Abstract
The stem cell paradigm was first demonstrated in hematopoietic stem cells. Whilst classically it was cytokines and chemokines which were believed to control stem cell fate, more recently it has become apparent that the stem cell niche and highly conserved embryonic pathways play a key role in governing stem cell behavior. One of these pathways, the hedgehog signaling pathway, found in all organisms, is vitally important in embryogenesis, performing the function of patterning through early stages of development, and in adulthood, through the control of somatic stem cell numbers. In addition to these roles in health however, it has been found to be deregulated in a number of solid and hematological malignancies, components of the hedgehog pathway being associated with a poor prognosis. Further, these components represent viable therapeutic targets, with inhibition from a drug development perspective being readily achieved, making the hedgehog pathway an attractive potential therapeutic target. However, although the concept of cancer stem cells is well established, how these cells arise and the factors which influence their behavior are not yet fully understood. The role of the hedgehog signaling pathway and its potential as a therapeutic target in hematological malignancies is the focus of this review.
Background
Stem cells
Adult somatic stem cells are defined as undifferentiated cells with three key properties: long life, multipotency, and the capacity to self-renew.Citation1 However, recent evidence has shown that although their phenotype is tightly defined, these cells are functionally heterogeneous.Citation2 The properties of stem cell self-renewal and survival are controlled by highly conserved embryonic signaling pathways, including the hedgehog (Hh), epithelial-mesenchymal transition, WNT, and Notch signaling pathways, the HOX transcription factors, and the BMI1/polycomb transcriptional regulators.Citation3
Cancer stem cells
There is experimental evidence from a number of malignancies to suggest the presence of a small population of very primitive cells that share many of the properties of somatic stem cells. These cells have been termed cancer stem cells (CSCs).Citation1
Whilst there is still much to learn in respect of the CSC, various experimental models have shown these cells to be quiescent, resistant to therapy, capable of self-renewal, and the initiation of tumors in secondary transplanted hosts.Citation4 Malignancies with evidence of CSC origin include hematological cancersCitation4,Citation5 and solid tumors including: prostate,Citation6 pancreas,Citation7 lung,Citation8 and certain neurological malignancies.Citation9
Although the concept of the CSC is largely accepted, the model by which it is capable of generating a tumor, or leukemia, remains contentious.Citation10 The stochastic model argues tumors are biologically homogeneous, with CSC behavior being determined by intrinsic or extrinsic factors; tumor heterogeneity arising because these factors are unpredictable.Citation11 In contrast, the hierarchy model argues CSCs are highly organized in unidirectional cellular hierarchies (the pattern of normal tissue growth). In the hierarchical model, the CSCs are biologically unique.Citation12 The fundamental difference between the models lies in which cell, or cells, are capable of behaving as a CSC with discrimination relying on accurate, well-defined experimental design.Citation13
The CSC hypothesis is of considerable clinical importance, potentially explaining minimal residual disease, relapse and disease progression, and highlighting the need to target these cells in order to effect a cure.Citation14 CSCs have been shown to be innately less sensitive to treatment, to continually develop genomic and epigenomic changes, and to uniquely interact with the stem cell niche.Citation14,Citation15 The influence each of these factors has on the CSCs treatment-resistant phenotype is however, unclear.
The pathways involved in self-renewal are of intense interest, with many, if not all being implicated in neoplastic proliferation when deregulated.Citation15 It is the behavior of one of these pathways, the Hh signaling pathway, which is the focus of this review.
Hh signaling pathway
The Hh signaling pathway was initially discovered in 1980 by Nüsslein-Volhard and Weischaus whilst studying embryonic patterning in the Drosophila fruit fly, with absence of the Hh protein giving the Drosophila a characteristic “hairy” or “prickly” appearance.Citation16,Citation17 Subsequent work has shown the Hh pathway to be highly conserved across species and vitally important in embryogenesis, performing the function of patterning during the early stages of development through the expansion and contraction of stem cell numbers. In adult organisms, through its ability to affect stem cell behavior in responsive tissues, it is involved in aspects of tissue maintenance and regeneration – proliferation, apoptosis, chromatin modeling, and self-renewal, acting in concert with other stimuli and the stem cell niche.Citation18
Canonical signaling
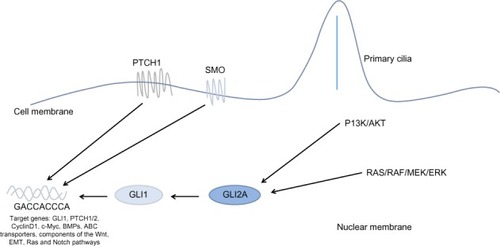
Classically, the Hh signaling pathway is believed to be ligand-dependent. Three Hh ligands (Sonic [SHH], Indian [IHH], and Desert [DHH]) have been identified in vertebrates, affecting stem cell behavior in a time- and concentration-dependent manner.Citation19 SHH is widely expressed, particularly during embryogenesis, with SHH deficiency being embryonically lethal.Citation17 IHH is produced in hematopoietic cells, bone, and cartilage,Citation20 whilst DHH is found in the peripheral nervous system and testes.Citation21 Hh ligands are initially synthesized as an inactive 45 kDa precursor, undergoing post-translational modifications to form a 19 kDa amino-terminal active signaling molecule.Citation22 This cholesterol and palmitoyl modification, catalyzed by Hh acyltransferase,Citation23 not only enhances ligand activity but also modifies its diffusion capacity.Citation24 The Hh ligands bind to the 12 trans-membrane receptor protein Patched 1 (PTCH1), causing its internalization and removing its repression of the 7-span trans-membrane protein Smoothened (SMO), allowing pathway activity.Citation25 In vertebrates, activity of the Hh pathway appears intrinsically related to primary immotile cilia; in the absence of ligand, PTCH1 is located within the primary cilia. Following ligand binding, and the internalization of PTCH1, SMO is able to concentrate in the primary cilia where it interacts with the GLI transcription factors shifting the balance toward pathway activation.Citation25 Whilst the intricacies of this interaction remain poorly understood, studies suggest these receptors do not physically interact, rather PTCH1 is thought to regulate SMO through an intermediary, with studies suggesting that oxysterols, including vitamin D3, are involved.Citation26 SMO subsequently causes accumulation of the full length active form of the zinc transcription factors GLI-2 and GLI-3 in the nucleus, and potentiates the activity of other positive regulators of the pathway including serine threonine kinase 36 (STK36) and kinesin family member 7 (KIF7), resulting in transcription of key downstream targets such as GLI-1 and PTCH1, regulators of chromatin formation, cell cycle activity, cell mobility, and apoptosis, eg, bone morphogenetic protein 4, forkhead box protein M1, and WNT2a,Citation27 . Of the transcription factors, GLI-1 functions as a positive regulator, GLI-3 a transcriptional repressor, and GLI-2 both a positive and negative transcriptional regulator determined by post-transcriptional and post-translational modifications.Citation28 It is the balance between these transcription factors which determines pathway activity.Citation29
Figure 1 Canonical Hedgehog (Hh) signaling.
Abbreviations: DISP, Dispatched; KIF 7, Kinesin family member 7; EMT, epithelial mesenchymaltransition; SCF(β-TRCP), Skp1-Cullin1-F-Box.
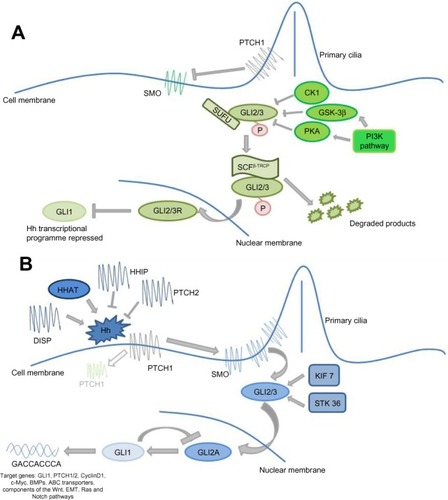
Alternatively, the Hh ligands can bind to a number of membrane-associated glycoproteins: Patched 2 (PTCH2), Hh-interacting protein (HHIP), and Dispatched (DISP). PTCH2, although structurally similar to PTCH1, has distinct functional properties and notably different tissue distribution, PTCH1 being broadly expressed whilst PTCH2 is primarily restricted to the skin and testes.Citation30,Citation31 HHIP is an endogenous Hh ligand inhibitor, with a binding affinity comparable to PTCH1, preventing pathway activation.Citation32 DISP, a 12 trans-membrane receptor protein, is not involved in Hh ligand synthesis or processing but rather facilitates ligand movement, thereby modulating canonical pathway activity.Citation33
In the inactive state the transcription factors GLI-2 and GLI-3 are retained in the cytoplasm in a protein complex associated with the inhibitory molecule, suppressor of fused (SUFU)Citation34 and non-specifically phosphorylated by casein kinase (CKI), glycogen synthase 3β (GSK3β), and protein kinase A (PKA). This complex subsequently undergoes E3 ubiquitin-mediated proteolysis by the Skp1-Cullin1-F-box protein (SCFβ-TRCP) to the truncated repressor form which, on translocating to the nucleus, strongly inhibits the Hh pathway,Citation35 . It is the complex interplay between the active and inactive state of the pathway and the positive and negative feedback loops that maintain the careful balance of Hh signaling in normal tissue.
Non-canonical signaling
The notion of non-canonical signaling has arisen following observations that the pathway response does not always appear to follow the classical canonical signaling paradigm, although the evidence is not conclusive. Three scenarios of non-canonical Hh signaling have been described, : direct interaction with components of other pathways, atypical interaction of components, and activity independent of GLI-mediated transcription, defined as Type I (PTCH-dependent) and Type II (SMO-dependent).Citation36 In reality, it is likely the canonical and non-canonical pathways act in parallel.
Downstream targets
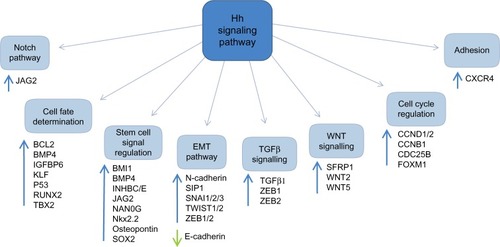
There are numerous downstream targets of the Hh pathway: BCL-2, the ATP-binding cassette transporter family members; the multi-drug resistance protein-1 and components of the epithelial-mesenchymal transition, WNT, and Notch signaling pathways, . There is already evidence to show up-regulation and involvement of many of these pathways in chemo-resistance in human malignancies.Citation37,Citation38 Additionally, studies have highlighted several of these downstream targets to be over-expressed in both acute myeloid leukemia (AML) and chronic myeloid leukemia (CML), with expression linked to chemo-resistance and poorer survival.Citation39
Hh signaling in hematopoiesis
In vertebrates, hematopoiesis is broadly divided into two major phases, primitive (embryonic) and definitive.Citation40 The Hh signaling pathway has a complex role in both embryonic and adult hematopoiesis. This role appears to be dependent on developmental stage, cell lineage, and whether the hematopoietic system is under regenerative pressure.Citation27,Citation41 Whilst evidence has shown it to be vital for early hematopoietic development,Citation20 there remains controversy over its role in normal hematopoiesis in adult organisms,Citation41–Citation43 although some of this may be explained by experimental method. Interestingly, recent early phase clinical trials looking at SMO inhibition have shown little or no hematopoietic toxicity potentially indicating Hh signaling may be dispensable in certain situations.Citation44 Importantly, abrogation of canonical Hh signaling by knockout of SMO does not adversely affect steady-state normal hematopoiesis.Citation42,Citation45
Hh signaling in malignancy
Crucially, abnormal Hh signaling has been associated with diverse human malignancies including basal cell carcinoma,Citation46 medulloblastoma,Citation47 pancreatic,Citation48 and lung cancer.Citation49 Interestingly, data suggest different mechanisms of action in the various tumor environments. Constitutive pathway activation through loss-of-function mutations,Citation46 epigenetic modifications,Citation50 or reduced expression of the negative regulators PTCH, HHIP, and SUFUCitation51 or gain-of-function mutations and epigenetic changesCitation52 in the positive regulator SMO have been observed in a number of solid malignancies.Citation53 To date, no mutations have been identified in hematological malignancies; however, epigenetic modifications have been observed in a cohort of pediatric AML patients, correlating with disease status.Citation54 Ligand-dependent canonical pathway activation involves autocrine or paracrine Hh signaling.Citation55 Autocrine Hh signaling has been identified in multiple myeloma (MM),Citation56 prostate,Citation57 and lung cancer.Citation58 Paracrine Hh signaling has been observed in lymphoma,Citation59 colon, and pancreatic cancer.Citation60
Hh signaling in myeloid malignancies
In myeloid malignancies, Hh signaling has been found to be vital in the maintenance and expansion of the CSC or “leukemic stem cell” (LSC), either as a survival and proliferation signal or through direction of the LSC fate.Citation27,Citation61–Citation64
CML is a clonal myeloproliferative disease driven by the Philadelphia chromosome which encodes the constitutively active BCR-ABL tyrosine kinase. For CML to develop, this mutation must originate in a multi-potent hematopoietic stem cell (HSC), producing the leukemia-initiating cell or LSC.Citation65 Acquisition of BCR-ABL by an HSC results in a number of functional changes, including increased proliferation, differentiation block, inhibition of apoptosis, and altered cell adhesion and stromal interactions, producing the clinical phenotype of CML.Citation66
The development of tyrosine kinase inhibitors (TKIs) has revolutionized the treatment of CML, with the majority of patients now achieving long-term survival with a good quality of life.Citation67 TKI therapy, however, is not without side effects, nor is it effective in all patients; TKI resistance and disease persistence despite TKI therapy remain ongoing issues. Persistent disease is believed to be due to lack of dependence on BCR-ABL signaling for survival and TKI resistance in CML stem cells.Citation68
Hh signaling appears to be intimately involved in the persistence and expansion of these CML stem cells.Citation35,Citation43,Citation61 Two different SMO-deficient murine models have shown supporting results – reduced incidence of leukemia in primary and secondary transplant recipients and a prolonged latency in primary transplantation.Citation43,Citation61 Additionally, these groups demonstrated improved survival, with an accompanying reduction in the LSC population following treatment with the SMO antagonist cyclopamine; combination therapy with a TKI and cyclopamine resulting in the largest reduction in LSCs in vitro and in vivo. These findings are supported by studies using clinical grade SMO inhibitors alone, and combined with TKIs, showing an improved survival, with a reduced incidence of leukemia in secondary transplant recipients and a marked reduction in measures of self-renewal.Citation69 Supporting work in NOD scid gamma mice, looking at molecular targets, has shown targeting the Hh pathway with dasatinib and GDC-0449 resulted in reduced expression of GLI-1, GLI-2, BCL-2, and Cyclin D2, and increased expression of p21, pATM, pChk2, and γH2AX.Citation70 Interestingly, low PTCH1 expression has been found to be an independent predictor of imatinib failure and reduced overall survival.Citation71
AML is an extremely heterogeneous clonal disorder. Whilst there is clear evidence to support the CSC theory in AML,Citation4,Citation72 recent work has suggested the LSC population is phenotypically variable, and may not be confined to a single clonal subpopulation. Further, whether this LSC arises following progenitor cell acquisition of abnormal self-renewal potential or from an HSC remains unclear.Citation4,Citation5,Citation72
There is increasing evidence showing the Hh pathway is deregulated in AML. Leukemic cell lines and primary AML cells express components of the Hh pathway, SHH and GLI-1.Citation73 GLI-1 expression correlating with cytogenetic risk, inferior event-free survival and a reduced overall survival, with GLI-1 conferring drug resistance through UGT1A-dependent glucuronidation.Citation74 Further, high GLI-1 expression predicts poor remission status and reduced overall survival in secondary AML.Citation75 GLI-2 has been shown to be a negative prognostic indicator in a number of microarrays. Additionally, aberrant Hh signaling has been linked to drug resistance with inhibition of the pathway restoring chemosensitivity in AML.Citation36,Citation62 Further, pathway inhibition with PF-04449913 sensitized AML cell lines and primary cells to the standard chemotherapy agent cytarabine; additional inhibition of SMO modulated cell cycle and self-renewal signaling.Citation76 In pediatric AML the hypo- and hypermethylation of pathway promoters were highly associated with AML diagnosis and relapse.Citation54 The complex interplay between the intrinsic and extrinsic signals governing LSC behavior may, however, mean targeting a single pathway, such as the Hh pathway, is not sufficient to eradicate these CSCs. For example, in an MLL-AF9-mediated murine model of myeloid leukemia Hh signaling was completely dispensable for the development of acute leukemia.Citation42,Citation45
The role of the Hh pathway in myelodysplastic syndrome (MDS) is not so well understood. However, there is increasing evidence to show it is deregulated. Analysis of primary MDS samples found overexpression of SHH, DHH, PTCH1, and SMO.Citation77 Further, there was a correlation between ligand expression and the progenitor/stem cell marker c-KIT.Citation77
Interestingly, work has shown the Hh pathway to act on the microenvironmental stromal cells, thereby regulating HSC behavior,Citation64 with the Hh ligands acting in an autocrine and/or paracrine manner.Citation59,Citation62,Citation78 Moreover, HHIP was highly expressed in primary stromal cells, but not in primary peripheral blood, bone marrow, or cord blood CD34+ selected cells, with stromal HHIP expression suppressing leukemic cell proliferation.Citation79 Further, studies have demonstrated HHIP expression in AML and MDS-derived bone marrow stromal cells to be markedly reduced, with these cells supporting the proliferation of leukemic cells; pretreatment with azacitidine increased stromal cell HHIP expression, with a parallel reduction in leukemic cell proliferation in co-culture.Citation79
Myeloproliferative neoplasms is a term encompassing several BCR-ABL-negative neoplasms – myelofibrosis (MF), polycythemia vera, and essential thrombocythemia – characterized by stem cell-derived clonal myeloproliferation and the Janus kinase 2 (JAK2V617F)Citation80 or Calreticulin mutation.Citation81 There are limited data available on the Hh pathway in these malignancies. GLI-1 and PTCH1 are increased 20–100-fold in primary myeloproliferative neoplasms samples, with pathway activity demonstrated in a murine bone marrow transplant model of MF using a GLI-luciferase reporter system. Combining the SMO antagonist LDE225 with INC424, a JAK1/2 dual kinase inhibitor, in this murine model saw a reduction in mutant allele burden and an improved clinical phenotype.Citation82 Another murine model of MF (Gata1low) has shown alterations in the Hh pathway at the gene level in the bone marrow and spleen, components of the Hh pathway coordinating with TGFβ, p53, and mTOR-related genes to produce the biological phenotype of MF.Citation83 However, a recent Phase II clinical trial using IPI-926 in MF saw the majority of patients taken off therapy due to side effects or no response.Citation84 How the Hh inhibitors work in combination with other novel drugs remains to be fully ascertained.
Hh signaling in lymphoid disorders
Hh signaling is vital for normal B- and T-cell development; with Hh ligands, produced by either the bone marrow or lymphoid organ microenvironment, determining lymphoid cell behavior.Citation63
B-cell disorders
B-cell acute lymphoblastic leukemia (B-ALL) is thought to develop from the malignant transformation of immature hematopoietic progenitor cells. Unlike AML, in which the CSC hypothesis was first demonstrated and is widely accepted, the B-ALL LSC has yet to be isolated or characterized,Citation85 blasts from all stages of maturation being able to reconstitute and establish a leukemic phenotype in vivo.Citation86 Interestingly however, up-regulation of components of the Hh pathway has been observed in precursor B-ALL,Citation87 SMO inhibition reducing in vitro and in vivo measures of self-renewal.Citation88
B-cell chronic lymphocytic leukemia (B-CLL) is a clonal disorder of mature, differentiated lymphocytes. Although not a CSC disease, both components of the Hh pathway (GLI-1, GLI-2, and SUFU) and key downstream targets (BCL-2, BCL-XL) are increased, and importantly correlate with clinical outcome.Citation89 Expression of BCL-2 is increased in the presence of active Hh signaling and down-regulated upon inhibition of the pathway.Citation59 Further, cyclopamine increased apoptosis of B-CLL cells in vitro, in stromal co-culture conditions and in combination with fludarabine.Citation59,Citation89 Gene expression of components of the Hh pathway is extremely variable in CLL patients, with expression correlating with response to SMO inhibition. Sixty percent of treated patient samples responded to at least one SMO antagonist; likelihood of response correlating with elevated GLI-1 and PTCH1 expression and the presence of trisomy 12 (a poor prognostic indicator). This work also showed evidence of autocrine DHH signaling, potentially enabling non-canonical signaling in B-CLL cells.Citation90 In contrast, another study found PTCH1, SMO, and GLI-1 to be reduced in primary CLL samples, although there was considerable heterogeneity – about 25% showing high transcript levels compared to normal B-lymphocytes. GANT61, a direct GLI inhibitor, induced apoptosis in a time- and concentration-dependent manner in association with STAT3 phosphorylation.Citation91 A further study found SMO and GLI-1 to be significantly down-regulated in B-CLL cells compared to normal B cells.Citation92 Moreover, whilst cyclopamine and knockdown of SMO had only minor specific effects on B-CLL cell survival in vitro, GANT61 caused significant apoptosis in B-CLL cells but not normal B cells.Citation92 Combined treatment with GANT61 and fludarabine causing increased apoptosis compared to either agent alone. Interestingly however, whilst CLL viability improved in the presence of the stromal cell lines HS-5 and M210-B4, known to produce Hh ligands,Citation59,Citation92 the same effect was not achieved with soluble SHH indicating other factors are involved. So, whilst these results support previous reports of Hh pathway activity in CLL, they would support redundancy of canonical Hh signaling in vitro.
The term lymphoma encompasses a broad spectrum of diseases from the relatively indolent to the aggressive and rapidly fatal; each has a complex and heterogeneous pathogenesis. Broadly there are two categories, Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL). Components of the Hh pathway and key downstream targets (BCL-2 and BCL-XL) are expressed in a variety of NHL cell lines and primary tissue,Citation59,Citation93 with expression of the downstream targets being influenced by the Hh pathway.
Whilst an Eμ-myc murine model of Burkitt’s lymphoma, an extremely aggressive form of NHL, demonstrated the importance of stromal co-culture for lymphoma cell survival and expansion, stroma could be replaced with soluble SHH or IHH.Citation59 Moreover, Burkitt’s cells underwent apoptosis in the absence of Hh signaling both in vitro and in vivo.
In diffuse large B-cell lymphoma (DLBCL), an aggressive form of NHL, response to Hh signaling is dependent on subtype, predominantly inducing cell cycle arrest in germinal center B-cell type, and apoptosis in the activated B-cell type.Citation93 In addition to responding to the Hh ligands, these DLBCL cells were also shown to synthesize and secrete Hh ligands, supporting a role for autocrine signaling.Citation94 Pharmacological and silencing techniques have shown expression of the AKT genes to be modulated by the Hh pathway in DLBCL at the transcriptional level,Citation95 with another study showing that the Hh pathway activates the NF-kβ pathway.Citation96
In another form of aggressive NHL, mantle cell lymphoma, a therapy-resistant murine model showed up- regulation of the GLI transcription factors at the gene level,Citation97 confirming previous work showing the GLI transcription factors to be over-expressed in mantle cell lymphoma, both in cell lines and primary lymphoma cells, compared to normal B cells.Citation98 Further, targeting the GLI transcription factors with antisense oligonucleotides down-regulated BCL-2 and Cyclin D1 resulting in decreased proliferation and increased susceptibility to chemotherapy.Citation98
Whilst these represent high grade forms of NHL, the Hh pathway has also been found to be deregulated and amenable to therapeutic intervention in low-grade lymphomas. In Waldenstrom’s macroglobulinemia, a characteristically indolent form of lymphoma secreting immunoglobulin M (IgM), GLI-2 has been found to influence IgM levels. Further, GANT61 resulted in a significant reduction in IgM secretion across a number of Waldenstrom’s macroglobulinemia cell lines potentially through the interleukin-6 receptor; this effect was not seen with cyclopamine.Citation99
In classical HL, whilst the Hh ligands and GLI transcription factors GLI-1 and -2 were expressed at a relatively low level, GLI-3 was highly expressed in all cell lines. Furthermore, immunohistochemistry for GLI-3 showed strong, uniform nuclear expression in virtually all Hodgkin/Reed-Stenberg cells whilst expression was variable in nodular lymphocyte predominant HL and NHL.Citation100 Interestingly GLI-3 in thymic stromal cells has been shown to regulate T-cell selection and thymocyte differentiation;Citation101 whether it is responsible for a similar role in HL remains to be determined.
MM is a CSC disorder, arising from the proliferation of a clonal population of plasma cells, and associated with the production of monoclonal immunoglobulin. Despite a variety of therapeutic approaches MM is incurable with a relapsing natural history; it is the MM CSC which is believed to be resistant to standard therapies, including lenalidomide, bortezomib, dexamethasone, and cyclophosphamide.
In MM, Hh pathway activity has been implicated in the maintenance and differentiation of the CSC.Citation56,Citation78 Pathway inhibition, with cyclopamine, or neutralization, using the monoclonal antibody 5E1, resulted in contraction of the CSC compartment, whereas exogenous Hh ligand caused expansion.Citation78 In addition, using LDE225 to target canonical signaling, and Forskolin a GLI-1 modulating compound, thereby bypassing PTCH1 and SMO and targeting non-canonical signaling, investigators have shown both mechanisms are amenable to therapeutic intervention in vitro in cell lines and patient samples.Citation56 Supporting evidence, using LDE225, confirmed differentiation was significantly induced and de-differentiation blocked in both cell lines and primary cells in vitro.Citation102 Moreover, there was a marked discrepancy in Hh pathway component expression, activity, and cyclopamine sensitivity between the stem cell and differentiated cell compartments.Citation78 Interestingly, Hh pathway gene expression was significantly altered in vivo suggesting the cell microenvironment markedly affects expression of Hh pathway components.Citation78
T-cell disorders
T-cell disorders, characterized by the abnormal proliferation of T-cell precursors, are generally aggressive, and far rarer than B-cell disorders. Interestingly, in comparison to B-ALL and B-cell lymphomas there are significant similarities between T-ALL and T-cell lymphomas; many arguing these entities are variations of the same disease, the two conditions often being treated in the same way.Citation103
The Hh pathway has been shown to be important in T-cell development;Citation104 it has also been implicated in the etiology of T-cell malignancies. In ALK+ anaplastic large cell lymphoma cell lines and primary tissue, SHH was amplified. In addition, GLI-1 expression at both the gene and protein level was increased; both SHH and GLI1 expression being influenced by the PI3K/AKT pathway although the mechanism remains unclear.Citation105 Supporting the importance of these components in cell survival, inhibition of SHH signaling with cyclopamine or silencing GLI-1 with ribonucleic acid interference resulted in cell cycle arrest and apoptosis.Citation105
Epigenetic modifications of the Hh pathway
Epigenetic modifications are stable, heritable alterations in gene expression caused without a change in coding DNA but rather DNA methylation and histone modification.Citation106 The bromodomain and extra-terminal domain (BET) protein family, responsible for reading differentially acetylated histones and thereby communicating changes in transcription potential, comprises four distinct genes, BRD2, -3, and -4 which are ubiquitously expressed and BRDT which is restricted to the testes. Importantly, there is increasing evidence to support the epigenetic modification of components of the Hh pathway. Work has predominantly focused on neural tissue and medulloblastoma, finding GLI-1 and GLI-2, but not GLI-3, to be acetylated, with a careful regulatory balance controlling GLI acetylation, and thereby transcriptional activity.Citation107 Transcriptional activity occurs through histone deacetylation, whilst the binding of RENKCTD11, an endogenous histone deacetylase inhibitor, to SCF(Β-TrCP)3-like E3 ubiquitin ligase complex, promotes GLI acetylation inhibiting transcription.Citation108 Recent work has shown BET proteins to further modulate GLI transcription and thereby pathway activity.Citation109 Targeting BRD4 with JQ1, a small-molecule bromodomain inhibitor, preventing BRD4 acetyl-lysine binding resulted in a global down-regulation of GLI associated genes in both patient and genetically engineered mouse model-derived Hh-driven tumors.Citation109 It remains to be seen whether this will translate into clinical practice. Further, how epigenetic modification of other components of the pathway will affect Hh antagonism in clinical practice and whether these too will be amenable to manipulation remains to be investigated.
Targeting the Hh pathway in hematological malignancies
There is increasing evidence to support the role of the Hh signaling pathway in hematological malignancies, with components representing viable therapeutic targets, making it a very attractive potential treatment strategy. Further, from a drug development perspective, inhibition of SMO and GLI-1 can be readily achieved, .
Table 1 Hedgehog inhibitors
The rationale for Hh inhibition in hematological malignancies is to target the LSC population, eradicating these therapy resistant cells, potentially affecting a cure. However, whilst there is evidence that the Hh pathway is important for LSC maintenance and expansion in CML and MM, its role in other diseases such as AML, MF, CLL, and lymphoma appears more complex, representing a delicate balance between the diseased cells and the stromal microenvironment. Whether Hh antagonism will be therapeutically useful in hematologic malignancies not only depends on its ability to target the diseased cells but also on the anticipated level of toxicity, both hematologic and systemic. To date, there appears to be little hematologic toxicity reported, particularly in solid tumor studies.Citation130
Whilst experimental data suggest Hh antagonism may address the persistence of CSC and the protective effect of the tumor microenvironment in both myeloid and lymphoid malignancies, clinical trials are only in the early stages, . Interestingly, it is only the SMO antagonists which are currently being trialed clinically. Excitingly however, several of these clinical grade SMO inhibitors are now in Phase II trial; with a number of Phase III trials in solid tumors and the licensing of vismodegib for use in basal cell carcinoma in 2012 hinting these early results could translate into clinical practice.
Table 2 Clinical trials involving hedgehog inhibitors
In hematological malignancies, results of a Phase Ia study using PF-04449913 in refractory, resistant, or intolerant myeloid malignancies were presented in 2011.Citation131 Thirty-two patients were enrolled, with early indications of efficacy seen across all diseases.Citation131 Notably, one patient with AML achieved a complete remission albeit with incomplete blood recovery and five had a >50% reduction in bone marrow blast counts. Additionally, one patient with MF achieved a >50% reduction in extramedullary disease and five attained stable disease. Results of the current Phase II trials are eagerly awaited.
Conclusion
Deregulated expression of components of the Hh signaling pathway is found throughout the huge range of hematological malignancies; the pathway appearing to either determine CSC behavior directly or indirectly via the stromal microenvironment.
As our knowledge and understanding of the Hh and other conserved embryonic pathways in hematologic malignancies increases, our ability to manipulate and potentially use these as therapeutic targets can only increase. Further, the sheer number of compounds coming onto the market, targeting both canonical and non-canonical mechanisms and the array of clinical trials, makes this an exciting time looking to tailor therapy and minimize treatment-related side effects.
Disclosure
M Copland has received research funding from Bristol-Myers Squibb and Novartis, honoraria from/attended Advisory Boards for Bristol-Myers Squibb, Pfizer, Ariad and Novartis, Bristol-Myers Squibb and Pfizer and travel funding from Bristol-Myers Squibb and Novartis. V Campbell is funded by the Wellcome Trust via the STMTI programme. M Copland is funded by Leukaemia and Lymphoma Research (Grant No: 11017). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript apart from those discussed.
References
- NguyenLVVannerRDirksPEavesCJCancer stem cells: an evolving conceptNat Rev Cancer201212213314322237392
- CopleyMRBeerPAEavesCJHematopoietic stem cell heterogeneity takes center stageCell Stem Cell201210669069722704509
- ZonLIIntrinsic and extrinsic control of haematopoietic stem-cell self-renewalNature2008453719330631318480811
- BonnetDDickJEHuman acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cellNat Med1997377307379212098
- GoardonNMarchiEAtzbergerACoexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemiaCancer Cell201119113815221251617
- CollinsATBerryPAHydeCStowerMJMaitlandNJProspective identification of tumorigenic prostate cancer stem cellsCancer Res20056523109461095116322242
- BuglinoJAReshMDPalmitoylation of hedgehog proteinsVitam Horm20128822925222391306
- O’FlahertyJDBarrMFennellDThe cancer stem-cell hypothesis: its emerging role in lung cancer biology and its relevance for future therapyJ Thorac Oncol20127121880189023154562
- SinghSKClarkeIDTerasakiMIdentification of a cancer stem cell in human brain tumorsCancer Res200363185821582814522905
- ValentPBonnetDDe MariaRCancer stem cell definitions and terminology: the devil is in the detailsNat Rev Cancer2012121176777523051844
- WangJCDickJECancer stem cells: lessons from leukemiaTrends Cell Biol200515949450116084092
- DickJEStem cell concepts renew cancer researchBlood2008112134793480719064739
- CleversHThe cancer stem cell: premises, promises and challengesNat Med201117331331921386835
- ReyaTMorrisonSJClarkeMFWeissmanILStem cells, cancer, and cancer stem cellsNature2001414685910511111689955
- AlisonMRLimSMNicholsonLJCancer stem cells: problems for therapy?J Pathol2011223214716121125672
- Nusslein-VolhardCWieschausEMutations affecting segment number and polarity in DrosophilaNature198028757857958016776413
- InghamPWMcMahonAPHedgehog signaling in animal development: paradigms and principlesGenes Dev200115233059308711731473
- KatohYKatohMHedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activationCurr Mol Med20099787388619860666
- InghamPWPlaczekMOrchestrating ontogenesis: variations on a theme by sonic hedgehogNat Rev Genet200671184185017047684
- CridlandSOKeysJRPapathanasiouPPerkinsACIndian hedgehog supports definitive erythropoiesisBlood Cells Mol Dis200943214915519443245
- BitgoodMJShenLMcMahonAPSertoli cell signaling by Desert hedgehog regulates the male germlineCurr Biol1996632983048805249
- LeeJJEkkerSCvon KesslerDPAutoproteolysis in hedgehog protein biogenesisScience19942665190152815377985023
- BuglinoJAReshMDHhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic HedgehogJ Biol Chem200828332220762208818534984
- PorterJAvon KesslerDPEkkerSCThe product of hedgehog autoproteolytic cleavage active in local and long-range signallingNature199537465203633667885476
- RohatgiRMilenkovicLScottMPPatched1 regulates hedgehog signaling at the primary ciliumScience2007317583637237617641202
- TaipaleJCooperMKMaitiTBeachyPAPatched acts catalytically to suppress the activity of SmoothenedNature2002418690089289712192414
- BhardwajGMurdochBWuDSonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulationNat Immunol20012217218011175816
- SasakiHNishizakiYHuiCNakafukuMKondohHRegulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signalingDevelopment1999126173915392410433919
- Ruiz i AltabaAMasCSteccaBThe Gli code: an information nexus regulating cell fate, stemness and cancerTrends Cell Biol200717943844717845852
- CarpenterDStoneDMBrushJCharacterization of two patched receptors for the vertebrate hedgehog protein familyProc Natl Acad Sci U S A1998952313630136349811851
- RahnamaFToftgardRZaphiropoulosPGDistinct roles of PTCH2 splice variants in Hedgehog signallingBiochem J2004378Pt 232533414613484
- ChuangPTMcMahonAPVertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding proteinNature1999397672061762110050855
- KawakamiTKawcakTLiYJMouse dispatched mutants fail to distribute hedgehog proteins and are defective in hedgehog signalingDevelopment2002129245753576512421714
- ChenMHWilsonCWLiYJCilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conservedGenes Dev200923161910192819684112
- IrvineDACoplandMTargeting hedgehog in hematologic malignancyBlood2012119102196220422223823
- JenkinsDHedgehog signalling: emerging evidence for non-canonical pathwaysCellular Signal200921710231034
- ReedJCBcl-2 family proteins: strategies for overcoming chemoresistance in cancerAdv Pharmacol1997415015329204157
- LingVCharlesFKettering Prize. P-glycoprotein and resistance to anticancer drugsCancer19926910260326091348966
- KurinnaSKonoplevaMPallaSLBcl2 phosphorylation and active PKC alpha are associated with poor survival in AMLLeukemia20062071316131916642043
- KellerGLacaudGRobertsonSDevelopment of the hematopoietic system in the mouseExp Hematol199927577778710340392
- TrowbridgeJJScottMPBhatiaMHedgehog modulates cell cycle regulators in stem cells to control hematopoietic regenerationProc Natl Acad Sci U S A200610338141341413916968775
- GaoJGravesSKochUHedgehog signaling is dispensable for adult hematopoietic stem cell functionCell Stem Cell20094654855819497283
- DierksCBeigiRGuoGRExpansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activationCancer Cell200814323824918772113
- CohenDJTargeting the hedgehog pathway: role in cancer and clinical implications of its inhibitionHematol Oncol Clin North Am201226356558822520980
- HofmannIStoverEHCullenDEHedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesisCell Stem Cell20094655956719497284
- XieJMuroneMLuohSMActivating Smoothened mutations in sporadic basal-cell carcinomaNature1998391666290929422511
- BermanDMKarhadkarSSHallahanARMedulloblastoma growth inhibition by hedgehog pathway blockadeScience200229755861559156112202832
- ThayerSPdi MaglianoMPHeiserPWHedgehog is an early and late mediator of pancreatic cancer tumorigenesisNature2003425696085185614520413
- ParkKSMartelottoLGPeiferMA crucial requirement for Hedgehog signaling in small cell lung cancerNat Med201117111504150821983857
- MartinSTSatoNDharaSAberrant methylation of the Human Hedgehog interacting protein (HHIP) gene in pancreatic neoplasmsCancer Biol Ther20054772873315970691
- SladeIMurrayAHanksSHeterogeneity of familial medulloblastoma and contribution of germline PTCH1 and SUFU mutations to sporadic medulloblastomaFam Cancer201110233734221188540
- ShahiMHLorenteACastresanaJSHedgehog signalling in medulloblastoma, glioblastoma and neuroblastomaOncol Rep200819368168818288402
- CarDSabolMMusaniVOzreticPLevanatSEpigenetic regulation of the Hedgehog-Gli signaling pathway in cancerPeriod Biol20101124419423
- OchsMFarrarJConsidineMGenome wide promoter methylation patterns predict AML subtype outcomes and identify novel pathways characterizing diagnostic and relapsed disease in childrenPresented at: 54th ASH Annual Meeting and ExpositionDecember 8–11, 2012Atlanta. GA, USA
- ScalesSJde SauvageFJMechanisms of Hedgehog pathway activation in cancer and implications for therapyTrends Pharmacol Sci200930630331219443052
- BlottaSJakubikovaJCalimeriTCanonical and noncanonical Hedgehog pathway in the pathogenesis of multiple myelomaBlood2012120255002501322821765
- KarhadkarSSBovaGSAbdallahNHedgehog signalling in prostate regeneration, neoplasia and metastasisNature2004431700970771215361885
- WatkinsDNBermanDMBurkholderSGHedgehog signalling within airway epithelial progenitors and in small-cell lung cancerNature2003422692931331712629553
- DierksCGrbicJZirlikKEssential role of stromally induced hedgehog signaling in B-cell malignanciesNat Med200713894495117632527
- TheunissenJWde SauvageFJParacrine Hedgehog signaling in cancerCancer Res200969156007601019638582
- ZhaoCChenAJamiesonCHHedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemiaNature2009458723977677919169242
- KobuneMTakimotoRMuraseKDrug resistance is dramatically restored by hedgehog inhibitors in CD34+ leukemic cellsCancer Sci2009100594895519245435
- KobuneMKatoJKawanoYAdenoviral vector-mediated transfer of the Indian hedgehog gene modulates lymphomyelopoiesis in vivoStem Cells200826253454217962696
- KobuneMItoYKawanoYIndian hedgehog gene transfer augments hematopoietic support of human stromal cells including NOD/SCID-beta2m−/− repopulating cellsBlood200410441002100915105288
- DeiningerMWGoldmanJMMeloJVThe molecular biology of chronic myeloid leukemiaBlood200096103343335611071626
- HochhausAHughesTClinical resistance to imatinib: mechanisms and implicationsHematol Oncol Clin North Am200418364165615271397
- HochhausAO’BrienSGGuilhotFSix-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemiaLeukemia20092361054106119282833
- HamiltonAHelgasonGVSchemionekMChronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survivalBlood201211961501151022184410
- IrvineDAZhangBAllanEKCombination of the hedgehog pathway inhibitor LDE225 and nilotinib eliminates chronic myeloid leukemia stem and progenitor cellsPresented at: 51st ASH Annual Meeting and ExpositionDecember 5–8, 2009New Orleans, LA
- OkabeSTauchiTTanakaYKatagiriSOhyashikiKEffects of the hedgehog inhibitor GDC-0449, alone or in combination with dasatinib, on BCR-ABL-positive leukemia CellsStem Cells Dev201221162939294822642671
- Alonso-DominguezJMGrinfeldJAlikianMPTCH1 expression at diagnosis reliably predicts treatment failure in imatinib-treated chronic myeloid leukaemia patientsPresented at: 54th ASH Annual Meeting and ExpositionDecember 8–11, 2012Atlanta, GA
- LapidotTSirardCVormoorJA cell initiating human acute myeloid leukaemia after transplantation into SCID miceNature199436764646456487509044
- BaiLYChiuCFLinCWDifferential expression of Sonic hedgehog and Gli1 in hematological malignanciesLeukemia200822122622817928882
- ZahreddineHACuljkovic-KraljacicBAssoulineSThe sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidationNature20145117507909324870236
- CampbellVTholouliEQuigleyMTEvidence that activated hedgehog signaling predicts for poor clinical outcome in acute myeloid leukemiaPresented at: 54th ASH Annual Meeting and ExpositionDecember 8–11, 2012Atlanta, GA
- FukushimaNMinamiYHayakawaFTreatment with hedgehog inhibitor, PF-04449913, attenuates leukemia-initiation potential in acute myeloid leukemia cellsPresented at: 55th ASH Annual Meeting and ExpositionDecember 7–10, 2013New Orleans, LA
- XavierJMDuarteASSPericoleFVHedgehog pathway is deregulated in myelodysplastic syndrome progenitor bone marrow cellsPresented at: 55th ASH Annual Meeting and ExpositionDecember 7–10, 2013New Orleans, LA
- PeacockCDWangQGesellGSHedgehog signaling maintains a tumor stem cell compartment in multiple myelomaProc Natl Acad Sci U S A2007104104048405317360475
- KobuneMIyamaSKikuchiSStromal cells expressing hedgehog-interacting protein regulate the proliferation of myeloid neoplasmsBlood Cancer J20122e8722961059
- LevineRLWadleighMCoolsJActivating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosisCancer Cell20057438739715837627
- KlampflTGisslingerHHarutyunyanASSomatic mutations of calreticulin in myeloproliferative neoplasmsN Engl J Med2013369252379239024325356
- BhagwatNKellerMDRampalRKImproved efficacy of combination of JAK2 and hedgehog inhibitors in myelofibrosisPresented at: 55th ASH Annual Meeting and ExpositionDecember 7–10, 2013New Orleans, LA
- ZingarielloMMartelliFCiaffoniFCharacterization of the TGF-beta1 signaling abnormalities in the Gata1low mouse model of myelofibrosisBlood2013121173345336323462118
- SasakiKGGotlibJRMesaRAA phase 2 study of IPI-926, an oral hedgehog inhibitor, in patients with myelofibrosisJ Clin Oncol2014325s (suppl; abstr 7111)
- BerntKMArmstrongSALeukemia stem cells and human acute lymphoblastic leukemiaSemin Hematol2009461333819100366
- le ViseurCHotfilderMBomkenSIn childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell propertiesCancer Cell2008141475818598943
- LangFBaduraSRuthardtMRiegerMAOttmannOGModulation of leukemic stem cell self-renewal and cell fate decisions by inhibition of hedgehog signalling in human acute lymphoblastic leukemia (ALL)Presented at: 54th ASH Annual Meeting and ExpositionDecember 8–11, 2012Atlanta, GA
- LinTLWangQHBrownPSelf-renewal of acute lymphocytic leukemia cells is limited by the Hedgehog pathway inhibitors cyclopamine and IPI-926PloS One2010512e1526221203400
- HegdeGVPetersonKJEmanuelKHedgehog-induced survival of B-cell chronic lymphocytic leukemia cells in a stromal cell microenvironment: a potential new therapeutic targetMol Cancer Res20086121928193619074837
- DeckerSZirlikKDjebatchieLTrisomy 12 and elevated GLI1 and PTCH1 transcript levels are biomarkers for Hedgehog-inhibitor responsiveness in CLLBlood20121194997100722130798
- WangCLuKWangXGLI1 Inhibitor GANT61 Deregulates stat3 Phosphorylation In Chronic Lymphocytic Leukemia CellsBlood201312221
- DeschPAsslaberDKernDInhibition of GLI, but not Smoothened, induces apoptosis in chronic lymphocytic leukemia cellsOncogene201029354885489520603613
- PatoleSPPatoleASRhenDSPatterned carbon nanotube growth using an electron beam sensitive direct writable catalystNanotechnology2009203131530219597250
- SinghRRKimJEDavuluriYHedgehog signaling pathway is activated in diffuse large B-cell lymphoma and contributes to tumor cell survival and proliferationLeukemia20102451025103620200556
- AgarwalNKQuCJKunkallaKLiuYDVegaFGLI1 directly regulates the transcription of AKT genes in diffuse large B-cell lymphomaBlood201212021Presented at: 54th ASH Annual Meeting and ExpositionDecember 8–11, 2012Atlanta, GA, USA
- QuCJLiuYDKunkallaKAgarwalNKVegaFSmoothened (SMO) activates NF-Kb pathway through activation of PKC beta/CARMA1 and TRAF6 stabilization in diffuse large B-cell lymphomaBlood201212021Presented at: 54th ASH Annual Meeting and ExpositionDecember 8–11, 2012Atlanta, GA, USA
- HegdeGVNordgrenTMMungerCMNovel therapy for therapy-resistant mantle cell lymphoma: multipronged approach with targeting of hedgehog signalingInt J Cancer2012131122951296022511234
- HegdeGVMungerCMEmanuelKTargeting of sonic hedgehog-GLI signaling: a potential strategy to improve therapy for mantle cell lymphomaMol Cancer Ther2008761450146018524848
- AmarsaikhanNDennisonJRBoiSKNeilMSElsawaSFGLI transcription factors modulate IgM secretion in waldenstrom macroglobulinemiaBlood201312221
- GreavesWOKimJESinghRRGlioma-associated oncogene homologue 3, a hedgehog transcription factor, is highly expressed in Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphomaHum Pathol201142111643165221531006
- Hager-TheodoridesALDessensJTOutramSVCromptonTThe transcription factor Gli3 regulates differentiation of fetal CD4- CD8-double-negative thymocytesBlood200510641296130415855276
- YangSBDRLohYSBlockade of the hedgehog signaling pathway by the novel agent NVP-LDE225 induces differentiation, prevents de-differentiation and inhibits proliferation of multiple myeloma stem cells in vitroBlood2012120120Presented at: 54th ASH Annual Meeting and ExpositionDecember 8–11, 2012Atlanta, GA, USA
- HoelzerDGokbugetNT-cell lymphoblastic lymphoma and T-cell acute lymphoblastic leukemia: a separate entity?Clin Lymphoma Myeloma20099Suppl 3S214S22119778844
- El AndaloussiAGravesSMengFHedgehog signaling controls thymocyte progenitor homeostasis and differentiation in the thymusNat Immunol20067441842616518394
- SinghRRCho-VegaJHDavuluriYSonic hedgehog signaling pathway is activated in ALK-positive anaplastic large cell lymphomaCancer Res20096962550255819244133
- ShihAHAbdel-WahabOPatelJPLevineRLThe role of mutations in epigenetic regulators in myeloid malignanciesNat Rev Cancer201212959961222898539
- CanettieriGDi MarcotullioLConiSGrecoAGulinoATurning off the switch in medulloblastoma: the inhibitory acetylation of an oncogeneCell Cycle20109112047204820505339
- CanettieriGDi MarcotullioLGrecoAHistone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylationNat Cell Biol201012213214220081843
- TangYGholaminSSchubertSEpigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibitionNat Med201420773274024973920
- KimJLeeJJGardnerDBeachyPAArsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effectorProc Natl Acad Sci U S A201010730134321343720624968
- CooperMKPorterJAYoungKEBeachyPATeratogen-mediated inhibition of target tissue response to Shh signalingScience19982805369160316079616123
- FirestoneAJWeingerJSMaldonadoMSmall-molecule inhibitors of the AAA plus ATPase motor cytoplasmic dyneinNature2012484739212512922425997
- ChenJKTaipaleJCooperMKBeachyPAInhibition of Hedgehog signaling by direct binding of cyclopamine to SmoothenedGenes Dev200216212743274812414725
- LauthMBergstromAShimokawaTToftgardRInhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonistsProc Natl Acad Sci U S A2007104208455846017494766
- HymanJMFirestoneAJHeineVMSmall-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockadeProc Natl Acad Sci U S A200910633141321413719666565
- KimJTangJYGongRItraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growthCancer Cell201017438839920385363
- LeeJWuXPasca di MaglianoMA small-molecule antagonist of the hedgehog signaling pathwayChembiochem20078161916191917886323
- ManettiFFaureHRoudautHVirtual screening-based discovery and mechanistic characterization of the acylthiourea MRT-10 family as smoothened antagonistsMol Pharmacol201078465866520664000
- RohnerASpilkerMELamJLEffective targeting of hedgehog signaling in a medulloblastoma model with PF-5274857, a potent and selective smoothened antagonist that penetrates the blood-brain barrierMol Cancer Ther2012111576522084163
- StantonBZPengLFSmall-molecule modulators of the Sonic Hedgehog signaling pathwayMol Biosyst201061445420024066
- PetrovaERios-EstevesJOuerfelliOGlickmanJFReshMDInhibitors of Hedgehog acyltransferase block Sonic Hedgehog signalingNat Chem Biol20139424724923416332
- ChenJKTaipaleJYoungKEMaitiTBeachyPASmall molecule modulation of Smoothened activityProc Natl Acad Sci U S A20029922140711407612391318
- WangYArvanitesACDavidowLSelective identification of hedgehog pathway antagonists by direct analysis of smoothened ciliary translocationACS Chem Biol2012761040104822554036
- IncardonaJPGaffieldWLangeYCyclopamine inhibition of sonic hedgehog signal transduction is not mediated through effects on cholesterol transportDevelopmental Biology2000224244045210926779
- GendreauSBHawkinsDHoCPreclinical characterization of BMS-833923 (XL139), a hedgehog (HH) pathway inhibitor in early clinical developmentMol Cancer Ther20098B192
- WongHAlickeBWestKAPharmacokinetic-pharmacodynamic analysis of vismodegib in preclinical models of mutational and ligand-dependent Hedgehog pathway activationClin Cancer Res201117144682469221610148
- PelusoMOCampbellVTHarariJAImpact of the smoothened inhibitor, IPI-926, on smoothened ciliary localization and hedgehog pathway activityPloS One201493e9053424608250
- PanSFWuXJiangJQDiscovery of NVP-LDE225, a potent and selective smoothened antagonistACS Med Chem Lett20101313013424900187
- MunchhofMJLiQShavnyaADiscovery of PF-04449913, a potent and orally bioavailable inhibitor of smoothenedACS Med Chem Lett20123210611124900436
- OhashiTOguroYTanakaTDiscovery of the investigational drug TAK-441, a pyrrolo[3,2-c]pyridine derivative, as a highly potent and orally active hedgehog signaling inhibitor: Modification of the core skeleton for improved solubilityBioorg Med Chem201220185507551722898254
- JamiesonCCortesJEOehlerVPhase 1 dose-escalation study of PF-04449913, an oral hedgehog (Hh) inhibitor, in patients with select hematologic malignanciesBlood201111821195196Presented at: 53rd ASH Annual Meeting and ExpositionDecember 10–13, 2011San Diego, CA, USA