKGunnRNSearleGECharacterising the plasma-target occupancy relationship of the neurokinin antagonist GSK1144814 with PETJ Psychopharmacol201428324425324429221
- Largent-MilnesTMYamamotoTNairPSpinal or systemic TY005, a peptidic opioid agonist/neurokinin 1 antagonist, attenuates pain with reduced toleranceBr J Pharmacol20101615986100120977451
- YamamotoTNairPJacobsenNEThe importance of micelle-bound states for the bioactivities of bifunctional peptide derivatives for delta/mu opioid receptor agonists and neurokinin 1 receptor antagonistsJ Med Chem200851206334634718821747
- SandweissAStarkJLargent-MilnesTMAn opioid agonist/NK1 antagonist inhibits nociception in an animal model of neuropathic pain while lacking accumbens dopamine releaseInternational Narcotics Research Conference2015Phoenix, AZ
- IngiTKitajimaYMinamitakeYNakanishiSCharacterization of ligand-binding properties and selectivities of three rat tachykinin receptors by transfection and functional expression of their cloned cDNAs in mammalian cellsJ Pharmacol Exp Ther199125939689751662278
