Abstract
Stem cell therapy and tissue engineering represent a forefront of current research in the treatment of heart disease. With these technologies, advancements are being made into therapies for acute ischemic myocardial injury and chronic, otherwise nonreversible, myocardial failure. The current clinical management of cardiac ischemia deals with reestablishing perfusion to the heart but not dealing with the irreversible damage caused by the occlusion or stenosis of the supplying vessels. The applications of these new technologies are not yet fully established as part of the management of cardiac diseases but will become so in the near future. The discussion presented here reviews some of the pioneering works at this new frontier. Key results of allogeneic and autologous stem cell trials are presented, including the use of embryonic, bone marrow-derived, adipose-derived, and resident cardiac stem cells.
Introduction
It is well known that cardiovascular disease is a main cause of morbidity and mortality worldwide.Citation1 Traditional medical and surgical therapies have had success in the treatment of many cardiovascular diseases, such as coronary artery disease and valvular diseases, but have had limited success in the therapy of damaged myocardium. Acute ischemic myocardial damage and chronic myocardial failure have been challenging conditions for which to provide an adequate long-term prognosis, although a recent study by Beltrami et al,Citation2 demonstrated the ability of cardiac cells (cardiomyocytes) to divide after the occurrence of myocardial infarction (MI), and reentering the human cell cycle, but that may not be enough to provide the needed quantity of cells to restore the damage; the common belief before that study was that myocytes are unable to divide depending on the interpretation of the scar formation after the infarction.
This aspect widens our perspective of the management approach – from being dependent solely on medical, percutaneous coronary intervention (PCI) and a surgical approach, to include a new side for management that includes the application of stem cell therapy – as these conditions have so far exceeded the reach of traditional medicine. The use of stem cells and tissue engineering has been tested in the laboratories and clinical trials as a potential solution for future treatment.
When engineering tissue for use as a cardiovascular therapy, there are three main points to consider: scaffolds, cell sources, and signaling factors.
Scaffolds
A “scaffold” is a substitute that provides a structural platform for a new cellular microenvironment that supports new tissue formation. It allows cell attachment, migration, differentiation, and organization that can aid in delivering soluble and bound biochemical factors.Citation3
Cell sources
The choice of cells to populate a scaffold depends on the purpose of the new tissue graft. The new cells will synthesize the bulk of the mass of a tissue matrix, and will form the integrating connections with existing native tissues. They also maintain tissue homeostasis in general and provide various metabolic supports to other tissues and organs. Terminally differentiated cells have been used with variable degrees of success and there are some limitations to their use in tissue engineering, but stem cells, and more recently adult stem cells, have become the major players in most new tissue replacement strategies.Citation4 Their favorable properties are being harnessed to drive most new tissue engineering processes.Citation5
Signaling factors
Signaling factors can influence, and even direct, a new tissue’s phenotype. Their application has been learned from signals observed during native tissue formation and they have direct and indirect effects on cell metabolism, migration, and organization.Citation3
Stem cell types used for cardiac repair
Xenogeneic cells from nonhuman species have limitations in therapeutic strategies due to significant differences in antigens between species, potentially leading to graft rejection. Meanwhile, allogeneic cells from human donors are likely to have greater success after implantation. Allogeneic stem cells include umbilical cord-derived cells, fetal cardiomyocytes, and embryonic mesenchymal stem cells (EmSCs). These cells, however, are still potentially subjected to immune surveillance and rejection.
To eliminate the potential for allogeneic rejection, autologous cells from the same individual have become a central focus of stem cell research. This category of cells includes skeletal myoblasts, adipose-derived stem cells (AdSCs), resident cardiac stem cells (RCSCs) and bone marrow-derived (BMD) stem cells, such as CD34+ cells, induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs), multipotent adult progenitor cells, and endothelial progenitor cells (EPCs).
Allogeneic sources
Fetal cardiomyocytes
Fetal cardiomyocytes have significant potential for integration and regeneration.Citation6,Citation7 However, there are concerns, including immunogenicity, malignant potential, ethical questions, as well as limited availability. For these reasons, other cell types have surpassed this source as likely candidates for use in cardiac regenerative therapy.
EmSCs
EmSCs have broad potential to differentiate into cells from all three embryonic germ layers. In addition, intact cardiomyocytes have been produced in vitro as well.Citation8 However, there are concerns about the use of EmSCs, due to their association with teratoma formation in rodent modelsCitation9 and concerns about their potential malignant transformation. Moreover, the ethical and legal issues surrounding the use of human EmSCs have obstructed further exploration and shifted the existing attention onto other alternative sources for stem cell therapy in cardiac repair.
Human umbilical cord blood-derived cells
Human umbilical cord blood-derived cells are presently used for repopulating bone marrow in patients treated for bone marrow illnesses such as acute leukemia. Human cord blood contains a big number of non-hematopoietic stem cells that show fewer class II human leukocyte antigens and appears not to trigger an immune response, thus dropping the risk of rejection. This reduced immunological activity provides a striking option for regenerative therapy.Citation10 A significant reduction in infarct size has also been shown after intramyocardial injection of human cord blood derived cells in animal models.Citation11
Autologous sources
AdSCs
AdSCs have been considered as a source of adult stem cells for cardiac repair. Adipose tissue includes a heterogeneous mixture of MSCs, hematopoietic stem cells, and EPCs. Consequently, the major clinical advantages of this type of cell is their availability, easy harvesting, and relatively low cost. Preclinical studies have shown that AdSCs are associated with improvement in ventricular function in animal models of MI. The mechanism of neoangiogenesis formation has hypothesized paracrine effects as a possible mechanism of action for AdSCs.Citation12,Citation13
Skeletal myoblasts
Skeletal myoblasts can be harvested by muscle biopsy from the individual and grafted to cardiac tissue. Preclinical animal studies have verified their ability to engraft, create myotubules, and improve cardiac function after transfer into infarcted myocardium.Citation14 Clinical human trials such as the Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial and others have demonstrated that epicardial injection of skeletal myoblasts during coronary artery bypass graft (CABG) surgery is feasible with likely functional benefits (see for study summaries).Citation15,Citation16 A major limitation, however, is that they remain devoted to the skeletal muscle lineage and have been associated with arrhythmias due to separation of cardiomyocytes by islands of skeletal muscle cells, therefore interfering with the propagation of electrical potentials.Citation15
Table 1 Clinical effects of cell therapy for acute or chronic heart failure with different types of stem cells
BMD stem cells
BMD stem cells are widely studied due to their versatility and ease of collection. Of these, the most frequently tested adult stem cells are BMD mononuclear cells. Encouraging results have been reported in animal models of ischemic heart failure.Citation17
iPSCs
“iPSCs” are autologous adult cells that can be converted into pluripotent cells. Through specific alterations, adult cells can be reprogrammed to express embryonic genes, allowing them to differentiate into tissues other than their specific lineage. Interestingly, a recent publication has led to the discovery of a cardiac progenitor population of cells expressing both platelet-derived growth factor receptor-alpha and kinase insert domain receptor derived from human iPSCs that can give rise to smooth muscle cells (SMCs), cardiomyocytes, and vascular endothelial cells (ECs).Citation18 Also, vascular ECs from human iPSCs can be attained from several distinct progenitor populations.
RCSCs
RCSCs have recently been discovered with great interest. Previously, the heart was considered to lack self-renewal capabilities as a terminally differentiated organ. Evidence has now shown, however, that the heart demonstrates continuous cell division following various injuries; for example, post-MI.Citation2 Several studies have addressed, identified, and isolated RCSCs, which are capable of differentiating into multiple cell types, such as cardiomyocytes or vascular SMCs (VSMCs).Citation19,Citation20 Reduction in the infarct size and improvement of left ventricular (LV) function were noticed in rodent models of MI after RCSC culture and injection.Citation21 These cells are an attractive option for cardiac repair. However, effective harvesting techniques need to be perfected (see for study summaries).
The best-characterized and most well-studied RCSC population is c-kit+/Lin− cells, originally described in the rat heart.Citation19 The c-kit+/Lin− RCSCs, isolated and expanded in culture, exhibit all the properties of induced stem cells. When injected into the injured myocardium, they were shown capable of restoring the cardiac pattern and function in various animal models of ischemic heart model, albeit to a variable extent.Citation22–Citation24 Human c-kit+ cells have also recently been tested as a potential therapy in patients suffering from ischemic cardiomyopathy with promising results.Citation19,Citation24–Citation28
Origin, production, and purification of cardiac stem cells
Since 1960, different experiments and discoveries in the field of stem cells have been made. Emerging clinical application trials started to evolve in the past 15 years and include but are not limited to the study of cardiac regeneration.
Embryologic development of stem cells in the heart
Different theories addressed the origin of stem cells in the heart. The origins of the cells in the heart may affect organ function, which implies that cells for tissue engineering and regenerative medicine should be selected cautiously. The myocardial cells in the adult heart originate from mesodermal precursors during the embryonic period of the heart’s growth. Signaling pathways orchestrate the formation of the first and second heart fields,Citation29 which become cardiomyocytes, and the subsequent growth of the four-chambered heart from a linear heart tube.Citation30,Citation31 After the early framework for the heart is formed, some mesodermal cells from the proepicardium are enrolled to form the epicardium, the single layer of cells enveloping the heart.Citation32
Next, in transition from an epithelial to mesenchymal layer, epicardial cells become migratory mesenchymal cells. These cells enter the underlying myocardium and differentiate into VSMCs as well as fibroblasts of the vascular interstitium and adventitia.
While the origin of the coronary endothelium is controversial, the present understanding is that this endothelium has a well-defined progenitor population of SMCs and fibroblasts in the proepicardium.Citation33,Citation34 It is interesting that only after the main structure of the coronaries is established do the ECs conquer the aorta and create the coronary circulation.Citation35
Differentiation of stem cells into cardiomyocytes
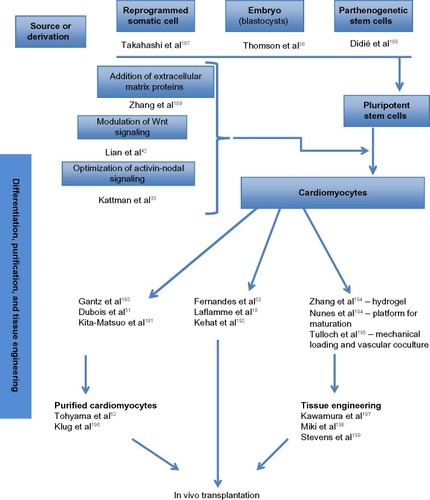
The derivation of cardiomyocytes from human pluripotent stem cells follows a distinct developmental lineage throughout mesoderm induction. There has been a recent increase in the understanding of this process since the ability to derive cardiomyocytes from human EmSCs and iPSCs has driven a transition from using pluripotent cells to form cardiomyocytes for tissue engineeringCitation36 (summarized in ). The use of cardiomyocytes was first introduced when neonatal rat ventricular cardiomyocytes, which were developed for cardiac tissue engineering,Citation34 were seeded into gel-foam and implanted onto an infarcted adult rat heart.Citation35
Differentiation protocols for human EmSCs to cardiomyocytes involve adding bone morphogenetic protein 4 (BMP4) and recombinant human activin A to induce the cardiac mesoderm to reproduce the main foundations of embryonic development.Citation18,Citation37,Citation38 Laflamme et alCitation18 produced cardiomyocyte populations with over 50% cardiac purity (consisting of nodal cells, ventricular cells, and atrial cells). In this protocol, they induced endogenous expression of recognized Wnt ligands, and after mesodermal Wnt induction, successful cardiac differentiation requires subsequent inhibition of this pathway.Citation39 This biphasic signaling profile, which normally occurs during development,Citation40,Citation41 offers an approach to evaluate the efficiency of cardiac differentiation in different human EmSC and human iPSC lines and can be boosted by the addition of exogenous Wnt3a followed by inhibition of Wnt signaling with dickkopf-related protein 1 (DKK1) to increase cardiogenesis.Citation39
Yang et alCitation37 developed a different approach mimicking the three-dimensional (3D) setting of an evolving embryo using small cell groups called “embryoid bodies”. These cells are exposed to basic fibroblast growth factor (bFGF), activin A, BMP4, vascular endothelial growth factor (VEGF), and, after mesodermal induction, DKK1 in a hypoxic environment for 10–12 days. A positive cardiovascular progenitor population expressing kinase insert domain receptor and platelet-derived growth factor receptor-alpha could be isolated on the 4th day, which revealed a differentiation of greater than 50% cardiac troponin T (cTnT)+ cardiomyocytes in multiple lines of human EmSC and human iPSCs.Citation33 Progenitor cardiovascular cells derived using this protocol also give rise to low levels of ECs (CD31+), fibroblasts (discoidin domain receptor 2 [DDR2]+), and VSMCs.
Another approach used the glycogen synthase kinase 3 (GSK3) inhibitor to activate Wnt/β-catenin signaling and mesoderm differentiation, which yielded high levels of cardiogenesis to produce greater than 80% cardiomyocytes.Citation42
An alternative approach uses transdifferentiation and reprogramming of fibroblasts directly into cardiomyocytes at the in vivo stage, using gene therapy, avoiding the pluripotent stage.Citation43,Citation44 However, reproducing this high-yield work has proven to be a challenge,Citation45 with very few transduced cells demonstrating contractile activity and several of these cells demonstrating only a partially reprogrammed, cardiomyocyte-like phenotype.Citation46 This approach, though inefficient at this time, will is likely to have potential in the future as therapy for patients who are not surgical candidates for revascularization using tissue-engineered replacement.
Cardiac purity after differentiation
Once differentiation has been achieved, there are several strategies to improve the yield and the purity of the tissue sample. One proposed protocol involved Percoll density gradient centrifugation.Citation18 This, however, reveled an injury to the cardiomyocytes and low survival rate at transplantation stage. Since then, multiple studies have used different strategies using genetic selection in which a cardiac-specific agent drives expression of an antibiotic-resistance gene, permitting cardiomyocytes to last while other cell types are lost.Citation47–Citation49 Cell-sorting techniques have been developed for cardiac-specific surface markers including vascular cell adhesion molecule (VCAM1)Citation50 and signal regulatory protein alpha (SIRPA).Citation51
Another strategy used involved culturing cells in a lactate-rich medium that would eradicate or kill the cells that do not have enough mitochondria to survive (which most of the time are the non cardiac cells). 100% purity is hard to achieve. It has been reported that usage of unsorted cardiomyocytes with purities greater than 50% does not result in the formation of teratomas in animal model (rodent) studies,Citation18 though occasional epithelial cysts have been detected.Citation53 These results are promising and imply that practically high-purity human EmSC- and iPSC-derived cardiomyocytes can be developed for clinical usage (summarized in ).
Cell-based therapy for heart failure
The treatment of post-MI heart failure remains an important and difficult task due to the high prevalence of post-MI heart failure and low levels of success with traditional medical and surgical therapies.Citation54 An evolving strategy for the treatment of advanced ischemic cardiomyopathy is regenerative medicine. This involves the transplantation of pluripotent progenitor cells into the area of the infarcted myocardium with the expectancy of new functioning myocyte and vascular cell production.
The adult mammalian heart has been traditionally considered as a post-mitotic organ without intrinsic capacity for regeneration. Clinically, it behaves in this manner, with little recovery after ischemic damage. New understandings of cell biology, however, have caused a shift in such opinions and introduced a new avenue for potential therapy. Several reports have shown new cardiomyocyte cell-cycle production ranging from 0.0005% to 3% in normal adult hearts,Citation55–Citation57 and the dating of adult hearts with 14C has also concluded that during a lifetime the human heart renews approximately 50% of its myocytes.Citation58 For these reasons, animal experiments have been performed and the interest generated from these experiments has led to clinical trials to evaluate stem cells as therapy for damaged adult human hearts.Citation59
Myocardial regeneration with allogeneic stem cell therapy
Allogeneic cells can be grown in large quantities in advance, stored before their use, and made available at short notice, which make them a suitable option for highly prevalent diseases and will permit their use soon after an acute insult to either prevent or diminish pathological remodeling of the heart.
As yet unanswered questions about the use of allogeneic stem cells in the treatment of patients with acute myocardial injury need to be addressed, including what method of delivery is best for cell administration and what is the best cell population to deliver.
To be widely available and compatible with the current clinical standard of care for acute myocardial injury, an intracoronary method for delivery at the time of the primary revascularization is feasible. Catheter-based direct myocardial injection during revascularization surgery is also realistic.
For choice of cell populations, MSCs have been tested. These cells secrete a broad range of favorable cytokines. However, they also secrete factors that negatively modulate cardiomyocyte apoptosis, inflammation, scar formation, and pathological remodeling, as described by Ranganath et al.Citation60 Also, only approximately 3%–4% of the cells delivered by intracoronary technique are retained in the myocardium, as reported by Dauwe and Janssens,Citation61 as well as Teng et al,Citation62 and MSCs can clump together, becoming entrapped in the microvasculature and obstructing entry into the myocardium.
Groups of allogeneic cell types in small aggregates known as “cardiospheres” have also been tested for therapy in a rat module of infarcted hearts.Citation63 Allogeneic cardiosphere-derived cell (CDC) transplantation resulted in an improvement in fractional area change (12%), ejection fraction (EF; 20%), and fractional shortening (10%), which was sustained for at least 6 months. Also, allogeneic CDCs stimulated regeneration through endogenous mechanisms, such as increased myocardial VEGF, insulin-like growth factor 1, and hepatocyte growth factor, and the recruitment of c-kit+ angiogenesis.Citation63
One promising cell type to undergo testing for cell therapy is the RCSC, which has advanced beyond more primitive stem cells into a definite cardiac lineage but has not yet terminally differentiated or matured. Unlike other cell types such as BMD stem cells, which were mentioned by Abdel-Latif et alCitation64 and Hofmann et al,Citation65 RCSCs have a high tropism for the myocardium. When administered through the systemic circulation, the majority of RCSCs home to and nest in the damaged myocardium.Citation66
Under appropriate conditions it is possible to replicate and expand RCSCs up to 1×1011 cells without noticeable alteration of karyotype, loss of differentiating properties, or the phenotype of the differentiated progeny.Citation67 These cells produce a range of beneficial pro-survival, anti-inflammatory, and cardiovascular regenerative growth factors, such as insulin-like growth factor 1, hepatocyte growth factor, activins, transforming growth factor-beta 1, and neuregulin-1, among others.Citation68
It is postulated that it is because of the secretion of these regenerative factors that allogeneic stem cell therapy is successful. The factors produced by the allogeneic cells may stimulate endogenous stem cells of the target tissue while the transplanted cells themselves may survive only transiently. Thus, they might not directly participate in the production of progeny that contribute to the regenerated tissue. As a result, although the therapeutic cells are allogeneic, a lasting regenerative response may be autologous, carried out by host RCSCs.Citation69
Myocardial regeneration with autologous stem cell therapy
Notwithstanding the values of autologous therapy, autologous cells require time for preparation and are not suited for highly prevalent diseases. A study done using an intracoronary infusion of AdSCs within hours of percutaneous revascularization in patients presenting with acute MI showed improved left ventricular ejection fraction (LVEF) with reduced scar formation.Citation70
Meanwhile, intracoronary injection of autologous BMD stem cells in patients with chronic heart failure had been shown to result in a corresponding 15% improvement in EF and a 30% reduction in infarct size.Citation71 Direct myocardial injection of CD133+ BMD stem cells into infarct border zones led to an improvement in LVEF from 37% to 47%.Citation72
Also, cardiac stem cells have been shown to differentiate into multiple cell types, including cardiac myocytes, SMCs, and ECs.Citation19 Infusion of autologous Lin−/c-kit+ cardiac stem cells into patients with post-MI resulted in LVEF improvement from 30.3% to 38.5%.Citation28
In patients with refractory angina after conventional revascularization therapy, compelling evidence supporting stem cell therapy emerged from a randomized trial of catheter-based intramyocardial (endoventricular) injection of CD34+ progenitor cells, arranged following granulocyte colony stimulating factor-induced cell mobilization and “leukapheresis” (separation of white blood cells).Citation73 It showed a trend in efficacy for endpoints such as fewer angina episodes, lowered nitroglycerin usage, improved exercise time, and improved functional class (as per the New York Heart Association Functional Classification) when compared with placebo. These results were similar to a recent trial (Efficacy and Safety of Targeted Intramyocardial Delivery of Auto CD34+ Stem Cells for Improving Exercise Capacity in Subjects With Refractory Angina [RENEW] study).Citation73,Citation74 In another trial, when patients were injected with bone marrow mononuclear stem cells endocardially, a trend toward improvement in chronic ischemia refractory to medical treatment was shown. Although there was no significant difference.Citation75 In conjunction with coronary arterial bypass grafting, another study showed that intramyocardial injection of high-dose BMD mononuclear cells yields improved results compared with CABG alone.Citation76 When CD133+ cells are injected through the transepicardial route during coronary surgery, there has been an improved LVEF and perfusion at 6 months post-operatively.Citation72
Clinical trials of cell therapy for heart failure
Many studies have addressed the efficacy of stem cell injection in the cardiac disease patient. Here, we mention some of these studies and divide them depending on the timeframe of the intervention (summarized in and and ).
Figure 2 Clinical trials of cell therapy for acute and chronic, ischemic and nonischemic heart failure.
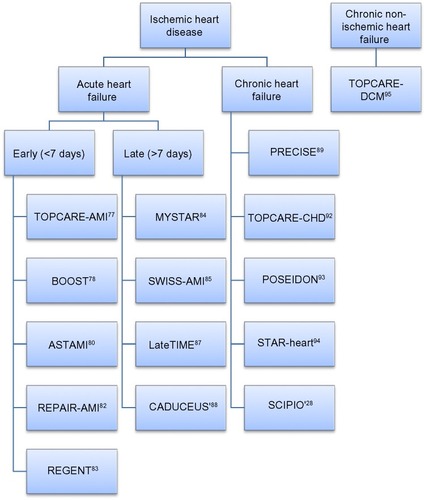
Table 2 Cells number and purification methods
Acute heart failure
Intervention within <7 days
Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI)
This was a safety and feasibility trial targeting patients with acute MI successfully reperfused by stent implantation. They used an intracoronary infusion of circulating progenitor cells (CPCs) (approximately 245±72×106 Ficoll-separated, cultured CPCs) and bone marrow cells (approximately 10±7×106 Ficoll-separated BMD cells) 4 days after the infarction. It showed a trend of improvement in regional wall motion in the infarcted zone and reduction of end-systolic LV volume immediately after the injection as well as during the 4-month follow-up. The efficacy was similar between patients receiving BMD stem cells and patients receiving blood-derived CPCs.Citation77
BOne marrOw transfer to enhance ST-elevation infarct regeneration (BOOST)
This study targeted patients who had acute MI and had stent implanted using PCI. They had a transfusion of a single dose of autologous bone marrow stem cells through intracoronary route. The number of cells used was approximately 2.5×109 of unfractionated BMD cells. The results showed a slight improvement in LVEF of 7%, which was, however, not sustained at 18 months.Citation78,Citation79
Autologous Stem-cell Transplantation in Acute Myocardial Infarction (ASTAMI)
This trial targeted patients with acute ST-segment elevation myocardial infarction (STEMI). They harvested autologous bone marrow cells, which were delivered through intracoronary injections during PCI for stent implantation. The number of cells used was approximately 7×107 Ficoll-separated BMD stem cells. In this trial, there was no improvement in LVEF at 6 months after delivery of injected cells.Citation80
Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI)
This was a randomized and double-blinded study targeting patients who had PCI after acute MI. They divided the population into two groups receiving bone marrow cells versus placebo infusion. The total number of cells used was estimated to be 2.4×108. The initial 4 months’ result showed an increase in LVEF in the bone marrow group compared with in the placebo group. At the 2-year follow-up, cell-treated patients in this trial had had fewer myocardial ischemias and there were fewer patients who met the combined endpoint of death, MI, or need for revascularization. Although the results were not statistically significant, there was a trend toward sustained improvement in EF and LV end-systolic volume.Citation81,Citation82
Myocardial REGENeraTion by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT)
REGENT was a randomized, multicenter study targeting patients with acute STEMI within 12 hours of the symptoms, to assess the myocardial regeneration by intracoronary infusion of selected populations of BMD stem cells. They used autologous BMD cells and divided them into three groups: selected CD34+ group (median number of cells 1.90×106), nonselected bone marrow cell group (median number of cells 1.78×108), and placebo group. The treatment with BMD stem cells did not lead to a significant improvement in LVEF or LV volumes. However, there was a trend in favor of cell therapy in patients with the most severely impaired LVEF and prolonged periods between symptoms and revascularization.Citation83
Intervention within >7 days MYocardial STem cell Administration after acute myocardial infaRction (MYSTAR)
The hypothesis relied on, is the injected stem cells homing which will be ideal 21 days after the incident of MI but not later than 42 days. They used autologous bone marrow stem cells by intracoronary and intramyocardial injections at two different timings. Patients were divided into four groups: early intracoronary nonselected BMD stem cells (between 21 and 42 days), early combined intracoronary with intramyocardial BMD stem cells, late (3 months) intracoronary injection, and late combined injections. The mean number of injected cells in the early group and late group was 1.56±0.40×109 and 1.55±0.44×109, respectively. The results showed that the early and late delivery of cells induced mild, but not clinically relevant, improvements. There was a reduction in infarct size and an improvement in LVEF at both 3 months and 9 to 12 months after acute myocardial ischemia. The main improvements in the early and late groups were an estimated 3.5% and 3.9%, respectively, improvement in LVEF, as well as a 3.5% and 3.4%, respectively, improvement in infarct size.Citation84
SWiss Multicenter Intracoronary Stem cells Study in Acute Myocardial Infarction (SWISS-AMI)
In this trial, BMD mononuclear stem cells were injected through the intracoronary route for patients who had STEMI, received a successful PCI intervention within 24 hours, and had an EF of less than 45%. They used an intracoronary injection of an estimated 153×106 and 139.5×106 of BMD stem cells in the early group (5–7 days) and in the late group (3–4 weeks), respectively. It showed that the infusion of BMD stem cells, either at 5–7 days or 3–4 weeks after acute MI did not improve LV function at 4 months.Citation85
Use of Adult Autologous Stem Cells in Treating People 2 to 3 Weeks after having a Heart Attack (LateTIME)
This trial evaluated the use of bone marrow mononuclear (BMD) stem cells with infusion at 2 to 3 weeks after acute anterior-wall MI versus placebo in patients with EF less than 45% successfully treated with primary PCI. Single intracoronary infusion of 150×106 of autologous BMD stem cells was injected within 12 hours of aspiration and cell preparation. The results showed no substantial improvement in LVEF measured after 6 months.Citation86,Citation87
CArdiosphere-Derived aUtologous Stem CElls to reverse ventricUlar dySfunction (CADUCEUS)
This was a randomized prospective trial targeting patients within 2–4 weeks of acute MI with an LVEF of 25%–45%. Cells were obtained from the right ventricular endocardium through biopsy. Patients received either a low dose (12.5×106) or a high dose (25×106) of cells versus a control group (which received no cells). The cells were introduced through the intracoronary route using an angioplasty catheter over a period of 15 minutes. This study was done to assess the safety of CDCs. There was a significant decrease in the scar size and increase in viable myocardium at 6 and 12 months in the treatment group compared with in the control group, but there was no significant difference between the two groups at 6 months in terms of EF improvement.Citation88
Chronic heart failure
AdiPose-deRived stEm and Regenerative Cells In the Treatment of Patients with non revaScularizable ischEmic myocardium (PRECISE)
PRECISE was a randomized clinical trial of AdSCs for patients with no revascularization option by either surgery or PCI. The autologous AdSCs were harvested by liposuction and the mean of the total number of cells used was 42×106 in the treatment group. This trial, which had an AdSCs group versus a placebo group, showed that harvesting and transendocardial injection of AdSCs are safe and feasible. Also, the treatment appeared to result in scar stabilization.Citation89 However, data preceding this trial, by Perin et al, explored the transendocardial administration of BMD stem cells in patients with ischemic cardiomyopathy and a mean LVEF of less than 40%.Citation90 There was no major improvement in LVEF at 6 or 12 months in the treated group when compared with control subjects. There was, however, a substantial improvement in exercise capacity, as well as ischemic burden, as measured by single-photon emission computed tomography (SPECT), found at 6 and 12 months.Citation91
Transplantation of progenitor cells and recovery of left ventricular function in patients with chronic ischemic heart disease (TOPCARE-CHD)
This study targeted patients with an MI of more than 3 months who were stable on medical therapy since the attack. Autologous BMD stem cells (mean number of 214±98×106) were injected through the intracoronary route. The study found that the treatment was associated with a reduction in natriuretic peptide serum levels (as natriuretic peptide serum levels are a solid marker for chronic heart failure) and improved the survival of patients with chronic heart failure post-MI.Citation92
Percutaneous stem cell injection delivery effects on neomyogenesis: Comparison of Allogeneic versus Autologous Bone Marrow-Derived Mesenchymal Stem Cells Delivered by Trans-Endocardial Injection in Patients with Ischemic Cardiomyopathy (POSEIDON)
This trial targeted patients with ischemic cardiomyopathy with EF of less than 50%. It compared autologous (extracted by bone marrow aspiration) and allogenic (extracted by bone marrow aspiration from healthy donors) MSCs. The researchers divided the population into three groups according to the number of infused cells: 20×106 for the first group, 100×106 for the second group, and 200×106 for the third group. Treatment was associated with lower rates of treatment-emergent serious adverse effects, including immunologic reactions. MSC injection favorably affected patient quality of life, functional capacity, and ventricular remodeling.Citation93
The acute and long-term effects of intracoronary Stem cell Transplantation in patients with chronic heARt failure (STAR-heart)
This study included a large group of patients with chronic ischemic heart failure within a time interval of 8.5±3.2 years between the infarct intervention and the admission to the clinical care. The study evaluated autologous BMD stem cells in patients with an LVEF of 35% or less and a remote history of MI. During this unblinded study, patients who refused cell therapy served as controls. The BMD stem cells were delivered into the infarct-related coronary artery through the intracoronary route and the estimated number of injected cells was 6.6±3.3×107. At 3 months, the treated group had had substantial improvements in cardiac index and calculated LVEF with an increase of almost 7%. There was also a drop in New York Heart Association class and in both end-systolic (≈15 mL) and end-diastolic (≈10 mL) ventricular volumes. These improvements persisted at 12 and 60 months after treatment, while no changes were noted in the control group. Also, the mortality rate of the treated group was remarkably lower than that of the control group (0.75% vs 3.68% per year). This trial provided the first long-term evidence that cell therapy can considerably affect mortality in heart failure patients.Citation94
Stem Cell Infusion in Patients with Ischemic cardiOmyopathy (SCIPIO)
SCIPIO was the first randomized, open-label, Phase I clinical trial in humans to evaluate autologous c−/kit+ RCSCs in patients with ischemic heart failure (LVEF equal to or less than 40%) at an average of 3.7 years post-MI. RCSCs were harvested from the right atrial appendage during CABG in 33 patients (20 RCSC-treated vs 13 control subjects), with 1×106 cells injected per patient. The RCSC-treated patients received an intracoronary infusion of cells at a mean of 113 days after CABG. In the treated group, cardiac magnetic resonance imaging showed an increase in LVEF at 4 months (from 27.5%±1.6% to 35.1%±2.4% [P=0.004, n=8]) and at 12 months (41.2%±4.5% [P=0.013, n=5]). Infarct size, measured as late gadolinium enhancement by perfusion magnetic resonance imaging, decreased by −9.8±3.5 g after RCSCs infusion at 12 months in six patients who completed 1-year follow-up. In the treated group, the LV infarcted mass decreased by −14.7±3.9 g and LV non-infarcted mass increased by +31.5±11.0 g at 12 months’ follow-up. The study concluded that the improvements in LVEF, decrease in infarcted LV mass, and increase in non-infarcted LV mass seen at 4 months and persisting for up to 12 months in a subgroup of patients were consistent with cardiac regeneration.Citation27
Chronic nonischemic heart failure
Transplantation of progenitor cells and recovery of left ventricular function in patients with nonischemic dilatative cardiomyopathy (TOPCARE-DCM)
This trial targeted patients who had nonischemic dilated cardiomyopathy with an EF of less than 40% and LV end-diastolic diameter of more than 60 mm who were stable for at least 6 months of medical therapy. The patients received autologous an estimated 259±135×106 of BMD stem cells. The researchers used intracoronary infusion by PCI. The results of this trial were correlated with improvements in cardiac contractility and intracoronary circulation flow in patients with dilated cardiomyopathy, as well as a significant improvement in natriuretic peptide serum levels, although the latter was noticed after the 1-year follow-up but not before.Citation95
Cardiac tissue engineering
Cardiac muscles, present in the pericardium, heart walls, and valves, form a dense network of striated and cross-linked cells. The muscle contains sarcomeres with sliding filaments of actin and myosin. The electrical and mechanical gap junctions permit the heart to contract and relax in a coordinated fashion and conduct these impulses throughout the heart walls through the arteries and ventricles.Citation96
The major diseases originating from cardiac muscles include MI, arrhythmias, ventricular dilatation, valvular diseases, and heart failure. It has been reported that the lack of donor organs, scar tissue formation, calcification of grafts, degradation, and inflammation of the affected tissue microenvironment are the most common problems associated with cardiac diseases. To overcome these hurdles, cardiac tissue engineering aims at assembling tissue constructs that can restore basic cardiac function by incorporating cellular components within scaffolds which in turn provide a framework of optimal structural, mechanical, and electrophysiological properties.Citation97,Citation98
Initially, cellular therapy involved the injection of “naked” cells into the site of injury, and although this technique has shown some promise in experiments,Citation80,Citation90 low cellular retention and engraftment rate limits its potential use for complete restoration of cardiac function.Citation18,Citation99,Citation100
Recently, much emphasis has been placed on tissue engineering methods that mimic the biological and biomechanical components of the native myocardial tissue and maintain transplanted cell function and survival. The three most common methods being investigated in this regard are biomimetic scaffolds, decellularized tissue scaffolds, and scaffold-free constructs.
Biomimetic scaffolds
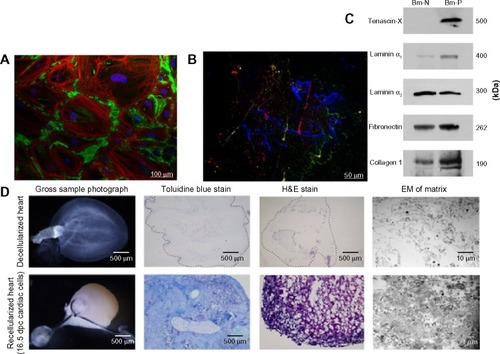
Biomimetic scaffolds are made of either natural or synthetic polymers or a hybrid natural/synthetic copolymer. Natural polymers (as seen in ), such as collagen,Citation4,Citation101 fibrin,Citation102 alginate,Citation103 Matrigel®,Citation104 chitosan,Citation105 and hyaluronic acidCitation106 are biodegradable protein and polysaccharide molecules with a similar structure to the native component of the tissue extracellular matrix (ECM), making them more biocompatible and less immunogenic than synthetic polymers with a higher capacity for cell adhesion and influence on various cellular functions.Citation106 The disadvantage of using natural polymer-based scaffolds is their limited mechanical and biodegradable properties that are not easily tailored.
Figure 3 Biomimetic scaffolds
Abbreviations: Bm-N, biomatrix from patient with a normal heart; Bm-P, biomatrix from patients with heart failure; dpc, day post conception.
On the other hand, synthetic polymers used for cardiac tissue engineering, such as polyethylene glycol, polyhydroxyethylmethacrylate, polylactide-glycolic acid, and poly(N-isopropylacrylamide), are easily tailored with predictable mechanical and chemical properties, but they may elicit an inflammatory response that might affect cell survival.Citation106–Citation109
In general, biomimetic scaffolds should be non-immunogenic and mechanically stable, mimic cardiac tissue flexibility, allow physiological electrical propagation, allow sufficient oxygen and nutrient delivery to cells, and have a degradation rate appropriate to the rate of native ECM replacement.Citation97,Citation110
Biomimetic scaffolds are used alone, as acellular scaffolds, or in combination with cells.Citation111–Citation114 Preparation and delivery can be further divided into either in vitro culture with cells to produce 3D engineered cardiac tissuesCitation115–Citation117 or in vivo injection either directly in situ or with minimally invasive through catheter-based systems.Citation103,Citation118
The pathological process that leads to heart failure post-MI includes an initial inflammatory response, loss of cardiomyocytes, and loss of the ECM, which leads to scar formation and ventricular wall thinning.Citation110,Citation119,Citation120 Injecting acellular biomimetic scaffolds at the site of MI, with or without bioactive molecules, provides structural mechanical support,Citation121,Citation122 decreases the amount of fibrosis and ventricular dilation, and promotes angiogenesis and the recruitment of native stem cells.Citation101
Scaffolds combined with cells form environmental substrates to replace the ECM and enhances the retention and survival of the transplanted cells, with one study reporting the increased early survival and retention of cardiomyoblasts in collagen scaffolds transplanted into rats in which MI was induced compared with cardiomyoblasts in saline suspension.Citation123
Selection of the cell type, scaffold material, and incorporation of bioactive molecules and bioreactors should be considered in constructing the optimal biomimetic scaffolds for treating cardiac diseases. “Bioreactors” are defined as specialized chambers designed to culture tissue-engineered constructs under controlled conditions.Citation124,Citation125 They have been developed to provide electrical and/or mechanical stimulation during tissue culture in order to promote uniform cellular distribution inside scaffolds, support the structure formation of 3D scaffold constructs, provide adequate oxygen and nutrient supply during culture, and help induce structural and functional cell maturation.Citation126–Citation128
For instance, it was found that electrical stimulation of neonatal rat myocyte in a collagen scaffold enhanced the conductive and contractile properties of the construct, increased cell alignment, and caused phenotypic changes resembling more mature myocytes compared with the non-stimulated scaffold.Citation129
Recent advancements in nanomaterial science have further increased the efficiency of scaffolds. Davis et al used self-assembling peptides that rapidly form nanofibers and form a 3D scaffold at physiological pH and osmolarity.Citation130 After injecting these peptides and the formation of the scaffold onto the free LV wall of adult male mice, the scaffold promoted the recruitment of endogenous endothelial and SMCs, and when compared with Matrigel scaffolds, fewer ECs were seen and they were localized around the edges. Furthermore, injecting neonatal mice cardiomyocytes with these self-assembling peptides resulted in the survival of these cells and the recruitment of endogenous cells that stained positively for myocyte progenitor markers.Citation130
Also utilizing nanomaterial technology, Dvir et al incorporated gold nanowire within an alginate scaffold seeded with neonatal rat cardiomyocytes, which improved both electrical communication between cardiac cells and tissue formation, producing thicker tissue and better-aligned cells than cells in alginate scaffolds without the nanowires.Citation131
Recently, there has been an interest in engineering cell-based cardiac pumps and tissue-engineered ventricles. Although technically challenging, such models can provide a potential concept for engineering a biological LV assist device or a biological drug pumping system. Furthermore, creating a hollow cardiac organoid structure can provide a flexible platform that can be adapted for the long-term study of cardiac pump function in vitro. Khait and Birla fabricated a cardiac pump prototype by surrounding a tubular graft with a monolayer of cardiomyocytes with the resulting generation of intraluminal pressure,Citation125 while Lee et al fabricated a cardiac organoid chamber using neonatal rat myocytes seeded in a collagen/Matrigel matrix then casted on a mold with a balloon catheter to control chamber size. Spontaneous beating was observed after 7–10 days of culture and in a cryo-injury model of MI led to diminished global chamber pressure and decreased intrinsic pulse rate.Citation132
Decellularization to build a new heart for transplantation
Whole-organ engineering is a promising field for the future of heart failure treatment.Citation133 Since donor organs for transplantation are a limited resource, creating replacement organs from cadaveric allogeneic or xenogeneic sources could reduce the need for living donors. Tissue engineering also avoids problems with synthetic materials or mechanical devices for heart failure, which are subject to foreign-body reactions and hardware or software malfunction. The shape and structure of biologic scaffolds are also superior to those of synthetic matrices, and their complexity is not reproducible.
Thus the use of decellularized matrices has the potential to overcome the need to artificially recreate the conditions for ECM deposition.Citation134–Citation138 Tissues used to prepare these biologic scaffolds are typically autogenic, allogeneic, or xenogeneic in origin.Citation139 Thus the decellularization technique (as seen in ) is very crucial to the success of the treatment.
From a tissue engineering perspective, the major objectives of decellularization are therefore to retain the structural integrity, composition and biological activity of the scaffold while completely removing the cellular and nuclear materials to eradicate any chance of unwanted immunological responses and other adverse side effects due to cross-presentation.Citation140,Citation141 If performed properly, the decellularization technique can be applied not only to tissues, but also to entire organs.Citation142 Commonly used decellularized scaffolds in cardiac applications include adipose tissue, cartilages, and heart tissues of allogeneic, autologous,Citation143–Citation145 as well as xenogeneic origin.Citation135,Citation146 Studies show rapid repopulation with cardiomyocytes in natural matrix obtained from porcine tissues.Citation147–Citation150
The optimum method of decellularization remains variable, as it depends on the specific requirements of the application, such as high density, high pressure, and stiffness, which play vital roles in determining the method for decellularization.Citation11,Citation151 Chemical, enzymatic, microwave radiation,Citation152 mechanical, and physical separations are the different methods used so far. Studies on the comparison of different techniques have been reported in the literature.Citation153,Citation154 It is known that a combination of physical, chemical, and enzymatic methods yields better results in the decellularization of both the whole organ and small tissues in cardiac applications.Citation155,Citation156 What is required for decellularization and recellularization is an organ to serve as a scaffold; a detergent, osmolar solution, or enzymatic solution to remove native cellular material; a choice of stem cells for repopulation; and a culture environment, or bioreactor, to promote new cell adhesion, growth, and integration.
Creating a clear scaffold for the reintroduction of stem cells is performed by coronary perfusion of a decellularizing agent. The choice of agent affects the level of antigenicity of the remaining tissue and its tensile strength, and an ideal decellularization process would remove antigenic material and preserve structural integrity.Citation157 Detergents, organic solvents, and enzyme solutions that solubilize cell membranes can weaken the ECM. More vigorous agents, including ionic detergents such as sodium dodecyl sulfate (SDS) or enzymatic digestion with trypsin, are effective at removing antigens but can cause degradation of collagen.Citation158,Citation159 A gentler decellularization technique uses hypotonic saline to osmotically eliminate cells, but this can leave behind some antigenic material.Citation160
SDS was used to decellularize a ventricular porcine myocardium, processed into hemocompatible and biocompatible hydrogels, and then injected into a porcine model with MI.Citation161 A reduction in fibrosis, lack of thrombogenicity, and growth of cardiac muscles were observed. This was the first time that such percutaneous delivery of ECM using a transendocardial approach was carried out.Citation162 Recent decellularization with SDS perfusion has been found successful in small animal organs and whole organs.Citation155 With these promising results, such porcine heart models are being gradually translated to clinical applications.Citation142,Citation163
The choice of allogeneic or xenogeneic organ for decellularization can be difficult. Allogeneic hearts are size-appropriate and most structurally compatible for implantation. They are a limited resource and have human antigens, which can be recognized readily by immune defenses, and can transmit disease within the same species. Meanwhile, xenogeneic hearts have lesser degrees of anatomical fit but are an abundant resource and cause less inter-species disease transmission. Porcine hearts in particular are favored for a xenogeneic scaffold.Citation164 Glutaraldehyde can be used to prevent acute immune rejection of the xenogeneic graft by cross-linking proteins, making them unrecognizable to host immune defenses, but delayed rejection is still possible.Citation165
Ott et al pioneered the first bioengineered heart from decellularized organ matrix recellularized with stem cell infusion in 2008. The cellular components of rat hearts were dissolved in detergent solution, leaving an intact collagen skeleton. The hearts were injected with cardiac and vascular progenitor cells while supported in an organic reactor that simulated the preload and afterload of cardiac physiology. The result after 8 days of incubation was the detection of macroscopic contractions and overall pump strength equivalent to 2% of the adult heart or 25% of the function of a 16-week fetal heart.Citation4
For larger models, porcine hearts have been decellularized for re-injection with cellsCitation121,Citation142,Citation163 and a human heart has been tested as a scaffold for repopulation with human mesenchymal cells and murine cardiomyocytes.Citation166
Decellularized injectable scaffolds and solubilized pericardial gels are currently the two areas of research in which decellularization techniques have been found extremely helpful.Citation134,Citation167–Citation172 Some of the other works include blends of natural and synthetic matrices to take advantage of both components.Citation173 However, the technology of decellularization is still in its initial stage of development.
The future of this therapy will depend on achieving the correct combination of support structure and stem cells. One goal will be to provide a non-antigenic scaffold that will give durable, long-term support. Another will be to identify the mixture of cell lines that can provide contractility for adequate cardiac output and vascularity to sustain cardiac perfusion.
The major issue is the translation of basic research into clinical human use. Apart from its initial success, there have been reports of drawbacks with this method due to aging, incomplete decellularization,Citation174 toxic effects, changes in biochemical properties,Citation137 and changes in the cell’s pathological immunogenicity.Citation175–Citation178 For instance, there has been a failure of clinically approved, tissue-engineered, decellularized porcine heart valves in pediatric patients; this was attributed to calcific depositions and incomplete decellularization of the implant.Citation174
Scaffold-free constructs
Recent advancement in utilizing scaffolds as a substitute for the damaged ECM and supporting the viability and survival of cells used for cardiac tissue engineering is promising. However, the low cellular concentration inside the scaffold compared with the dense myocardial tissue of the native heart combined with the inflammatory reaction and fibrous tissue formation caused by scaffold degradation led to the consideration of building tissue engineering constructs without the use of scaffolds.
Shimizu et al developed a cell sheet engineering technique to construct scaffold-free 3D cardiac tissue. Using a temperature-responsive culture surface, they were able to detach two-dimensional (2D) myocardial layers as an intact confluent sheets and stack them to produce a 3D cardiac construct.Citation173
Cell sheets are obtained by using specialized cell-culture surfaces that are covalently grafted with the temperature-responsive polymer poly(N-isopropylacrylamide).Citation180 The surface is hydrophobic and allows attachment of cells when the temperature is 37°C; when the temperature is lowered to 32°C, the surface becomes hydrophilic and causes the detachment of the cultured cells as an intact layer without disrupting the deposited ECM or the cell-to-cell junction proteins,Citation181 both of which usually happen when using traditional enzymatic degradation substances such as trypsin.
When these 2D monolayers are stacked, they can rapidly attach and form cell-to-cell connections due to the presence of the intact deposited ECM. Shimizu et al also demonstrated that stacking monolayers of neonatal rat cardiomyocytes results in a spontaneously pulsating tissue with rapid electrical coupling between the layers, which was supported by the observation of the rapid formation of functional gap junctions.Citation182
Miyahara et al used this technique to generate a mono-layer of adipose tissue-derived MSCs. Four weeks after inducing MI in rats, they transplanted the 2D monolayer onto the scared myocardium. After transplantation, the sheet induced angiogenesis and grew to a thick striatum that included a few myocytes and prevented the progression of ventricular wall dilation and improved cardiac function.Citation183
Similarly, Miyagawa et al transplanted 3D sheets of stacked monolayers of neonatal rat cardiomyocytes 2 weeks after inducing MI in rats. The 3D sheets became attached to the native myocardium, expressed connexin-43, showed angiogenesis and improved overall cardiac function.Citation184
However, trying to apply such a method clinically is still challenging. The thickness limit for these 3D sheets was 80 μm, or three layers, as reported by Shimizu et al;Citation182 stacking more than three layers at once resulted in core tissue necrosis.Citation185 Several methods have been proposed to increase the vascularity and thickness of these sheets. Coculture with ECs and treatment with VEGF and in vitro vascularization bioreactors, allowing for a multi-step stacking of three-layer sheets, are examples of methods proposed to increase 3D sheet thickness.Citation186
Conclusion
Stem cell therapy is likely to be a fundamental treatment strategy in the not-too-distant future. Traditional medical and surgical therapies have reached a point of maximal clinical utility and new strategies are required to treat the large population of patients with acute or chronic heart failure worldwide. Encouragingly, animal models have shown that stem cell therapy is safe and effective. As for the human clinical trials, many have demonstrated the safety and feasibility of the therapy; others have shown no significant effect, but, on the other hand, the majority of those trials have shown a trend of positive effect, whether on EF or on scar regression. What is needed now is further clinical testing and refinement to achieve better, more reliable, and more reproducible benefits. Larger randomized controlled trials could demonstrate the utility of stem cell therapy and comparison of studies could help identify the cell lineages that are the most effective, whether alone or in combination. With continued support, the proposed paracrine effects of implanted stem cells on resident stem cells could also be elucidated. Possible stem cells signaling factors therapy alone can potentially be employed, removing the time and burden of autologous stem cell harvest, or the antigenicity and rejection of allogeneic implantation. The need for the treatment of ischemic and nonischemic heart failure is highly demanded. Therefore, ongoing advancement in this new frontier is expected to continue. More organized infrastructure and collaborative research efforts, guided by evidence-based information, and adequate funding are needed to meet the important challenges ahead. However, the holy grail of tissue engineering and regenerative medicine seems to progressively take shape and hold tremendous promise to benefit humankind and treat presently incurable diseases.
Acknowledgments
The authors acknowledge the financial support to Dr Shum-Tim granted by the Natural Sciences and Engineering Research Council of Canada (NSERC) and Fonds de Recherché du Québec – Santé (FRSQ) – The Cell.
Disclosure
The authors declare no conflicts of interest in this work.
References
- LozanoRNaghaviMForemanKGlobal and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet201238098592095212823245604
- BeltramiAPUrbanekKKajsturaJEvidence that human cardiac myocytes divide after myocardial infarctionN Eng J Med20013442317501757
- ReddiAHHugginsCBInfluence of geometry of transplanted tooth and bone on transformation of fibroblastsProc Soc Exp Biol Med197314336346374578252
- OttHCMatthiesenTSGohSKPerfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heartNat Med200814221322118193059
- PaschosNKBrownWEEswaramoorthyRHuJCAthanasiouKAAdvances in tissue engineering through stem cell-based co-cultureJ Tissue Eng Regen Med2014 Epub23
- ScorsinMHagegeAAMarotteFDoes transplantation of cardiomyocytes improve function of infarcted myocardium?Circulation1997969 SupplII-188II-193
- LiRKJiaZQWeiselRDCardiomyocyte transplantation improves heart functionAnn Thorac Med1996623654660
- XuCPoliceSRaoNCarpenterMKCharacterization and enrichment of cardiomyocytes derived from human embryonic stem cellsCirc Res200291650150812242268
- LeeASTangCCaoFEffects of cell number on teratoma formation by human embryonic stem cellsCell Cycle20098162608261219597339
- GluckmanETen years of cord blood transplantation: from bench to bedsideBr J Haematol2009147219219919796268
- HenningRJBurgosJDVaskoMHuman cord blood cells and myocardial infarction: effect of dose and route of administration on infarct sizeCell Transplant200716990791718293889
- WangLDengJTianWAdipose-derived stem cells are an effective cell candidate for treatment of heart failure: an MR imaging study of rat heartsAm J Physiol Heart Circ Physiol20092973H1020H103119574490
- ValinaCPinkernellKSongYHIntracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarctionEur Heart J200728212667267717933755
- TaylorDAAtkinsBZHungspreugsPRegenerating functional myocardium: improved performance after skeletal myoblast transplantationNat Med1998489299339701245
- MenaschéPAlfieriOJanssensSThe Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantationCirculation200811791189120018285565
- DibNMichlerREPaganiFDSafety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: four-year follow-upCirculation2005112121748175516172284
- OrlicDKajsturaJChimentiCBone marrow cells regenerate infarcted myocardiumNature2001410682970170511287958
- LaflammeMAChenKYNaumovaAVCardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat heartsNat Biotechnol20072591015102417721512
- BeltramiAPBarlucchiLTorellaDAdult cardiac stem cells are multipotent and support myocardial regenerationCell2003114676377614505575
- MessinaEDe AngelisLFratiGIsolation and expansion of adult cardiac stem cells from human and murine heartCirc Res200495991192115472116
- OhHBradfuteSBGallardoTDCardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarctionProc Natl Acad Sci U S A200310021123131231814530411
- BeltramiAPAdult cardiac stem cells are multipotent and support myocardial regenerationCell2003114676377614505575
- WeissmanILAndersonDJGageFStem and progenitor cells: origins, phenotypes, lineage commitments, and trans-differentiationsAnnu Rev Cell Dev Biol20011738740311687494
- TangXLRokoshGSanganalmathSKIntracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarctionCirculation2010121229330520048209
- BearziCRotaMHosodaTHuman cardiac stem cellsProc Natl Acad Sci U S A200710435140681407317709737
- LinkeAMüllerPNurzynskaDStem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac functionProc Natl Acad Sci U S A2005102258966897115951423
- UrbanekKCesselliDRotaMStem cell niches in the adult mouse heartProc Natl Acad Sci U S A2006103249226923116754876
- BolliRChughARD’AmarioDCardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trialLancet201137898061847185722088800
- Pérez-PomaresJMde la PompaJLSignaling during epicardium and coronary vessel developmentCirc Res2011109121429144222158650
- TianXHuTZhangHSubepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteriesCell Res20132391075109023797856
- CossetteSMisraRThe identification of different endothelial cell populations within the mouse proepicardiumDev Dyn2011240102344235321932312
- TomanekRJFormation of the coronary vasculature: a brief reviewCardiovasc Res199631Spec NoE46E518681345
- KattmanSJWittyADGagliardiMStage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell linesCell Stem Cell20118222824021295278
- YeLZimmermannWHGarryDJZhangJPatching the heart: cardiac repair from within and outsideCirc Res2013113792293224030022
- LiRKJiaZQWeiselRDMickleDAChoiAYauTMSurvival and function of bioengineered cardiac graftsCirculation199910019 SupplII63II6910567280
- ThomsonJAItskovitz-EldorJShapiroSSEmbryonic stem cell lines derived from human blastocystsScience19982825391114511479804556
- YangLSoonpaaMHAdlerEDHuman cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived populationNature2008453719452452818432194
- BurridgePWKellerGGoldJDWuJCProduction of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogrammingCell Stem Cell2012101162822226352
- PaigeSLOsugiTAfanasievOKPabonLReineckeHMurryCEEndogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cellsPloS One201056e1113420559569
- UenoSWeidingerGOsugiTBiphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cellsProc Natl Acad Sci U S A2007104239685969017522258
- NaitoATShiojimaIAkazawaHDevelopmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesisProc Natl Acad Sci U S A200610352198121981717170140
- LianXHsiaoCWilsonGRobust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signalingProc Natl Acad Sci U S A201210927E1848E185722645348
- QianLHuangYSpencerCIIn vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytesNature2012485740059359822522929
- SongKNamYJLuoXHeart repair by reprogramming non-myocytes with cardiac transcription factorsNature2012485740059960422660318
- ChenJXKraneMDeutschMAWangLRav-AchaMInefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5Circ Res20121111505522581928
- AddisRCEpsteinJAInduced regeneration – the progress and promise of direct reprogramming for heart repairNat Med201319782983623836233
- KimCMajdiMXiaPNon-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiationStem Cells Dev201019678379520001453
- PasumarthiKBFieldLJCardiomyocyte enrichment in differentiating ES cell cultures: strategies and applicationsMethods Mol Biol200218515716811768986
- AndersonDSelfTMellorIRGohGHillSJDenningCTransgenic enrichment of cardiomyocytes from human embryonic stem cellsMol Ther200715112027203617895862
- UosakiHFukushimaHTakeuchiAEfficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expressionPloS One201168e2365721876760
- DuboisNCCraftAMSharmaPSIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cellsNat Biotechnol201129111011101822020386
- TohyamaSHattoriFSanoMDistinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytesCell Stem Cell201312112713723168164
- FernandesSNaumovaAVZhuWZLaflammeMAGoldJMurryCEHuman embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in ratsJ Mol Cell Cardiol201049694194920854826
- Writing Group MembersLloyd-JonesDAdamsRJAmerican Heart Association Statistics Committee and Stroke Statistics SubcommitteeHeart disease and stroke statistics – 2010 update: a report from the American Heart AssociationCirculation20101217e46e21520019324
- RumyantsevPPGrowth and Hyperplasia of Cardiac Muscle Cells1st edLondonTaylor and Francis19911371
- SoonpaaMHFieldLJSurvey of studies examining mammalian cardiomyocyte DNA synthesisCirc Res199883115269670914
- AnversaPKajsturaJVentricular myocytes are not terminally differentiated in the adult mammalian heartCirc Res19988311149670913
- BergmannOBhardwajRDBernardSEvidence for cardiomyocyte renewal in humansScience200932459239810219342590
- MichlerREStem cell therapy for heart failureCardiol Rev201422310511624595173
- RanganathSHLevyOInamdarMSKarpJMHarnessing the mesenchymal stem cell secretome for the treatment of cardiovascular diseaseCell Stem Cell201210324425822385653
- DauweDFJanssensSPStem cell therapy for the treatment of myocardial infarctionCurr Pharm Des201117303328334021919877
- TengCJLuoJChiuRCShum-TimDMassive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplastyJ Thorac Cardiovasc Surg2006132362863216935119
- MalliarasKLiTSLuthringerDSafety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cellsCirculation2012125110011222086878
- Abdel-LatifABolliRTleyjehIMAdult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysisArch Intern Med20071671098999717533201
- HofmannMWollertKCMeyerGPMonitoring of bone marrow cell homing into the infarcted human myocardiumCirculation2005111172198220215851598
- EllisonGMVicinanzaCSmithAJAdult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repairCell2013154482784223953114
- EllisonGMTorellaDDellegrottaglieSEndogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heartJ Am Coll Cardiol201158997798621723061
- WaringCDVicinanzaCPapalamprouAThe adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formationEur Heart J201435392722273123100284
- Nadal-GinardBEllisonGMTorellaDThe cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adultStem Cell Res2014133 Pt B61563024838077
- HoutgraafJHden DekkerWKvan DalenBMFirst experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarctionJ Am Coll Cardiol201259553954022281257
- StrauerBEBrehmMZeusTRegeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT StudyJ Am Coll Cardiol20054691651165816256864
- StammCKleineHDChoiYHIntramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studiesJ Thorac Cardiovasc Surg2007133371772517320570
- LosordoDWSchatzRAWhiteCJIntramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trialCirculation2007115253165317217562958
- PovsicTJJungeCNadaAA phase 3, randomized, double-blinded, active-controlled, unblinded standard of care study assessing the efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina: design of the RENEW studyAm Heart J20131656854861.e223708155
- van RamshorstJBaxJJBeeresSLIntramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trialJAMA2009301191997200419454638
- ZhaoQSunYXiaLChenAWangZRandomized study of mononuclear bone marrow cell transplantation in patients with coronary surgeryAnn Thorac Med200886618331840
- AssmusBSchächingerVTeupeCTransplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI)Circulation2002106243009301712473544
- MeyerGPWollertKCLotzJIntracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trialCirculation2006113101287129416520413
- WollertKCMeyerGPLotzJIntracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trialLancet2004364942914114815246726
- LundeKSolheimSAakhusSIntracoronary injection of mononuclear bone marrow cells in acute myocardial infarctionN Engl J Med2006355121199120916990383
- AssmusBTonnTSeegerFHRed blood cell contamination of the final cell product impairs the efficacy of autologous bone marrow mononuclear cell therapyJ Am Coll Cardiol201055131385139420338501
- SchächingerVErbsSElsässerAREPAIR-AMI InvestigatorsImproved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trialEur Heart J200627232775278317098754
- TenderaMWojakowskiWRuzyłłoWREGENT InvestigatorsIntracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) TrialEur Heart J200930111313132119208649
- GyöngyösiMLangIDettkeMCombined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized studyNat Clin Pract Cardiovasc Med200961708119002124
- SürderDMankaRLo CiceroVIntracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular functionCirculation2013127191968197923596006
- TraverseJHHenryTDEllisSGCardiovascular Cell Therapy Research NetworkEffect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trialJAMA2011306192110211922084195
- TraverseJHHenryTDVaughanDECardiovascular Cell Therapy Research NetworkLateTIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarctionTex Heart Inst J201037441242020844613
- MakkarRRSmithRRChengKIntracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trialLancet2012379981989590422336189
- PerinECSanz-RuizRSánchezPLAdipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE TrialAm Heart J201416818895.e224952864
- PerinECDohmannHFBorojevicRTransendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failureCirculation2003107182294230212707230
- PerinECDohmannHFBorojevicRImproved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathyCirculation200411011 Suppl 1II213II21815364865
- AssmusBFischer-RasokatUHonoldJTOPCARE-CHD RegistryTranscoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD RegistryCirc Res200710081234124117379833
- HareJMFishmanJEGerstenblithGComparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trialJAMA2012308222369237923117550
- StrauerBEYousefMSchannwellCMThe acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart studyEur J Heart Fail201012772172920576835
- Fischer-RasokatUAssmusBSeegerFHA pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathyCirc Heart Fail20092541742319808371
- PatnaikSSWangBWeedBWertheimJALiaoJDecellularized scaffolds: concepts, methodologies, and applications in cardiac tissue engineering and whole-organ regenerationLiuQWangHTissue Regeneration: Where Nanostructure Meets BiologySingaporeWorld Scientific201477124
- ZimmermannWMelnychenkoIEschenhagenTEngineered heart tissue for regeneration of diseased heartsBiomaterials20042591639164714697865
- BursacNPapadakiMCohenRJCardiac muscle tissue engineering: toward an in vitro model for electrophysiological studiesAm J Physiol19992772 Pt 2H433H44410444466
- Müller-EhmsenJWhittakerPKlonerRASurvival and development of neonatal rat cardiomyocytes transplanted into adult myocardiumJ Mol Cell Cardiol200234210711611851351
- ReineckeHMurryCETaking the death toll after cardiomyocyte grafting: a reminder of the importance of quantitative biologyJ Mol Cell Cardiol200234325125311945017
- YangYLMotteSKaufmanLJPore size variable type I collagen gels and their interaction with glioma cellsBiomaterials201031215678568820430434
- ChristmanKLFokHHSieversREFangQLeeRJFibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarctionTissue Eng2004103–440340915165457
- LandaNMillerLFeinbergMSEffect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in ratCirculation2008117111388139618316487
- GiraudMNAyuniECookSSiepeMCarrelTPTevaearaiHTHydrogel-based engineered skeletal muscle grafts normalize heart function early after myocardial infarctionArtif Organs200832969270018684206
- LuWNLüSHWangHBFunctional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogelTissue Eng Part A20091561437144719061432
- LutolfMPHubbellJASynthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineeringNat Biotechnol2005231475515637621
- DobnerSBezuidenhoutDGovenderPZillaPDaviesNA synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodelingJ Card Fail200915762963619700140
- KloudaLMikosAGThermoresponsive hydrogels in biomedical applicationsEur J Pharm Biopharm2008681344517881200
- NelsonDMMaZFujimotoKLHashizumeRWagnerWRIntra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challengesActa Biomater20117111520619368
- LeorJAmsalemYCohenSCells, scaffolds, and molecules for myocardial tissue engineeringPharmacol Ther2005105215116315670624
- ChristmanKLLeeRJBiomaterials for the treatment of myocardial infarctionJ Am Coll Cardiol200648590791316949479
- DavisMEHsiehPCGrodzinskyAJLeeRTCustom design of the cardiac microenvironment with biomaterialsCirc Res200597181516002755
- HuangNFYuJSieversRLiSLeeRJInjectable biopolymers enhance angiogenesis after myocardial infarctionTissue Eng20051111–121860186616411832
- ReffelmannTKlonerRACellular cardiomyoplasty – cardiomyocytes, skeletal myoblasts, or stem cells for regenerating myocardium and treatment of heart failure?Cardiovasc Res200358235836812757870
- KellarRSShepherdBRLarsonDFNaughtonGKWilliamsSKCardiac patch constructed from human fibroblasts attenuates reduction in cardiac function after acute infarctTissue Eng20051111–121678168716411813
- RobinsonKALiJMathisonMExtracellular matrix scaffold for cardiac repairCirculation20051129 SupplI135I14316159805
- ZimmermannWHMelnychenkoIWasmeierGEngineered heart tissue grafts improve systolic and diastolic function in infarcted rat heartsNat Med200612445245816582915
- LeorJTuviaSGuettaVIntracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in SwineJ Am Coll Cardiol200954111014102319729119
- DetenAVolzHCBriestWZimmerHGCardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in ratsCardiovasc Res200255232934012123772
- YoshidaMOhHStem cell engineering for cardiac tissue regenerationCardiology2010115319119320145395
- SingelynJMDeQuachJASeif-NaraghiSBLittlefieldRBSchup-MagoffinPJChristmanKLNaturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineeringBiomaterials200930295409541619608268
- ZimmermannWHEschenhagenTCardiac tissue engineering for replacement therapyHeart Fail Rev20038325926912878835
- KutschkaIChenIYKofidisTCollagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat heartsCirculation20061141 SupplI167I17316820568
- KochMAVrijEJEngelEPlanellJALacroixDPerfusion cell seeding on large porous PLA/calcium phosphate composite scaffolds in a perfusion bioreactor system under varying perfusion parametersJ Biomed Mater Res A20109541011101820872752
- DvirTTimkoBPBrighamMDNanowired three-dimensional cardiac patchesNat Nanotechnol201161172072521946708
- BilodeauKMantovaniDBioreactors for tissue engineering: focus on mechanical constraints. A comparative reviewTissue Eng20061282367238316968176
- RadisicMMarsanoAMaidhofRWangYVunjak-NovakovicGCardiac tissue engineering using perfusion bioreactor systemsNat Protoc20083471973818388955
- TandonNMarsanoACannizzaroCVoldmanJVunjak-NovakovicGDesign of electrical stimulation bioreactors for cardiac tissue engineeringConf Proc IEEE Eng Med Biol Soc200820083594359719163486
- RadisicMParkHShingHFunctional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffoldsProc Natl Acad Sci U S A200410152181291813415604141
- DavisMEMotionJPNarmonevaDAInjectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cellsCirculation2005111444245015687132
- KhaitLBirlaRKCell-based cardiac pumps and tissue-engineered ventriclesRegen Med20072439140617635047
- LeeEJKim doEAzelogluEUCostaKDEngineered cardiac organoid chambers: toward a functional biological model ventricleTissue Eng Part A200814221522518333774
- BadylakSFTaylorDUygunKWhole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffoldsAnnu Rev Biomed Eng201113275321417722
- SingelynJMChristmanKLInjectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matricesJ Cardiovasc Transl Res20103547848620632221
- Schenke-LaylandKVasilevskiOOpitzFImpact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valvesJ Struct Biol2003143320120814572475
- MoroniFMirabellaTDecellularized matrices for cardiovascular tissue engineeringAm J Stem Cells20143112024660110
- BreuerCKMettlerBAAnthonyTSalesVLSchoenFJMayerJEApplication of tissue-engineering principles toward the development of a semilunar heart valve substituteTissue Eng20041011–121725173615684681
- IopLBonettiANasoFDecellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluationPloS One201496e9959324940754
- OberwallnerBBrodaracAChoiYHPreparation of cardiac extracellular matrix scaffolds by decellularization of human myocardiumJ Biomed Mater Res A201410293263327224142588
- FreytesDOStonerRMBadylakSFUniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffoldsJ Biomed Mater Res B Appl Biomater200884240841417618508
- WangBBorazjaniATahaiMFabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cellsJ Biomed Mater Res A20109441100111020694977
- WainwrightJMCzajkaCAPatelUBPreparation of cardiac extracellular matrix from an intact porcine heartTissue Eng Part C Methods201016352553219702513
- OzawaTMickleDAWeiselRDKoyamaNOzawaSLiRKOptimal biomaterial for creation of autologous cardiac graftsCirculation200210612 Suppl 1I176I18212354729
- WeberBEmmertMYSchoenauerRBrokoppCBaumgartnerLHoerstrupSPTissue engineering on matrix: future of autologous tissue replacementSemin Immunopathol201133330731521279358
- ZouYZhangYMechanical evaluation of decellularized porcine thoracic aortaJ Surg Res2012175235936821571306
- SongJJOttHCOrgan engineering based on decellularized matrix scaffoldsTrends Mol Med201117842443221514224
- ChangYChenSCWeiHJWuTJLiangHCLaiPHTissue regeneration observed in a porous acellular bovine pericardium used to repair a myocardial defect in the right ventricle of a rat modelJ Thorac Cardiovasc Surg2005130370571116153917
- BadylakSFKochupuraPVCohenISDoroninSVSaltmanAEGilbertTWThe use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardiumCell Transplant200615S29S4016826793
- OtaTGilbertTWBadylakSFSchwartzmanDZenatiMAElectromechanical characterization of a tissue-engineered myocardial patch derived from extracellular matrixJ Thorac Cardiovasc Surg2007133497998517382638
- RobinsonKALiJMathisonMRedkarACuiJChronosNAExtracellular matrix scaffold for cardiac repairCirculation20051129SI135I14316159805
- FuRHWangYCLiuSPDecellularization and recellularization technologies in tissue engineeringCell Transplant2014234–562163024816454
- AzhimANaritaYMuramatsuKMorimotoYTanakaMDecellularization of living tissue using microwave chemical process for tissue-engineered scaffold applicationsLimCTGohJCInternational Conference on Biomedical Engineering Proceedings. Vol 31, 6th World Congress of Biomechanics (WCB 2010)August 1–6, 2010SingaporeConjunction with 14th International Conference on Biomedical Engineering (ICBME) and 5th Asia Pacific Conference on Biomechanics (APBiomech)Berlin and HeidelbergSpringer2010934937
- AkhyariPAubinHGwanmesiaPThe quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue engineeringPart C Methods2011179915926
- HeMCallananAComparison of methods for whole-organ decellularization in tissue engineering of bioartificial organsTissue eng Part B Rev201319319420823083305
- GuyetteJPGilpinSECharestJMTapiasLFRenXOttHCPerfusion decellularization of whole organsNat Protoc201491451146824874812
- VenkatasubramanianRTGrasslEDBarocasVHLafontaineDBischofJCEffects of freezing and cryopreservation on the mechanical properties of arteriesAnn Biomed Eng200634582383216619131
- GilbertTWSellaroTLBadylakSFDecellularization of tissues and organsBiomaterials200627193675368316519932
- ZangMZhangQChangEIMathurABYuPDecellularized tracheal matrix scaffold for tissue engineeringPlast Reconstr Surg2012130353254022929238
- YangMChenCZWangXNZhuYBGuYJFavorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valvesJ Biomed Mater Res B Appl Biomater200991135436119507136
- CissellDDHuJCGriffithsLGAthanasiouKAAntigen removal for the production of biomechanically functional, xenogeneic tissue graftsJ Biomech20144791987199624268315
- WongMLWongJLAthanasiouKAGriffithsLGStepwise solubilization-based antigen removal for xenogeneic scaffold generation in tissue engineeringActa Biomater2013956492650123321301
- Seif-NaraghiSBSingelynJMSalvatoreMASafety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarctionSci Transl Med20135173173ra125
- WeymannALoganathanSTakahashiHDevelopment and evaluation of a perfusion decellularization porcine heart model – generation of 3-dimensional myocardial neoscaffoldsCirc J201175485286021301134
- BadylakSFXenogeneic extracellular matrix as a scaffold for tissue reconstructionTranspl Immunol2004123–436737715157928
- ManjiRAZhuLFNijjarNKGlutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejectionCirculation2006114431832716831988
- Sánchez FernándezPLFernández SantosMECostanzaSDecelularización de corazones humanos para el desarrollo de matrices cardiacas que sirvan como soporte a la bioingeniería de tejidos cardiacos funcionales [Decellularization of human hearts for the development of heart matrices serve as a support for functional cardiac tissue bioengineering]. Abstract 31Rev Esp Cardiol201164Suppl 34 Spanish
- Seif-NaraghiSBSalvatoreMASchup-MagoffinPJHuDPChristmanKLDesign and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineeringTissue Eng Part A20101662017202720100033
- PaulABinsalamahZMKhanAAA nanobiohybrid complex of recombinant baculovirus and Tat/DNA nanoparticles for delivery of Ang-1 transgene in myocardial infarction therapyBiomaterials201132328304831821840594
- PaulAEliasCBShum-TimDPrakashSBioactive baculovirus nanohybrids for stent based rapid vascular re-endothelializationSci Rep20133236623917680
- PaulAHasanAKindiHAInjectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repairACS Nano2014888050806224988275
- PaulAHasanARodesLSangaralingamMPrakashSBioengineered baculoviruses as new class of therapeutics using micro and nanotechnologies: principles, prospects and challengesAdv Drug Deliv Rev20147111513024503281
- PaulAShaoWAbbasiSShum-TimDPrakashSPAMAM dendrimer-baculovirus nanocomplex for microencapsulated adipose stem cell-gene therapy: in vitro and in vivo functional assessmentMol Pharm2012992479248822817267
- Vunjak-NovakovicGTandonNGodierAChallenges in cardiac tissue engineeringTissue Eng Part B Rev201016216918719698068
- SimonPKasimirMTSeebacherGEarly failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patientsEur J Cardiothorac Surg200323610021006 discussion 100612829079
- SchoenFJLevyRJCalcification of tissue heart valve substitutes: progress toward understanding and preventionAnn Thorac Med200579310721080
- SutherlandFWPerryTEYuYFrom stem cells to viable autologous semilunar heart valveCirculation2005111212783279115927990
- HawkinsJABreinholtJPLambertLMClass I and class II anti-HLA antibodies after implantation of cryopreserved allograft material in pediatric patientsJ Thorac Cardiovasc Surg2000119232433010649208
- LiangRWooSLTakakuraYMoonDKJiaFAbramowitchSDLong-term effects of porcine small intestine submucosa on the healing of medial collateral ligament: a functional tissue engineering studyJ Orthop Res200624481181916514641
- ShimizuTYamatoMIsoiYFabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfacesCirc Res2002903e4011861428
- OkanoTYamadaNSakaiHSakuraiYA novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide)J Biomed Mater Res19932710124312518245039
- KushidaAYamatoMKonnoCKikuchiASakuraiYOkanoTDecrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfacesJ Biomed Mater Res199945435536210321708
- ShimizuTSekineHYangJPolysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissuesFASEB J200620670871016439619
- MiyaharaYNagayaNKataokaMMonolayered mesenchymal stem cells repair scarred myocardium after myocardial infarctionNat Med200612445946516582917
- MiyagawaSSawaYSakakidaSTissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardiumTransplantation200580111586159516371930
- MasudaSShimizuTYamatoMOkanoTCell sheet engineering for heart tissue repairAdv Drug Deliv Rev200860227728518006178
- SekineHShimizuTSakaguchiKIn vitro fabrication of functional three-dimensional tissues with perfusable blood vesselsNat Commun20134139923360990
- TakahashiKTanabeKOhnukiMInduction of pluripotent stem cells from adult human fibroblasts by defined factorsCell2007131586187218035408
- DidiéMChristallaPRubartMParthenogenetic stem cells for tissue-engineered heart repairJ Clin Investig201312331285129823434590
- ZhangJKlosMWilsonGFExtracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich methodCirc Res201211191125113622912385
- GantzJAPalpantNJWeliksonREHauschkaSDMurryCELaflammeMATargeted genomic integration of a selectable floxed dual fluorescence reporter in human embryonic stem cellsPloS One2012710e4697123071682
- Kita-MatsuoHBarcovaMPrigozhinaNLentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytesPloS One200944e504619352491
- KehatIKhimovichLCaspiOElectromechanical integration of cardiomyocytes derived from human embryonic stem cellsNat Biotechnol200422101282128915448703
- ZhangDShadrinIYLamJXianH-QSnodgrassHRBursacNTissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytesBiomaterials201334235813582023642535
- NunesSSMiklasJWLiuJAschar-SobbiRXiaoYZhangBBiowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytesNat Methods201310878178723793239
- TullochNLMuskheliVRazumovaMVGrowth of engineered human myocardium with mechanical loading and vascular cocultureCirc Res20111091475921597009
- KlugMGSoonpaaMHKohGYFieldLJGenetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac graftsJ Clin Invest19969812162248690796
- KawamuraMMiyagawaSMikiKFeasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy modelCirculation201312611 Suppl 1S29S3722965990
- MikiKUenakaHSaitoABioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in ratsStem Cells Transl Med20121543043723197822
- StevensKRKreutzigerKLDuprasSKPhysiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissueProc Natl Acad Sci U S A200910639165681657319805339
- JanssensSDuboisCBogaertJAutologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trialLancet2006367950511312116413875
- HuikuriHVKervinenKNiemeläMFINCELL InvestigatorsEffects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarctionEur Heart J200829222723273218845667
- HirschANijveldtRvan der VleutenPAHEBE InvestigatorsIntracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trialEur Heart J201132141736174721148540
- TraverseJHHenryTDPepineCJCardiovascular Cell Therapy Research Network (CCTRN)Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trialJAMA2012308222380238923129008
- PerinECWillersonJTPepineCJCardiovascular Cell Therapy Research Network (CCTRN)Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trialJAMA2012307161717172622447880
- DuckersHJHoutgraafJHehrleinCFinal results of a phase IIa, randomised, open-label trial to evaluate the percutaneous intramyocardial transplantation of autologous skeletal myoblasts in congestive heart failure patients: the SEISMIC trialEuroIntervention20116780581221252013
- DibNDinsmoreJLababidiZOne-year follow-up of feasibility and safety of the first US, randomized, controlled study using 3-dimensional guided catheter-based delivery of autologous skeletal myoblasts for ischemic cardiomyopathy (CAuSMIC study)JACC Cardiovasc Interv20092191619463392
- AngKLChinDLeyvaFRandomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG aloneNat Clin Pract Cardiovasc Med200851066367018711405
- LosordoDWHenryTDDavidsonCACT34-CMI InvestigatorsIntramyocardial, autologous CD34+ cell therapy for refractory anginaCirc Res2011109442843621737787
- BartunekJBehfarADolatabadiDCardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologicsJ Am Coll Cardiol201361232329233823583246
- CastaldoCDi MeglioFMiragliaRCardiac fibroblast-derived extracellular matrix (biomatrix) as a model for the studies of cardiac primitive cell biological properties in normal and pathological adult human heartBiomed Res Int2013201335237023738325
- ChamberlandCMartinez-FernandezABeraldiRNelsonTJEmbryonic decellularized cardiac scaffold supports embryonic stem cell differentiation to produce beating cardiac tissueISRN Stem Cells201420142014 Article ID 625164