Abstract
Adult mesenchymal stem cells (MSCs) and epithelial stem cells play essential roles in tissue repair and self-healing. Oral MSCs and epithelial stem cells can be isolated from adult human oral tissues, for example, teeth, periodontal ligament, and gingiva. Cocultivated adult oral epithelial stem cells and MSCs could represent some developmental events, such as epithelial invagination and tubular structure formation, signifying their potentials for tissue regeneration. Oral epithelial stem cells have been used in regenerative medicine over 1 decade. They are able to form a stratified cell sheet under three-dimensional culture conditions. Both experimental and clinical data indicate that the cell sheets can not only safely and effectively reconstruct the damaged cornea in humans, but also repair esophageal ulcer in animal models. Oral MSCs include dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth (SHED), stem cells from apical papilla (SCAP), periodontal ligament stem cells (PDLSCs), and mesenchymal stem cells from gingiva (GMSCs). They are widely applied in both regenerative dentistry and medicine. DPSCs, SHED, and SCAP are able to form dentin–pulp complex when being transplanted into immunodeficient animals. They have been experimentally used for the regeneration of dental pulp, neuron, bone muscle and blood vessels in animal models and have shown promising results. PDLSCs and GMSCs are demonstrated to be ideal cell sources for repairing the damaged tissues of periodontal, muscle, and tendon. Despite the abovementioned applications of oral stem cells, only a few human clinical trials are now underway to use them for the treatment of certain diseases. Since clinical use is the end goal, their true regenerative power and safety need to be further examined.
Introduction
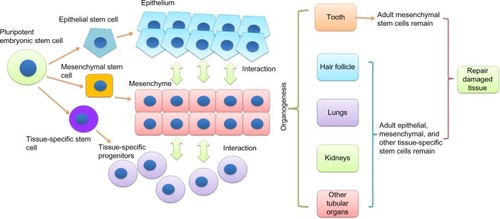
To restore the damaged tissues or organs, it is critical to understand the developmental process of specific tissues and then reproduce it. Stem cells play essential roles in organ development and tissue repair. In an organism, all the tissues are built from pluripotent embryonic stem cells.Citation1 The embryonic stem cells differentiate into multipotent stem cells, including epithelial, mesenchymal, and other tissue-specific stem cells.Citation2,Citation3 Interactions among these stem cells initiate and regulate developmental processes, resulting in the formation of highly specialized functional tissues and organs.Citation4,Citation5 Once the organism matures, the pluripotent embryonic stem cells evanesce and some multipotent adult stem cells remain in the developed tissue to sustain the homeostasis and repair injuriesCitation6 (). Many adult human tissues (such as bone marrow,Citation7 dental pulp,Citation8 adipose tissue,Citation9 dermis,Citation10 and umbilical cordCitation11) contain populations of mesenchymal stem cells (MSCs). To date, the developmental origin of MSCs is still unclear. Although it is commonly considered that MSCs derive from mesodermal origin,Citation12 evidence indicated that Sox1+ neuroepithelium and neural crest give rise to the earliest MSCs.Citation13 In many postnatal tissues, MSCs are mainly located in the perivascular niche.Citation14 Nevertheless, genetic lineage-tracing experiment showed that MSCs might have other localization.Citation15 MSCs are able to differentiate into mature specific mesenchymal cells as well as adipocytes, chondrocytes, and osteoblasts under inductive stimuli.Citation7,Citation16 Additionally, MSCs can also give rise to nonmesenchymal cell lineages, such as endothelial cells,Citation17 neuronal cells,Citation18 and keratinocytes.Citation19 Adult epithelial stem cells localized in the basal layer of various epithelial tissues such as skin epidermis and mucosal epithelium of the digestive and respiratory tracts. They are a dynamically heterogeneous cell population,Citation20 have slow-cycling,Citation21 retain long-term self-renewal potential and can serve as a single stem-cell pool.Citation22 Epithelial stem cells contribute the physiological renewal and wound healing in epithelial tissues by asymmetric divisions to generate the upper strata of the epidermis in skinCitation23 or specialized cells in the simple epithelia of the gut (which has only one cell layer that contains different cell lineages).Citation24 Transplantation and lineage-tracing experiments confirmed that epithelial stem cells give rise to not only all epithelial lineagesCitation25 but also neuroendocrine Merkel cells.Citation26,Citation27 MSCs and epithelial stem cells have been isolated from human oral tissues, including gingival, tooth, and periodontal ligament.Citation8,Citation28,Citation29 Immunodeficient animal transplantation, preclinical trial, and in vitro differentiation assays demonstrated that these oral stem cells have strong potentials on various organs and tissues regeneration. Here, we will review current understanding of oral mesenchymal and epithelial stem cells and their prospective applications in both regenerative dentistry and medicine.
Epithelial and mesenchymal stem cells regulate tooth development
Teeth share similar developmental processes with many organs, such as lungs, kidneys, and hair follicle. Unlike the internal organs, the loss of teeth and artificial tooth extraction are not life-threatening. Therefore, teeth are excellent targets for the analysis of developmental mechanisms. The interactions between epithelial and mesenchymal stem cells initiate teeth development and regulate their morphogenesis.Citation30 The details of epithelial–mesenchymal signaling in tooth development have been well documented by Thesleff et al.Citation31,Citation32 Here we focus on the fates of the epithelial and mesenchymal stem cells. In humans, tooth development begins in the middle of the sixth week of gestation.Citation33 In an embryo, the basal cells of the dental lamina (dental epithelial tissue) undergo proliferation and form a horseshoe-shaped band that invaginates into the underlying mesenchymal tissue (this process is called epithelium invagination).Citation34 The mesenchymal tissue, derived from neural crest cells,Citation35 initiates the proliferation of the dental epithelial cells and directs them to finally differentiate into the enamel-producing cells, ameloblasts. Cells from the mesenchymal tissue react to the signals from dental epithelial cells.Citation36 They differentiate into cementoblasts, periodontal ligament cells, odontoblasts, and other dental pulp cells (including neurons, endothelial cells, and fibroblasts). Once a functional tooth is formed, the dental epithelial cells no longer exist, whereas the mesenchymal cells remain in dental pulp and periodontal tissue ().Citation37
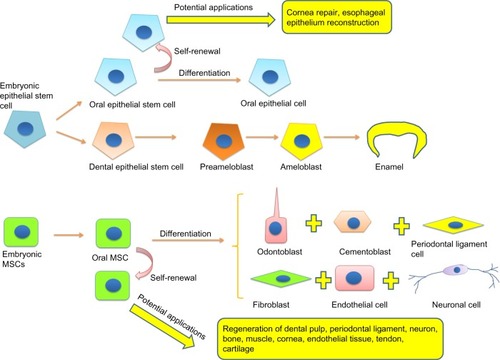
Figure 2 Differentiation of oral stem cells and their potential applications.
In 2007, Nakao et alCitation38 rebuilt a bioengineered tooth using completely dissociated epithelial and mesenchymal stem cells from a mouse embryo. The bioengineered tooth could erupt from an immunodeficient mouse oral cave and develop into a functional tooth.Citation39 Although their method is a breakthrough in tissue engineering, the clinical application is limited because it requires embryonic epithelial and mesenchymal stem cells. Using similar technology, they also regenerated functional hair follicle with adult epithelial and mesenchymal stem cells in immunodeficient mice.Citation40 However, the hair follicle itself is a lifelong regenerative organ which is very different from most of the organs, including tooth and internal organs. The regeneration of tooth and internal organs (such as lungs and kidneys) with adult stem cells remains largely unexplored. We have reconstructed a three-dimensional epithelium invagination-like tissue model using human adult oral epithelial cells and dental pulp stem cells (DPSCs).Citation41 We also demonstrated that DPSCs-derived spheroids could mimic common developmental processes of tubular organs, including cavitation and spontaneous differentiation in vitro.Citation42 Our data suggest that adult stem cells are able to represent some events of development under certain conditions that would provide an approach to the reconstruction of tooth and other organs by using adult epithelial and mesenchymal stem cells.
Adult epithelial stem cells from oral tissue and their applications
As we described earlier, the epithelial stem cells contribute toward tooth formation during development. Although it does not exist in developed human tooth, oral mucosal epithelium has drawn attention as an epithelial stem cell source for tissue engineering. In oral cavity, the mucosal epithelium covers the inner surface of the lips, floor of the mouth, gingiva, cheeks, hard palate, and tongue. Epithelial cells in the mucosal epithelium can be generally divided into three layers: basal, suprabasal, and superficial layers. Owing to the existence of epithelial progenitors in the basal layer, like most of the epithelial tissues (such as skin epidermis, intestinal crypt, and corneal limbus), the mucosal epithelium can constantly replace damaged or dead cells throughout the life of animals. These epithelial progenitors are characterized as quiescent and slow-cycling cells in vivo,Citation43 as well as epithelial stem cells in other tissues.Citation21 They express stem cell markers (α6 and β1 integrins, keratins 15 and 19, p63, α6β4, oct3/4, CD44H, p75, ATP-binding cassette subfamily G member 2 and K5) and give rise to cells in the suprabasal layer and finally differentiate into superficial epithelial cells.Citation44 Because the epithelial tissues of the body share common molecular and cellular characteristics, unlike MSCs, identification of epithelial stem cells in vitro is based not on their ability of multidifferentiation but on their self-renewing capacity. The mucosal epithelial stem cells (or progenitors) can be isolated from oral tissues by using p75 antibody-based cell-sorting technique. When cultivated in vitro, they showed high clonogenicity and proliferative capacity.Citation45 Under three-dimensional culture conditions, these oral epithelial progenitors are able to form a stratified cell sheet. Numerous studies have demonstrated that oral mucosal epithelial cell sheets promote corneal reconstruction when transplanted into the damaged sites.Citation46,Citation47 Autogenously grafted oral epithelial cell sheet has been used in patients with corneal limbal epithelial stem cell deficiency and was found to be safe and effective on corneal repair.Citation48 Oral epithelial stem cells have also been used for the reconstruction of esophageal epithelium. Endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets was performed on esophageal ulcer in a canine model. The transplanted cell sheets were able to adhere to and survive on the underlying muscle layers in the ulcer sites, providing an intact, stratified epithelium.Citation49 This evidence suggests that oral epithelial stem cells have the capacity to repair damaged epithelial tissues in the body. It has been reported that a collagen vitrigel sponge scaffold could promote regenerations of cornea and tracheal epithelium.Citation50,Citation51 A combination of oral mucosal epithelial cell sheet and the vitrigel scaffold might be a promising strategy for regeneration of various organs.
Despite these considerable regenerative applications of oral epithelial stem cells, numerous studies have shown evidence about their tumorigenic properties. White et alCitation52 demonstrated that oral epithelial stem cells contributed to oncogenic multipotency and metastasis that caused transgenic mice to develop multilineage tumors. Therefore, it is important to carefully test the carcinogenicity of oral epithelial stem cells before their clinical transplantation.
Adult MSCs from oral tissue and their applications
Defining oral MSCs
Oral MSCs can be broadly classified into two types: dental MSCs (which can form dentin–pulp complex in vivo) and nondental MSCs (which cannot form dentin–pulp complex). The dental MSCs include DPSCs,Citation8 stem cells from exfoliated deciduous teeth (SHED),Citation53 and stem cells from apical papilla (SCAP).Citation54 Nondental oral MSCs include periodontal ligament stem cells (PDLSCs)Citation55 and MSCs from gingiva (GMSCs).Citation56 So far, there is no sole surface molecule to identify MSCs from oral tissues. The expression of common MSC markers, such as STRO-1, p75, Oct-4, SOX-2, SSEA-4, CD29, CD44, CD73, CD90, CD105, CD133, and CD146, have been used to partly identify their stemness.Citation57 When being cultivated in vitro, they rapidly expand and display multipotency, with the capacity to give rise to osteo/odontogenic cells, chondrocytes, adipocytes, neuronal cells, muscle cells, cardiomyocytes, endothelial cells, hepatocyte-like cells, and islet-like cells.Citation58–Citation61 Because the behaviors of dental MSCs and nondental MSCs are different when being transplanted into immunodeficient animals,Citation8,Citation53–Citation56 it is necessary to choose appropriate cells for different tissue regeneration. For example, dental MSCs are good for the regeneration of dentin–pulp complex, bone, cartilage, and neuronal tissues, whereas nondental oral MSCs are suitable for periodontal ligament tissue, tendon, and muscle repair.
Dental pulp regeneration
Dental pulp is a connective tissue that contains different types of cells, such as endothelial cells, neurons, fibroblasts, and odontoblasts. Dental pulp is surrounded by a thin layer of dentin matrix and dentin in the pulp chamber. The blood supply to dental pulp is through the apical foramen at the end of the pulp chamber.Citation33 Since the diameter of apical fora-men is only about 250 μm, the microenvironment of dental pulp lacks oxygen and nutrition, which is good for stem cell maintenanceCitation62 but bad for the infection control. Once the dental pulp is infected with pathogens by trauma or caries, it is difficult to remove the pathogens through antimicrobial therapies, often resulting in the extirpation of the whole pulp. Since dental pulp has numerous functions (such as nutritive, protective, reparative, and sensory) in the maintenance of teeth, regeneration of dental pulp has clinical needs.
Gronthos et alCitation8 first isolated human DPSCs from dental pulp in impacted third molars. When in vitro expanded DPSCs were mixed with hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder and then transplanted into the dorsal surface of immunocompromised mice, a dentin–pulp-like complex was observed 6 weeks later. Other dental MSCs, such as SHED and SCAP, also showed the ability to generate the dentin–pulp-like complex after subcutaneous transplantation.Citation53,Citation54 However, no published reports have confirmed that PDLSCs and GMSCs could form a dentin–pulp-like complex under the same conditions, suggesting that the original location determines the fate of MSCs. Similar to HA/TCP ceramic powder, human dentin also has been used as a carrier for dentin–pulp regeneration. Batouli et alCitation63 loaded DPSCs onto a human dentin surface and then subcutaneously transplanted them into immunocompromised mice. At 8 weeks after transplantation, DPSCs were able to form a reparative dentin-like structure on the human dentin surface. Inside the dentin-like structure, blood vessels and connective tissue were observed, indicating dentin–pulp complex formation. Further study showed that the pretreatment of dentin could influence cellular behavior of subcutaneously transplanted DPSCs at the cell–dentin interface. On ethylenediaminetetraacetic acid (EDTA)-treated dentin cylinders, DPSCs could form a vascularized soft connective tissue similar to dental pulp, whereas on the NaOCl-treated dentin cylinders, DPSCs did not organize well.Citation64 These data suggested that dental MSCs are able to regenerate dental pulp on calcific carriers under certain conditions. However, in most cases, transplanted stem cells survive only in vascularized places (such as subcutaneous tissue). Without an abundant blood supply, most transplanted stem cells will undergo necrotic or apoptotic cell death. Since dental pulp is housed in the pulp chamber, the blood supply can only come from the narrow apical foramen. Thus regenerating dental pulp inside the pulp chamber by transplanting dental MSCs remains a major challenge. A study showed that SHED and DPSCs could regenerate a pulp-like tissue in emptied root canal space (6–7 mm in length) with enlarged diameter (>2 mm) after subcutaneous transplantation.Citation65 Since enlarged root canal passably increases the risk of infection, it is necessary to use different ways to increase angiogenesis and the viability of transplanted dental MSCs. Granulocyte-colony stimulating factor (G-CSF) showed stimulative effects on angiogenesis and stem cell mobilization.Citation66,Citation67 Iohara et alCitation68 and Murakami et alCitation69 demonstrated that G-CSF-treated DPSCs express a high level of trophic factors with properties of high proliferation, migration, and antiapoptotic effects and are endowed with regenerative potential. They transplanted G-CSF-mobilized autologous DPSCs with drug-approved G-CSF into dog pulpectomized tooth. At 14 days after transplantation, a pulp-like tissue with good vasculature and innervation was observed. The DPSCs differentiated into odontoblasts-like cells attached to the dentinal wall in the root canal and expressed enamelysin/matrix metalloproteinase 20, a marker for odontoblasts. Based on their results, a clinical trial has already been started in Japan. Although some safety issues need to be solved, the clinical applications of dental MSCs for pulp regeneration will not be far.
Periodontal ligament tissue regeneration
Periodontal ligament is the supporting tissue of tooth. It has the following functions: 1) suspends the tooth in its bony socket, the alveolus proper; 2) supplies nutrients to alveolus and cementum; 3) protects teeth; and 4) maintains homeostasis of teeth by PDLSCs.Citation33 Periodontal disease or periodontitis is a disease with chronic inflammation-induced collapse of periodontal ligament tissue that often results in alveolar bone destruction, and eventually tooth loss.Citation70 MSCs-based periodontal therapy has been demonstrated to inhibit inflammation, promote bone regeneration, and prevent tooth loss.Citation71 Since PDLSCs are isolated from periodontal ligament, and they are able to generate typical cementum/periodontal ligament-like structure in vivo, they are considered as the first choice for periodontal ligament regeneration. Akizuki et alCitation72 applied periodontal ligament cell sheets to dehiscence defects of the mesial roots in dogs. The cell sheet showed improvement in periodontal tissue healing with bone, periodontal ligament, and cementum formation. Ding et alCitation73 reported that allogeneic PDLSC sheets could significantly stimulate periodontal tissue regeneration and cure inflammation in a miniature pig periodontitis model. The transplanted PDLSCs moderated the inflammatory response of periodontitis in part by suppressing the activation of both T cells and B cells.Citation74 Vitamin C treatment was capable of inducing telomerase activity in PDLSCs, leading to a significant improvement in periodontal tissue regeneration compared with untreated control in a periodontal defects swine model.Citation75
Oral MSCs derived from human gingiva (GMSCs) also have been considered as a promising alternative cell source for periodontal regeneration.Citation76 In a class III furcation defects dog model, the transplanted GMSCs significantly enhanced the regeneration of the damaged periodontal tissue, including the alveolar bone, cementum, and functional periodontal ligament.Citation77 Moreover, autologous DPSCs also have been used for periodontal regeneration in animal models and have shown promising results.Citation78
Neural regeneration
Adult mammalian central nerve system (CNS) lacks regenerative power to replace the damaged neuronal cells (including neurons and glial cells) and reconstruct the dendritic connections. The reasons are considered to be that 1) neuronal progenitors have limited ability to regenerate functional neuronal cells; and 2) the local microenvironment, especially glial scar, inhibits neural regeneration. Transplanted stem cells therapy showed hopeful effects by providing neuronal progenitors and improving microenvironment in the injured site of CNS.Citation79
Both dental and nondental MSCs are mainly derived from neural crest stem cells (which also give rise to neuronal cells).Citation80 These two types of oral MSCs display neural crest stem cell features with expression of markers of neural progenitors, such as nestin, p75/NGFR, Pax6, and Tuj1.Citation81,Citation82 With neuronal stimulations, human dental MSCs (DPSCs, SHED, and SCAP) could differentiate into neural lineage in vitro.Citation53,Citation83,Citation84 We observed that under a serum-free condition, human DPSCs are able to form spheroids with positive expression of neuronal progenitor marker HuC/D. Even without neuronal induction, gene expression of neural markers CDH2, NFM, TUBB3, and CD24 in the spheroids were observed and increased in a culture time-dependent manner.Citation85 It has been reported that when transplanted into the injured site of the CNS, DPSCs and SHED enhanced neuronal recovery in animal models of the CNS injuries.Citation86,Citation87 Although some studies showed evidence that DPSCs and SHED could differentiate into functional neurons with a voltage-gated, tetrodotoxin-sensitive inward current in vitro,Citation83 expressed neuronal markers, and migrated into the CNS in vivo,Citation53,Citation88 most of the in vivo studies indicated that DPSCs and SHED did not differentiate into functional neuronal cells in the lesion site of animal models. The improvement of DPSCs and SHED on neuronal recovery is likely due to their neurotrophic products.Citation89 Moreover, implanted DPSCs and SHED directly inhibited multiple axon growth inhibitor signals generated by the glial scar, suggesting that they can improve the microenvironment.Citation90 To date, there is no in vivo study about the effect of SCAP and nondental MSCs (PDLSCs and GMSCs) on neuronal repair.
Bone regeneration
Bone development involves the aggregation of MSCs into mesenchymal condensations, which is partly similar to tooth development but without the epithelial invagination. There are two types of bone formation: intramembranous and endochondral. In endochondral bone formation, the mesenchymal condensations first undergo chondrogenesis and then ossification to form cartilage and bone.Citation91 During adulthood, bone possesses the intrinsic capacity for regeneration throughout life. In most bone injuries (fractures), the damaged bone tissue can be functionally regenerated by the local cells (including chondroblasts, osteoblasts, endotheliocytes, and fibroblasts). However, when the fractures are serious (such as large bone defects created by trauma, infection, tumor resection, and skeletal abnormalities) enough that self-healing cannot repair, an adequate supply of stem cells (such as bone marrow stem cells) is required for efficient bone regeneration.Citation92 Oral MSCs seem to be ideal candidates for bone regeneration. Both dental and nondental MSCs are able to differentiate into chondroblasts and osteoblasts under inductive conditions in vitro.Citation93–Citation96 An in vivo study showed that human DPSCs generated both osteoblasts and endotheliocytes, and eventually formed a bone-like structure with an integral blood supply similar to that of human adult bone in immunocompromised rats.Citation97 Zheng et alCitation98 reported that stem cells from miniature pig deciduous teeth were able to regenerate bone to repair critical-size mandibular defects in a swine model. In a clinical study, DPSCs and collagen sponge scaffold formed a biocomplex that could completely restore mandible bone defects in patients.Citation99 Seo et alCitation100 reported that SHED were able to repair the defects with substantial bone formation on the calvaria of immunocompromised mice. They also tested the bone regeneration capacity of PDLSCs and GMSCs encapsulated in a novel RGD (arginine–glycine–aspartic acid tripeptide)-coupled alginate microencapsulation system both in vitro and in vivo. Results confirmed that PDLSCs were able to repair the calvarial defects by promoting the formation of mineralized tissue, while GMSCs showed a significantly lesser osteogenic differentiation capability.Citation101
Muscle regeneration
Some research groups have focused on the muscle- and tendon-forming properties of oral stem cells. Armiñán et alCitation102 first reported that DPSCs could differentiate into cardiomyocyte-like cells when cocultivated with neonatal rat cardiomyocytes for about 4 weeks in vitro. Yang et alCitation103 demonstrated that DPSCs were able to differentiate into dystrophin-producing muscle cells in cardiotoxin-paralyzed muscles in a mouse model, which has implications for the study and treatment of muscular dystrophy.
Tendon and cartilage regeneration
Tendons have very limited ability for self-repair after injuries. Since periodontal ligaments are similar to tendons (they both have the ability to absorb mechanical forces of stress and strain), PDLSCs have been used for tendon regeneration. Gronthos et alCitation104 reported that ovine PDLSCs express scleraxis, a tendon-specific transcription factor in vitro. A recent study by the same group demonstrated that human PDLSCs and GMSCs encapsulated in RGD-coupled alginate microspheres, loaded with TGF-β3, could form tendon-like tissue after subcutaneous transplantation into immunocompromised mice. Compared with GMSCs and human bone marrow MSCs, PDLSCs showed a more organized structure, with more extracellular matrix and collagen, suggesting PDLSCs have better potential for tendon regeneration.Citation105
Cartilage (especially articular cartilage) also has very limited regenerative power. The treatment of cartilage injuries remains one of the most difficult challenges.Citation106 Cell sheet technology has achieved robust cartilage repair in animal models.Citation107,Citation108 A clinical study has just started in Japan by implanting cartilaginous cell sheets to patients.Citation109 PDLSCs and GMSCs have shown potential for cartilage regeneration. Moshaverinia et alCitation110 reported that RGD-coupled alginate hydrogel can be used to encapsulate PDLSCs and GMSCs for cartilage regeneration. After 4 weeks of chondrogenic induction in vitro, PDLSCs and GMSCs differentiated into chondrocyte-like cells. In animal studies, ectopic cartilage tissue regeneration was observed in the areas of transplanted RGD-PDLSCs and RGD-GMSCs. Although some studies demonstrated that DPSCs could express cartilaginous markers after induction,Citation111,Citation112 to date there is no report about the in vivo study.
Other regeneration
DPSCs have been demonstrated to have therapeutic potential for ischemic injuries. An in vivo study showed that DPSCs could increase angiogenesis and reduce infarct size in rats with acute myocardial infarction. However, the transplanted DPSCs did not differentiate into endothelial cells, smooth muscle cells, or cardiac muscle cells within the infarct, suggesting that DPSCs possibly stimulated angiogenesis through secretion of paracrine factors.Citation113 In models of mouse hind-limb ischemia, local transplantation of a highly vasculogenic subfraction of side population cells from dental pulp resulted in successful engraftment and an increase in the blood flow, including high density of capillary formation. In situ hybridization results showed that transplated DPSCs express an angiogenic marker (VEGF-A), chemokines (G-CSF, GM-CSF, CXCR4), and matrix-degrading enzymes (MMP1, MMP3) in the ischemic region 7 days after transplantation, implying that DPSCs promote neovascularization by paracrine actions of proangiogenic and chemotactic cytokines.Citation114
Govindasamy et alCitation115 explored the potential of DPSCs to differentiate into pancreatic cell lineage resembling islet-like cell aggregates in vitro. They demonstrated that DPSCs could differentiate into islet-like cell aggregates with positive expression of pancreatic markers C-peptide, Pdx-1, Pax4, Pax6, Ngn3, and Isl-1. Ishkitiev et alCitation59 reported that CD117+ SHED and DPSCs could differentiate into a pancreatic lineage in serum-free conditions. After pancreatic differentiation in vitro, the expression of pancreatic-specific endocrine markers insulin, glucagon, somatostatin, and pancreatic polypeptide, and exocrine marker alpha amylase-2a were detected by real-time reverse-transcription polymerase chain reaction in both SHED and DPSCs. A recent report showed that rat dental pulp cells could be converted into insulin-producing cells in vitro after being transfected with the transcription factors Pdx1 and Neurog3.Citation60
Ishkitiev et alCitation61,Citation116,Citation117 also reported that CD117+ SHED and DPSCs were able to differentiate into hepatocyte-like cells under both serum-containing and serum-free conditions in vitro, implying that SHED and DPSCs can be potential sources for hepatocyte regeneration. This hepatic differentiation of DPSCs and SHED could be promoted by an oral malodorous compound, hydrogen sulfide (H(2)S).Citation118
Moreover, human immature DPSCs (hIDPSCs) have been used for ocular surface reconstruction. Gomes et alCitation119 transplanted a tissue-engineered hIDPSCs sheet onto the corneal bed of a rabbit model of total limbal stem cell deficiency. After 3 months, hIDPSC transplantation improved functional corneal regeneration and integrated into the local ocular tissue.
Conclusion
Oral epithelial and mesenchymal stem cells are easily obtained as discarded biological materials. Their excellent regenerative ability can be applied not only in dentistry but also in various fields of regenerative medicine. As we conclude in , oral stem cells show their capability to repair cornea, dental pulp, periodontal, neural, bone, muscle, tendon, cartilage, and endothelial tissues without neoplasm formation. However, despite these experimental studies demonstrating the regenerative potential of oral stem cells, most of the studies lack strict quantitative analysis for testing the ability of these cells to self-renew, proliferate, and differentiate, especially in vivo. Moreover, before their clinical application, the experimental studies need to resolve the following issues: 1) massive cell death in the transplanted site (it has been reported that in the damaged spinal cord only a few percent of the transplanted oral stem cells could survive, and they have difficulty to integrate into the local tissue;Citation89 therefore, viability and functional differentiation of oral stem cells in vivo need to be improved); particularly for neuronal regeneration, 2) the interaction between transplanted oral stem cells and local cells or microenvironment needs to be analyzed; 3) in vivo cell lineage tracing of transplanted oral stem cells is required for understanding their fate and behavior; 4) since oral stem cells, especially oral epithelial stem cells, are often involved in neoplasia, the cellular and molecular mechanisms that allow oral stem cells to choose self-renewal, canceration, and differentiation should be well studied.
Table 1 Oral stem cells and their regenerative applications
Furthermore, although these experimental assays are very informative to discover the features of oral stem cells, they do not exactly reflect the physiological and pathological conditions of the damaged tissues in human body. Up to now, only a few human clinical trials are underway to use oral stem cells for the regeneration of cornea, dental pulp, and bone. For the confirmation of their true regenerative power, double-blind randomized controlled trials need to be performed. As the clinical trials require a large number of clinical-grade cells in a short time, banking and manufacturing of oral stem cells are the deal strategies for clinical use. In the light of present research, there are two approaches for preserving oral stem cells: cryopreservation and magnetic freezing. Both the approaches can successfully store oral stem cells for a long time (>10 years) with high cell survival rates after thawing. The procedure for banking oral stem cells is well described in an excellent review.Citation120 Since Hiroshima University first established a commercial teeth bank in Japan in 2004, numerous biocompanies for teeth banking started business across more than 20 countries in America, Europe, and Asia. With the advanced preservation technology, dentistry will serve as a gateway to a wide variety of regenerative therapies by effectively using these valuable stem cell resources.
Acknowledgments
This work was supported in part by the Nippon Dental University Research Project 4 Grant and Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (26861689). The authors thank Mr Nathaniel Green for proofreading.
Disclosure
The authors report no conflicts of interest in this work.
References
- BehjatiSHuchMvan BoxtelRGenome sequencing of normal cells reveals developmental lineages and mutational processesNature201451342242525043003
- DoetschmanTCEistetterHKatzMSchmidtWKemlerRThe in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardiumJ Embryol Exp Morphol19858727453897439
- OdoricoJSKaufmanDSThomsonJAMultilineage differentiation from human embryonic stem cell linesStem Cells200119319320411359944
- AufderheideEChiquet-EhrismannREkblomPEpithelial-mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchymeJ Cell Biol198710515996082440899
- VainioSKaravanovaIJowettAThesleffIIdentification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth developmentCell199375145588104708
- KørblingMEstrovZAdult stem cells for tissue repair–a new therapeutic concept?N Engl J Med2003349657058212904523
- PittengerMFMackayAMBeckSCMultilineage potential of adult human mesenchymal stem cellsScience1999284541114314710102814
- GronthosSMankaniMBrahimJRobeyPGShiSPostnatal human dental pulp stem cells (DPSCs) in vitro and in vivoProc Natl Acad Sci U S A20009725136251363011087820
- StremBMHicokKCZhuMMultipotential differentiation of adipose tissue-derived stem cellsKeio J Med200554313214116237275
- JoannidesAGaughwinPSchwieningCEfficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cellsLancet2004364942917217815246730
- BiebackKKernSKlüterHEichlerHCritical parameters for the isolation of mesenchymal stem cells from umbilical cord bloodStem Cells200422462563415277708
- DennisJECharbordPOrigin and differentiation of human and murine stromaStem Cells200220320521412004079
- TakashimaYEraTNakaoKNeuroepithelial cells supply an initial transient wave of MSC differentiationCell200712971377138817604725
- CaplanAIAdult mesenchymal stem cells for tissue engineering versus regenerative medicineJ Cell Physiol2007213234134717620285
- FengJMantessoADe BariCNishiyamaASharpePTDual origin of mesenchymal stem cells contributing to organ growth and repairProc Natl Acad Sci U S A2011108166503650821464310
- Nombela-ArrietaCRitzJSilbersteinLEThe elusive nature and function of mesenchymal stem cellsNat Rev Mol Cell Biol201112212613121253000
- OswaldJBoxbergerSJørgensenBMesenchymal stem cells can be differentiated into endothelial cells in vitroStem Cells200422337738415153614
- TropelPPlatetNPlatelJCFunctional neuronal differentiation of bone marrow-derived mesenchymal stem cellsStem Cells200624122868287616902198
- SasakiMAbeRFujitaYAndoSInokumaDShimizuHMesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell typeJ Immunol200818042581258718250469
- LeguéESequeiraINicolasJFHair follicle renewal: authentic morphogenesis that depends on a complex progression of stem cell lineagesDevelopment2010137456957720110322
- TakedaNJainRLeBoeufMRWangQLuMMEpsteinJAInterconversion between intestinal stem cell populations in distinct nichesScience201133460611420142422075725
- RitsmaLEllenbroekSIZomerAIntestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imagingNature2014507749236236524531760
- PlikusMVGayDLTreffeisenEWangASupapannachartRJCotsarelisGEpithelial stem cells and implications for wound repairSemin Cell Dev Biol201223994695323085626
- OshimaHRochatAKedziaCKobayashiKBarrandonYMorphogenesis and renewal of hair follicles from adult multipotent stem cellsCell2001104223324511207364
- BarkerNBartfeldSCleversHTissue-resident adult stem cell populations of rapidly self-renewing organsCell Stem Cell20107665667021112561
- MorrisonKMMiesegaesGRLumpkinEAMaricichSMMammalian Merkel cells are descended from the epidermal lineageDev Biol20093361768319782676
- Van KeymeulenAMascreGYouseffKKEpidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasisJ Cell Biol200918719110019786578
- Angelova VolponiAKawasakiMSharpePTAdult human gingival epithelial cells as a source for whole-tooth bioengineeringJ Dent Res201392432933423458883
- MrozikKGronthosSShiSBartoldPMA method to isolate, purify, and characterize human periodontal ligament stem cellsMethods Mol Biol201066626928420717790
- ThesleffIPartanenAMVainioSEpithelial-mesenchymal interactions in tooth morphogenesis: the roles of extracellular matrix, growth factors, and cell surface receptorsJ Craniofac Genet Dev Biol19911142292371725871
- ThesleffIVaahtokariAKettunenPAbergTEpithelial-mesenchymal signaling during tooth developmentConnect Tissue Res1995321–49157554939
- ThesleffIEpithelial-mesenchymal signalling regulating tooth morphogenesisJ Cell Sci2003116Pt 91647164812665545
- GartnerLPOral Histology and EmbryologyBaltimore, MDWilliams and Wilkins1989
- GoldbergMThe Dental Pulp: Biology, Pathology, and Regenerative TherapiesBerlin, Heidelberg, NYSpringer2014
- Le DouarinNMCreuzetSCoulyGDupinENeural crest cell plasticity and its limitsDevelopment2004131194637465015358668
- DassuleHRLewisPBeiMMaasRMcMahonAPSonic hedgehog regulates growth and morphogenesis of the toothDevelopment2000127224775478511044393
- NanciATen Cate’s Oral Histology: Development, Structure, and Function7th edSt Louis, MOMosby2007
- NakaoKMoritaRSajiYThe development of a bioengineered organ germ methodNat Methods20074322723017322892
- IkedaEMoritaRNakaoKFully functional bioengineered tooth replacement as an organ replacement therapyProc Natl Acad Sci U S A200910632134751348019666587
- ToyoshimaKEAsakawaKIshibashiNFully functional hair follicle regeneration through the rearrangement of stem cells and their nichesNat Commun201217378422510689
- XiaoLTsutsuiTThree-dimensional epithelial and mesenchymal cell co-cultures form early tooth epithelium invagination-like structures: expression patterns of relevant moleculesJ Cell Biochem201211361875188522234822
- XiaoLKumazawaYOkamuraHCell death, cavitation and spontaneous multi-differentiation of dental pulp stem cells-derived spheroids in vitro: a journey to survival and organogenesisBiol Cell Epub8292014
- NakamuraTEndoKKinoshitaSIdentification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signalingStem Cells200725362863817110619
- JonesKBKleinODOral epithelial stem cells in tissue maintenance and disease: the first steps in a long journeyInt J Oral Sci20135312112923887128
- SenSSharmaSGuptaAMolecular characterization of explant cultured human oral mucosal epithelial cellsInvest Ophthalmol Vis Sci201152139548955422064988
- NishidaKYamatoMHayashidaYCorneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epitheliumN Engl J Med2004351121187119615371576
- MaDHKuoMTTsaiYJTransplantation of cultivated oral mucosal epithelial cells for severe corneal burnEye (Lond)20092361442145019373264
- BurillonCHuotLJustinVCultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiencyInvest Ophthalmol Vis Sci20125331325133122064987
- OhkiTYamatoMMurakamiDTreatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine modelGut200655121704171016709659
- McIntosh AmbroseWSalahuddinASoSCollagen Vitrigel membranes for the in vitro reconstruction of separate corneal epithelial, stromal, and endothelial cell layersJ Biomed Mater Res B Appl Biomater200990281883119283827
- TaniATadaYTakezawaTRegeneration of tracheal epithelium using a collagen vitrigel-sponge scaffold containing basic fibroblast growth factorAnn Otol Rhinol Laryngol2012121426126822606930
- WhiteRANeimanJMReddiAEpithelial stem cell mutations that promote squamous cell carcinoma metastasisJ Clin Invest2013123104390440423999427
- MiuraMGronthosSZhaoMSHED: stem cells from human exfoliated deciduous teethProc Natl Acad Sci U S A2003100105807581212716973
- HuangGTSonoyamaWLiuYLiuHWangSShiSThe hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineeringJ Endod200834664565118498881
- SeoBMMiuraMGronthosSInvestigation of multipotent postnatal stem cells from human periodontal ligamentLancet2004364942914915515246727
- ZhangQShiSLiuYMesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitisJ Immunol2009183127787779819923445
- MaoJJProckopDJStem cells in the face: tooth regeneration and beyondCell Stem Cell201211329130122958928
- HuangGTGronthosSShiSMesenchymal stem cells derived from dental tissues vs those from other sources: their biology and role in regenerative medicineJ Dent Res200988979280619767575
- IshkitievNYaegakiKKozhuharovaAPancreatic differentiation of human dental pulp CD117+ stem cellsRegen Med20138559761223998753
- NozakiTOhuraKRegulation of miRNA during direct reprogramming of dental pulp cells to insulin-producing cellsBiochem Biophys Res Commun2014444219519824440707
- IshkitievNYaegakiKImaiTHigh-purity hepatic lineage differentiated from dental pulp stem cells in serum-free mediumJ Endod201238447548022414832
- CipolleschiMGDello SbarbaPOlivottoMThe role of hypoxia in the maintenance of hematopoietic stem cellsBlood1993827203120378104535
- BatouliSMiuraMBrahimJComparison of stem-cell-mediated osteogenesis and dentinogenesisJ Dent Res2003821297698114630898
- GallerKMD’SouzaRNFederlinMDentin conditioning codetermines cell fate in regenerative endodonticsJ Endod201137111536154122000458
- HuangGTYamazaTSheaLDStem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo modelTissue Eng Part A201016260561519737072
- NatoriTSataMWashidaMHirataYNagaiRMakuuchiMG-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cellsBiochem Biophys Res Commun200229741058106112359263
- IedaYFujitaJIedaMG-CSF and HGF: combination of vasculogenesis and angiogenesis synergistically improves recovery in murine hind limb ischemiaJ Mol Cell Cardiol200742354054817223129
- IoharaKMurakamiMTakeuchiNA novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regenerationStem Cells Transl Med20132752153323761108
- MurakamiMHoribeHIoharaKThe use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potentialBiomaterials201334369036904723988014
- TatakisDNKumarPSEtiology and pathogenesis of periodontal diseasesDent Clin North Am200549349151615978238
- RaczGZKadarKFoldesAImmunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitisJ Physiol Pharmacol201465332733924930504
- AkizukiTOdaSKomakiMApplication of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogsJ Periodontal Res200540324525115853971
- DingGLiuYWangWAllogeneic periodontal ligament stem cell therapy for periodontitis in swineStem Cells201028101829183820979138
- LiuOXuJDingGPeriodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1Stem Cells20133171371138223553748
- WeiFQuCSongTVitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activityJ Cell Physiol201222793216322422105792
- YangHGaoLNAnYComparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditionsBiomaterials201334297033704723768902
- YuXGeSChenSHuman gingiva-derived mesenchymal stromal cells contribute to periodontal regeneration in beagle dogsCells Tissues Organs2013198642843724777155
- KhorsandAEslaminejadMBArabsolgharMAutologous dental pulp stem cells in regeneration of defect created in canine periodontal tissueJ Oral Implantol201339443344323964777
- HornerPJGageFHRegenerating the damaged central nervous systemNature2000407680796397011069169
- XiaoLTsutsuiTHuman dental mesenchymal stem cells and neural regenerationHum Cell2013263919623817972
- HuangLLiangJGengYDirecting adult human periodontal ligament-derived stem cells to retinal fateInvest Ophthalmol Vis Sci20135463965397423661377
- XuXChenCAkiyamaKGingivae contain neural-crest- and mesoderm-derived mesenchymal stem cellsJ Dent Res201392982583223867762
- ArthurARychkovGShiSKoblarSAGronthosSAdult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cuesStem Cells20082671787179518499892
- SonoyamaWLiuYYamazaTCharacterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot studyJ Endod200834216617118215674
- XiaoLTsutsuiTCharacterization of human dental pulp cells-derived spheroids in serum-free medium: stem cells in the coreJ Cell Biochem2013114112624263623794488
- YangKLChenMFLiaoCHPangCYLinPYA simple and efficient method for generating Nurr1-positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapyCytotherapy200911560661719579137
- YamagataMYamamotoAKakoEHuman dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal miceStroke201344255155423238858
- WangJWeiXLingJHuangYGongQHuoYIdentification and characterization of side population cells from adult human dental pulp after ischemic cultureJ Endod201238111489149723063223
- SakaiKYamamotoAMatsubaraKHuman dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanismsJ Clin Invest20121221809022133879
- MeadBLoganABerryMLeadbeaterWSchevenBAIntravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injuryInvest Ophthalmol Vis Sci201354127544755624150755
- KhuranaJSBone pathology2nd edPhiladephia, PASpringer2009
- DimitriouRJonesEMcGonagleDGiannoudisPVBone regeneration: current concepts and future directionsBMC Med201196621627784
- YamadaYItoKNakamuraSUedaMNagasakaTPromising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrowCell Transplant20112071003101321054950
- GrazianoAd’AquinoRLainoGPapaccioGDental pulp stem cells: a promising tool for bone regenerationStem Cell Rev200841212618300003
- MoriGBrunettiGOrangerADental pulp stem cells: osteogenic differentiation and gene expressionAnn N Y Acad Sci20111237475222082364
- ChadipirallaKYochimJMBahuleyanBOsteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teethCell Tissue Res2010340232333320309582
- d’AquinoRGrazianoASampaolesiMHuman postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formationCell Death Differ20071461162117117347663
- ZhengYLiuYZhangCMStem cells from deciduous tooth repair mandibular defect in swineJ Dent Res200988324925419329459
- d’AquinoRDe RosaALanzaVHuman mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexesEur Cell Mater200918758319908196
- SeoBMSonoyamaWYamazaTSHED repair critical-size calvarial defects in miceOral Dis200814542843418938268
- MoshaveriniaAChenCXuXBone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffoldTissue Eng Part A2014203–461162124070211
- ArmiñánAGandíaCBartualMCardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cellsStem Cells Dev200918690791818983250
- YangRChenMLeeCHYoonRLalSMaoJJClones of ectopic stem cells in the regeneration of muscle defects in vivoPLoS One2010510e1354720975999
- GronthosSMrozikKShiSBartoldPMOvine periodontal ligament stem cells: isolation, characterization, and differentiation potentialCalcif Tissue Int200679531031717033723
- MoshaveriniaAXuXChenCApplication of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regenerationBiomaterials20143592642265024397989
- OrthPRey-RicoAVenkatesanJKMadryHCucchiariniMCurrent perspectives in stem cell research for knee cartilage repairStem Cells Cloning2014711724520197
- EbiharaGSatoMYamatoMCartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig modelBiomaterials201233153846385122369960
- YanHYuCRepair of full-thickness cartilage defects with cells of different origin in a rabbit modelArthroscopy200723217818717276226
- SatoMYamatoMHamahashiKOkanoTMochidaJArticular cartilage regeneration using cell sheet technologyAnat Rec (Hoboken)20142971364324293096
- MoshaveriniaAXuXChenCAkiyamaKSneadMLShiSDental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regenerationActa Biomater20139129343935023891740
- RizkARabieABHuman dental pulp stem cells expressing transforming growth factor β3 transgene for cartilage-like tissue engineeringCytotherapy201315671272523474328
- YuJHeHTangCDifferentiation potential of STRO-1+dental pulp stem cells changes during cell passagingBMC Cell Biol2010113220459680
- GandiaCArmiñanAGarcía-VerdugoJMHuman dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarctionStem Cells200826363864518079433
- IoharaKZhengLWakeHA novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulpStem Cells20082692408241818583536
- GovindasamyVRonaldVSAbdullahANDifferentiation of dental pulp stem cells into islet-like aggregatesJ Dent Res201190564665221335539
- IshkitievNCalenicBAoyamaIIiHYaegakiKImaiTHydrogen sulfide increases hepatic differentiation in tooth-pulp stem cellsJ Breath Res20126101710322368253
- IshkitievNYaegakiKCalenicBDeciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitroJ Endod201036346947420171365
- OkadaMIshkitievNYaegakiKHydrogen sulfide increases hepatic differentiation of human tooth-pulp stem cells compared with human bone-marrow stem cellsInt Endod J201447121142115024517624
- GomesJAGeraldes MonteiroBMeloGBCorneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cellsInvest Ophthalmol Vis Sci20105131408141419892864
- AroraVAroraPMunshiAKBanking stem cells from human exfoliated deciduous teeth (SHED): saving for the futureJ Clin Pediatr Dent200933428929419725233