Abstract
Maple syrup urine disease (MSUD) is an inborn error of metabolism caused by defects in the branched-chain α-ketoacid dehydrogenase complex, which results in elevations of the branched-chain amino acids (BCAAs) in plasma, α-ketoacids in urine, and production of the pathognomonic disease marker, alloisoleucine. The disorder varies in severity and the clinical spectrum is quite broad with five recognized clinical variants that have no known association with genotype. The classic presentation occurs in the neonatal period with developmental delay, failure to thrive, feeding difficulties, and maple syrup odor in the cerumen and urine, and can lead to irreversible neurological complications, including stereotypical movements, metabolic decompensation, and death if left untreated. Treatment consists of dietary restriction of BCAAs and close metabolic monitoring. Clinical outcomes are generally good in patients where treatment is initiated early. Newborn screening for MSUD is now commonplace in the United States and is included on the Recommended Uniform Screening Panel (RUSP). We review this disorder including its presentation, screening and clinical diagnosis, treatment, and other relevant aspects pertaining to the care of patients.
Introduction
Maple syrup urine disease (MSUD, MIM #248600) is an autosomal recessive disease characterized by disruption of the normal activity of the branched-chain α-ketoacid dehydrogenase (BCKAD) complex, the second step in the catabolic pathway for the branched-chain amino acids (BCAAs) that include leucine, isoleucine, and valine. Pathogenic homozygous or compound heterozygous variants in BCKDHA (MIM #608348), BCKDHB (MIM #248611), DBT (MIM #248610), or DLD (MIM #238331), which form the catalytic subunits of BCKAD, can result in MSUD, which is characterized by neurological and developmental delay, encephalopathy, feeding problems, and a maple syrup odor to the urine. Patients with this disorder have elevations of branch chain ketoacids in the urine in addition to elevated BCAAs in the plasma. MSUD is amenable to treatment through dietary restriction of BCAAs, and with early treatment, patients typically have good clinical outcomes. MSUD is therefore included on the Recommended Uniform Screening Panel (RUSP), a list of actionable, early onset disorders for which screening is recommended for all newborns in the United States. The use of tandem mass spectrometry (MS/MS) in newborn screening (NBS) has helped facilitate early detection of and timely medical intervention for patients with MSUD, thus improving clinical outcomes in affected individuals. In this review, we will discuss the pathophysiology, clinical presentation, screening and diagnosis, as well as treatment of patients with MSUD with a particular focus on the management of adult patients.
Pathophysiology of MSUD
MSUD is a metabolic disorder caused by decreased function of the BCKAD enzyme complex. Biallelic pathogenic variants in the catalytic components of BCKAD decrease its activity thereby increasing BCAA levels and causing toxicity within skeletal muscle and brain tissue.Citation1,Citation2 BCAA catabolism is essential for normal physiological functions.Citation3 The first step involves the conversion of leucine, isoleucine, and valine into their relevant α-ketoacids by branch-chain aminotransferase within the mitochondria (). Unlike most other amino acid metabolism, the majority of this process does not take place in the liver but rather in skeletal muscle.Citation3–Citation5 BCAAs can be found in protein-rich diets and are among the nine amino acids essential for human life, playing important roles in protein synthesis and function, cellular signaling, and glucose metabolism.Citation6,Citation7
Figure 1 Overview of BCAA catabolic pathway. The BCAAs undergo transamination that is catalyzed by the branched-chain aminotransferase (BCAT) and requires α- ketoglutarate, leading to the production of the α-ketoacids KIC, KMV, and KIV. These intermediates then undergo oxidative decarboxylation, catalyzed by the BCKAD complex.
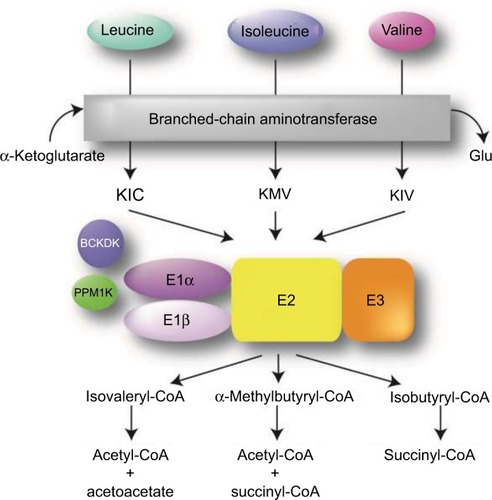
During the second step in BCAA catabolism, the BCKAD complex initiates oxidative decarboxylation of α-ketoacids.Citation4 This process results in the conversion of α-ketoacids into acetoacetate, acetyl-CoA, and succinyl-CoA as illustrated in . The BCKAD complex is made up of several components, including subunits E1α and E1β, E2, and E3. Increased BCAA levels within the body due to pathogenic defects in these components cause MSUD, leading to a variety of symptoms mentioned above, including dysfunction of the immune system, skeletal muscle, and central nervous system (CNS).Citation2–Citation4 The generation of various mouse models has been used to study MSUD and the effects of dietary BCAA restriction.Citation8,Citation9 In these models, as well as MSUD patients, excessive amounts of BCAAs build up and can cause severe tissue damage if left untreated.Citation10 Within the brain, BCAA metabolism functions to maintain glutamate levels.Citation2,Citation11,Citation12 Glutamate serves as a neurotransmitter within the CNS and plays important roles in brain development and cognitive functions such as learning and memory. Disorders of BCAA metabolism can cause abnormalities in glutamate synthesis leading to various neurological problems in patients.Citation13 Controlling plasma concentrations of BCAA levels is key to preventing these symptoms. Furthermore, the accumulation of leucine is highly neurotoxic.Citation2 Elevated levels of leucine can affect water homeostasis within the subcortical gray matter causing swelling within the brain, alter nitrogen homeostasis further depleting glutamate levels, increase oxidative stress, and compete with other important amino acids within the CNS such as tyrosine, which is involved in protein signaling. In addition, there is evidence that α-ketoisocaproic acid, an intermediate in the metabolism of leucine, is a major neuro-toxin contributing to the encephalopathic syndrome.
Although the specific outcomes of BCAA abnormalities have not been fully characterized, it is apparent that proper metabolism is critical for human health.Citation4 While genetic variants in certain components of this pathway lead to MSUD, several other disorders have been associated with abnormal BCAA metabolism, including insulin resistance and type 2 diabetes mellitus, liver disease, and certain types of cancer, broadening its respective roles in human health.Citation3,Citation4
Clinical presentation of MSUD
Clinical presentation
There are five distinct clinical phenotypes of MSUD, with no good genotype–phenotype correlation. However, the MSUD forms can be categorized based on age at onset, severity of symptoms, response to thiamine supplementation, and biochemical findings (). Classic and E3-deficient MSUD typically present in the neonatal period, while the intermediate, intermittent, and thiamine-responsive forms may present at any time of life, with decompensations occurring during periods of illness or stress. The clinical presentation of MSUD depends on the BCKAD residual activity, although the phenotypic classification relies on the leucine tolerance and metabolic response to illness.
Table 1 MSUD genetics, clinical phenotype, and biochemical features
Individuals with the classic neonatal form have <2% of BCKAD enzymatic activity and present with maple syrup odor in cerumen shortly after birth and in urine during the first week of life. If untreated, the neonate may develop irritability, lethargy, poor feeding, apnea, opisthotonus, “bicycling” movements, followed by coma and early death due to brain edema.Citation14,Citation15 In older individuals, increased levels of leucine (leucinosis) causes epigastric pain, anorexia, vomiting, muscle fatigue, altered level of consciousness, psychiatric symptoms, movement disorders, and ataxia.Citation16 Furthermore, clinical features similar to Wernicke encephalopathy have been reported in patients with acute decompensation.Citation17 During periods of catabolism of endogenous protein, such as fever, infections, exercise, trauma, or surgery, individuals with MSUD can develop neurological deterioration due to acute leucine intoxication.Citation18
The intermediate form of MSUD is characterized by up to 30% of BCKAD residual activity. These individuals may appear healthy during the neonatal period, although maple syrup odor in cerumen may be present. During the first years of life, they may experience feeding problems, poor growth, and intellectual disability, and are susceptible to similar neurologic features as individuals with the classic form.Citation19
In a third form, individuals with intermittent MSUD are completely asymptomatic, having normal growth and neurological development, even on an unrestricted diet. During catabolic states, clinical and biochemical features of the classic form may arise in patients, and should be controlled similarly to individuals with the severe form.
Thiamine-responsive MSUD is a rare phenotype associated with pathogenic variants in DBT that encodes the BCKAD E2 subunit. Affected individuals present with symptoms similar to the intermediate form and usually require a combination of BCAA dietary restriction and thiamine supplementation. However, there has been one case of full responsiveness to thiamine without dietary restriction.Citation20
Dihydrolipoamide dehydrogenase acts as the E3 subunit for three mitochondrial enzyme complexes: branched chain alpha-ketoacid dehydrogenase (BCKAD) complex, α-ketoglutarate dehydrogenase (αKGDH) complex, and pyruvate dehydrogenase (PDH) complex, so individuals with the E3-deficient MSUD form may present with a wide phenotypic spectrum, ranging from early-onset neurologic manifestations to adult-onset isolated liver disease. The most frequent is the severe form characterized by metabolic acidosis, encephalopathy, feeding difficulties, liver failure, and early death. Besides the biochemical hallmarks of MSUD, patients have increased levels of lactate, alanine, and α-ketoglutarate, which are related to mitochondrial dysfunction.Citation21,Citation22 Individuals with hepatic presentation may present with signs and symptoms at any age, and experience recurring episodes of hepatopathy that decrease with age and are often triggered by hypercatabolic states.Citation23 The different clinical variants of MSUD are summarized in .
Medical management
NBS
Historically, MSUD has had a worldwide incidence, occurring in one in 185,000 live births,Citation19 but is much more frequent in certain founder populations including the Old Order Men-nonites of Pennsylvania, where it may be observed in as many as one in every 200 live births.Citation24 Since MSUD is amenable to treatment through dietary restriction of BCAAs (or treatment with thiamine in the responsive forms) and outcomes are generally very good if it is detected and treated within the first few days of life, routine NBS for the disorder is recommended. MSUD screening is currently incorporated in NBS programs throughout the United States, five Cana-dian provinces, 22 European countries, two Latin American countries (Costa Rica and Uruguay), and eight countries in the Asia Pacific region.Citation25
NBS for MSUD has been performed since 1964 when the bacterial inhibition assay for leucine on dried blood spots (Guthrie specimen) was introduced.Citation26 Today, NBS for inborn errors of BCAA metabolism typically involves quantitative plasma amino acid profiling by MS/MS ().Citation27,Citation28 If elevations of BCAAs are detected, the laboratory may perform additional studies including urine organic acid analysis by gas chromatography (GC)-MS/MS, dinitrophenylhydrazine (DNPH) test, quantitative plasma amino acids by liquid chromatography (LC)-MS/MS, and confirmatory molecular testing in suspected cases. Diagnosis of MSUD relies upon the presence of clinical, molecular, and biochemical features, including elevations of BCAAs and alloisoleucine in plasma as well as the presence of branched -chain α-hydroxyacids and branched -chain α-ketoacids (BCKAs) in urine. MS/MS testing for MSUD examines the leucine–isoleucine concentration plus its ratio with other amino acids including alanine, glutamate, glutamine, tryptophan, methionine, histidine, phenylalanine, and tyrosine.Citation15,Citation29 MS/MS cannot distinguish isobaric amino acids (ions with the same mass) including leucine, isoleucine, alloisoleucine, and hydroxyproline and MS/MS-positive cases may require second-tier testing such as LC-MS/MS ().Citation30
Figure 2 Overview of MSUD testing algorithm in NBS
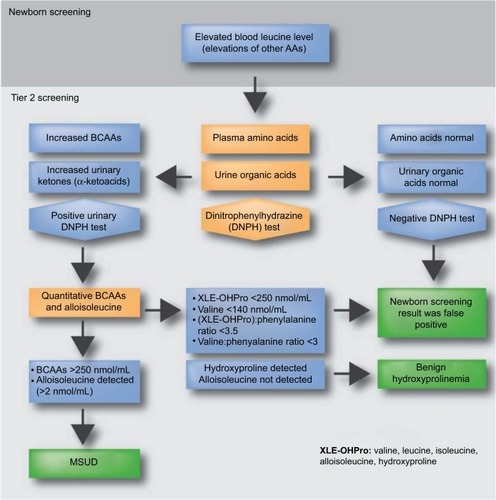
The milder variant forms of MSUD are often missed by NBS due to normal leucine levels in the newborn period.Citation31 Individuals with mild forms may have greater residual enzyme activities and they typically appear after the neonatal period, usually before the second year of life.Citation19 All classic MSUD patients have pathognomonic elevations of alloisoleucine; however, even second-tier testing can miss variant cases, which may only be detectable during acute illness or metabolic crises.Citation31 Second-tier testing can also distinguish MSUD from benign hydroxyprolinemia (4-hydroxy-l-proline oxidase deficiency, MIM #237000), which has no known clinical phenotype aside from elevated hydroxyproline. Since newborns with benign hydroxyprolinemia test positive for MSUD, they represent a false-positive result on NBS.
Biochemical workup
MSUD in a newborn may be suspected due to the presence of illness and/or an abnormal neonatal screening test result. The maple syrup-like odor characteristic of MSUD is usually present in the cerumen and may be detected as early as 12 hours after birth. Expanded NBS by MS/MS can show elevations of BCAAs on dried blood samples, but is unable to distinguish the isobaric amino acids as described above. In MSUD patients, plasma leucine, isoleucine, and valine are usually elevated, but may range from normal to slightly reduced. Elevations of BCAAs may be accompanied by decreased concentrations of other amino acids, leading to elevated concentration ratios ().Citation15,Citation29 Subsequent quantitative plasma amino acid profiling that includes alloisoleucine detection provides the strongest evidence supporting a diagnosis of MSUD. Urine organic acid analysis by GC-MS/MS to detect BCKA (including α-ketoisocaproate, α-keto-β-methylisovalerate, α-ketoisovalerate) also provides supporting evidence for a diagnosis of MSUD. Elevations of BCKAs can be detected 48–72 hours after birth and correspond with massive elevations of BCAAs. The DNPH test, a nonquantitative screening test, can be performed in lieu of urine organic acid testing and detects α-ketoacids in urine. Ketonuria can serve as a surrogate marker suggestive of underlying metabolic instability and can be detected using urine test strips, particularly in resource poor settings that do not have access to the DNPH test or other analytic methods.
Monitoring in adult patients
The DNPH test reagent can also be used in the outpatient setting for detection of urine BCKAs during episodes of metabolic decompensation. If detected early, many MSUD patients can be treated at home by dietary restriction of BCAAs (leucine in particular) and the administration of high-calorie “sick day” formulas accompanied by frequent follow-up testing. Treatment details are outlined in the subsequent sections.
Molecular genetics of MSUD
Previous genetic studies have determined that MSUD is an autosomal recessive disease caused by pathogenic variants in genes encoding the E1α, E1β, E2, and E3 components of BCKAD. In 1989, the first genetic variants linked to MSUD were discovered in the E1α subunit (BCKDHA) of the BCKAD complex.Citation32 Analysis of BCKDH activity in cultured fibroblasts showed that both the father and mother had levels that were 50% of the normal, while the patient’s levels were about 5% of normal.Citation32 DNA sequencing then confirmed that each parent was a carrier for different pathogenic variants in BCKDHA and that the affected proband was compound heterozygous.Citation32
Since then, over 190 different pathogenic or likely pathogenic variants have been identified in E1α and the other BCKAD components including E1β (BCKDHB), E2 (DBT), and E3 (DLD). All pathogenic variants that have been identified are homozygous or compound heterozygous variants within the same gene.Citation33–Citation35 Genetic testing is essential for a clinical diagnosis of MSUD and to determine which subunit is deficient, which may be helpful in the future for determining individualized therapies.Citation36,Citation37
Genetic counseling
Since MSUD is inherited in an autosomal recessive pattern, both parents of an individual with MSUD are most often unaffected carriers of the condition. A carrier for MSUD has one pathogenic variant in one of the previously described genes. When two carrier parents with mutations in the same gene reproduce, there is a 25% chance that any child will have MSUD. There is a 50% chance that any offspring will be a carrier and a 25% chance that they will be neither a carrier nor affected. There are many reproductive options available for couples who are both carriers of the same MSUD gene. Parents can choose to pursue natural conception with no genetic testing, consider adoption, or sperm or egg donation. Additionally, when carrier status has been molecularly confirmed, parents can also consider prenatal testing through chorionic villus sampling or amniocentesis or preimplantation genetic diagnosis with in vitro fertilization.
Special attention and care should be provided to young adults with MSUD who are beginning to take on more responsibilities regarding their health.Citation38 The new pressure of managing MSUD without assistance from a parent is added to what can already be a challenging time. Young adults with MSUD may feel isolated from peers based on their diagnosis and may struggle with how to discuss the diagnosis in new relationships. Typical activities, like going out to a restaurant with friends, can be complicated by dietary restrictions. It can also be uncomfortable for some young adults to continue to receive care through a facility for children. Young adults may face more concrete barriers, like difficulty obtaining various insurance coverages as well. Not all individuals will have the same experiences, but incorporation of a mental health professional into the care of these adolescents may help with the transitional period.Citation39
Medical nutrition management
Basics of management
One of the primary goals in treating MSUD is to manage diet by reducing BCAAs and provide adequate macronutrients to prevent catabolism and help maintain plasma BCAAs within targeted treatment ranges. Initiation of dietary management usually begins in the newborn period following a positive NBS result and clinical confirmation. Amounts of BCAAs are titrated in the diet by close monitoring of biochemical lab values and growth throughout infancy, childhood, and into adulthood ().Citation40 Long-term treatment requires careful manipulation of calories, restriction of dietary BCAAs, and supplementation using a BCAA-free amino acid mixture ( shows a list of available products) to provide non-BCAAs and other nutrients for protein synthesis.Citation41
Table 2 Recommended daily nutrient intake of BCAA, PRO, energy, and fluids for non-symptomatic individuals with MSUD
Table 3 Selected medical foods
Nutrition management consists of BCAA-free medical food specially formulated to provide 80%–90% of protein needs, and a majority of energy and micronutrient necessities throughout life.Citation42 Leucine requirements are usually achieved using breast milk or infant formula. Leucine from breast milk or infant formula is replaced by solid foods when the infant is developmentally ready. The dietary protein restriction is guided by leucine requirements. Valine and isoleucine need to be supplemented, as the content of these tend to be lower than leucine in medical foods. Micronutrient intake should be monitored and supplemented as needed.Citation42
Acute management
Individuals with MSUD are at risk for metabolic decompensation. Families and individuals should be equipped with an emergency protocol provided by the managing physician, such as a biochemical geneticist, for both the family and medical professionals caring for the patient. The goal in acute management is to suppress catabolism and promote protein anabolism. Patients must have a “sick day” prescription in order to manage illness at home, although this practice is not universal. Sick day protocol usually entails guidelines on increasing BCAA-free amino acid formula intake to 120% of the usual intake, decreasing leucine intake by 50%–100%, and providing small but frequent feedings throughout a 24 hour period. Monitoring BCAA plasma concentrations is necessary to guide appropriate diet adjustments during the illness.Citation42 The sick day protocol is usually initiated upon the first signs of an illness and for minor illnesses can be managed at home. In serious cases, more aggressive approaches should be taken (e.g., dialysis, hemofiltration, parenteral nutrition, and/or tube feedings).Citation40 Acute dietary treatment needs to be aggressive and include sufficient energy (up to 150% of the normal energy consumption), based on BCAA-free formula and fluid administration (up to 150 mL/kg).Citation40 In cases where gastrointestinal delivery is not tolerated, BCAA-free formulations exist (Coram Specialty Infusion Services).Citation42
Special considerations for management in adults
Acute metabolic management in adults
Adult patients in metabolic crisis can be difficult to manage. Typically, most expertise for management of inborn errors of metabolism rests in academic pediatric metabolic centers. Furthermore, most biochemical geneticists are trained in pediatrics and lack experience in treating adult patients. Finally, the treating physicians in intensive care and the hospital floors may not be familiar with the condition or how to manage it. All of these factors create a very challenging situation and underline the need for extreme vigilance in monitoring these patients.
Management strategies in adults are similar to those in children in that the primary goals are to 1) stop protein intake for 24–72 hours, 2) provide hydration and calorific support, 3) correct any metabolic abnormalities, 4) eliminate toxic metabolites, 5) address the underlying cause of the metabolic crisis, 6) consider cofactor supplementation, and 7) minimize associated clinical sequelae.
Once the patient shows clinical improvement, protein can be gradually reintroduced back in the range of 25%–50% of normal intake initially using total parenteral nutrition and then increased to 100% orally over the course of 3–5 days depending on the patient’s clinical course.
Calorific support is crucial to supporting protein anabolism. In the pediatric setting, the ratio of body weight/body surface is used to guide fluid infusion rate. This is not common practice in the adult setting and may well lead to confusion among treating physicians if these guidelines are given. Typical maintenance fluid rates for adults are 2–3 L of fluid per day. The 24 hour rates of 4.5 L per day would equal around 1.5 times maintenance fluid rates and represent a high fluid infusion rate of 187.5 mL/hour. We have seen successful treatment with infusion rates of 150–200 mL/hour of fluid for adult patients which should be titrated depending on the body size of the individual. Ideally, fluids should be given as 10%–12.5% dextrose. Most hospitals do not have high concentration dextrose infusions readily available. IV insulin should be considered to promote anabolism and should also be used to control the increase in glucose levels after the use of IV dextrose. A common error in the management of acute decompensated patients with MSUD is that treating physicians reduce infusion rates of IV dextrose if the blood sugar is significantly elevated, for example above 150–200 mg/dl. In this scenario, rather than decreasing the fluid rate, the rate of insulin infusion should be increased.
Metabolic abnormalities should be corrected as needed. Intervenous sodium bicarbonate should be considered when blood acidosis is below pH 7.2 or serum bicarbonate <14 Meq/L. Plasma sodium should be monitored closely, due to hyponatremia enhancing the risk of brain edema, and kept in the range of 140–145 Meq/L. Frequent monitoring of amino acids is essential and ideally daily amino acids should be obtained to guide management. Often, this is not possible due to the availability of a biochemical genetics lab or long turnaround time, and therefore, it is important to reintroduce protein after a maximum of 72 hours to guard against catabolism.
If symptoms are very severe and the patient is comatose with associated abnormalities such as refractory acidosis or electrolyte abnormalities, hemodialysis should be considered. This has been performed successfully in pediatric populations and detailed information on the dialysis technique has been described previously.Citation43
Normally, there is a precipitating metabolic stressor that causes acute decompensation in adults and children. The most common causes are infection, surgery, injury, or significant dietary change. These should be treated as appropriate – infection should be treated with antibiotics; before surgical procedures, the patient should receive IV fluid with 10% dextrose which should be continued during and after surgery; and dietary changes should be addressed promptly by the treating metabolic team.
Pregnancy and lactation
Unlike other inborn errors of metabolism, there are insufficient data concerning the potential harmful effects of elevated BCAAs on the developing fetus.Citation44 Careful attention and planning is important in managing pregnant patients with MSUD. During pregnancy, the mother must maintain plasma BCAA levels while increasing protein to support maternal changes and fetal health.Citation40 It is reported that there are increases in leucine tolerance during pregnancy and lactation.Citation44,Citation45 Calorie and protein needs are calculated based on the requirements for pregnancy. Supplemental vitamins and minerals may be necessary to meet pregnancy needs that are not met through medical foods. Wessel et al published a successful case management by providing adequate energy and BCAA-free protein during pregnancy, delivery, and the postpartum period.Citation45 The use of BCAA-free parenteral nutrition can be obtained through Coram Specialty Infusion Services during pre- and postpartum emergencies. This treatment will provide adequate energy and BCAA-free protein preventing elevated plasma leucine. Successful lactation in the same woman with MSUD was also reported. Management of pregnancy in an MSUD patient requires thorough observations between obstetrics and laboratory nutrition services. Careful planning and monitoring, such as reduced fasting time, is necessary for optimal outcomes during any surgical procedure.Citation42
Treatment options
Liver transplantation
Orthotopic liver transplantation in pediatric patients with classic MSUD has been a very successful treatment. In a study of 52 individuals with classic MSUD who underwent liver transplant, all were able to achieve an unrestricted diet posttransplant.Citation46 Interestingly, the risks associated with surgery and lifelong immunosuppression were similar to other pediatric liver transplant populations, with no reversal of cognitive deficit or progression of neurocognitive impairment.Citation47,Citation48 To date, there are no major studies looking at liver transplantation in an adult population. While orthotopic liver transplant is not a universal recommendation for classic MSUD, studies have shown it to be an effective therapy and should be considered in difficult to manage patients.
The most frequent transplant approach is to use liver from unrelated deceased individuals, but when the availability of deceased donor tissue is limited, living related donor liver can be used. It is important to point out that neither deceased unrelated or living related livers can restore the enzyme activity to normal levels, so acute metabolic intoxication can still occur posttransplantation.Citation49 In general, weight-adjusted leucine tolerance is more favorable in young patients. One hypothesis for this finding is that adolescent and adult recipients tend to consume more protein and typically have less control over their daily amino acid intake. Another reason could be related to the decrease in dietary leucine tolerance from childhood (when the need matches the demand for normal growth) to adulthood.Citation50
Sodium phenylbutyrate
Sodium phenylbutyrate (NaPBA), a nitrogen scavenging medication, is commonly used for the treatment of patients with urea cycle disorders (UCDs). It has been noted in these patients that NaPBA lowers BCAA amino acid levels. Bur-rage et al examined a cohort of 533 patients with UCDs, confirmed this BCAA reduction, and suggested follow-up studies to determine whether it could be a therapeutic option for MSUD.Citation51 There is no clinical role for NaPBA at present; however, ongoing studies are assessing its efficacy.
Summary
MSUD is an organic acidemia with a varying presentation and clinical course. Dietary management is key to successful long-term treatment. As treatment has improved, a new subfield of adult management is emerging which presents new challenges. Finally, new treatments are needed to better manage difficult cases and timely clinical diagnosis remains a challenge in nonclassical patients.
Acknowledgments
The authors would like to thank the Mayo Clinic Center for Individualized Medicine for their support.
Disclosure
The authors report no conflicts of interest in this work.
References
- LangCHLynchCJVaryTCBCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic miceAm J Physiol Regul Integr Comp Physiol20102993R935R94420554928
- YudkoffMDaikhinYNissimIHorynOLuhovyyBLazarowABrain amino acid requirements and toxicity: the example of leucineJ Nutr20051356 Suppl1531S1538S15930465
- LynchCJAdamsSHBranched-chain amino acids in metabolic signalling and insulin resistanceNat Rev Endocrinol2014101272373625287287
- BurrageLCNagamaniSCCampeauPMLeeBHBranched-chain amino acid metabolism: from rare Mendelian diseases to more common disordersHum Mol Genet201423R1R1R824651065
- WahrenJFeligPHagenfeldtLEffect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitusJ Clin Invest1976574987999947963
- BrosnanJTBrosnanMEBranched-chain amino acids: enzyme and substrate regulationJ Nutr20061361 Suppl207S211S16365084
- HarperAEMillerRHBlockKPBranched-chain amino acid metabolismAnnu Rev Nutr198444094546380539
- JoshiMAJeoungNHObayashiMImpaired growth and neurological abnormalities in branched-chain alpha-keto acid dehydrogenase kinase-deficient miceBiochem J2006400115316216875466
- VogelKRArningEWasekBLMcPhersonSBottiglieriTGibsonKMBrain-blood amino acid correlates following protein restriction in murine maple syrup urine diseaseOrphanet J Rare Dis201497324886632
- ZinnantiWJLazovicJInterrupting the mechanisms of brain injury in a model of maple syrup urine disease encephalopathyJ Inherit Metab Dis2012351717921541722
- YudkoffMDaikhinYGrunsteinLNissimISternJPleasureDAstrocyte leucine metabolism: significance of branched-chain amino acid transaminationJ Neurochem19966613783858522978
- YudkoffMDaikhinYLinZPNissimISternJPleasureDInterrelationships of leucine and glutamate metabolism in cultured astrocytesJ Neurochem1994623119212027906717
- ScainiGTononTde SouzaCFSerum markers of neurodegeneration in maple syrup urine diseaseMol Neurobiol Epub2016922
- LevinMLScheimannALewisRABeaudetALCerebral edema in maple syrup urine diseaseJ Pediatr199312211671688419609
- StraussKAWardleyBRobinsonDClassical maple syrup urine disease and brain development: principles of management and formula designMol Genet Metab201099433334520061171
- CarecchioMSchneiderSAChanHMovement disorders in adult surviving patients with maple syrup urine diseaseMov Disord20112671324132821484869
- ManaraRDel RizzoMBurlinaAPWernicke-like encephalopathy during classic maple syrup urine disease decompensationJ Inherit Metab Dis201235341341722350544
- ThompsonGNFrancisDEHallidayDAcute illness in maple syrup urine disease: dynamics of protein metabolism and implications for managementJ Pediatr19911191 Pt 135412066856
- ChuangDTShihVEMaple syrup urine disease (branched-chain ketoaciduria)ScriverCRBeaudetASlyWSValleDThe Metabolic and Molecular Bases of Inherited DiseaseNew York, NYMcGraw-Hill200119712006
- ScriverCRMackenzieSClowCLDelvinEThiamine-responsive maple-syrup-urine diseaseLancet1971176943103124100151
- MunnichASaudubrayJMTaylorJCongenital lactic acidosis, alpha-ketoglutaric aciduria and variant form of maple syrup urine disease due to a single enzyme defect: dihydrolipoyl dehydrogenase deficiencyActa Paediatr Scand19827111671716897145
- QuinonezSCLeberSMMartinDMThoeneJGBedoyanJKLeigh syndrome in a girl with a novel DLD mutation causing E3 deficiencyPediatr Neurol2013481677223290025
- BrassierAOttolenghiCBoutronADihydrolipoamide dehydrogenase deficiency: a still overlooked cause of recurrent acute liver failure and Reye-like syndromeMol Genet Metab20131091283223478190
- MortonDHStraussKARobinsonDLPuffenbergerEGKelleyRIDiagnosis and treatment of maple syrup disease: a study of 36 patientsPediatrics20021096999100812042535
- TherrellBLPadillaCDLoeberJGCurrent status of newborn screening worldwide: 2015Semin Perinatol201539317118725979780
- NaylorEWGuthrieRNewborn screening for maple syrup urine disease (branched-chain ketoaciduria)Pediatrics1978612262266416414
- ChaceDHHillmanSLMillingtonDSKahlerSGRoeCRNaylorEWRapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometryClin Chem199541162687813082
- ChaceDHKalasTANaylorEWUse of tandem mass spectrometry for multianalyte screening of dried blood specimens from newbornsClin Chem200349111797181714578311
- StraussKAMortonDHBranched-chain ketoacyl dehydrogenase deficiency: maple syrup diseaseCurr Treat Options Neurol20035432934112791200
- OglesbeeDSandersKALaceyJMSecond-tier test for quantification of alloisoleucine and branched-chain amino acids in dried blood spots to improve newborn screening for maple syrup urine disease (MSUD)Clin Chem200854354254918178665
- PuckettRLLoreyFRinaldoPMaple syrup urine disease: further evidence that newborn screening may fail to identify variant formsMol Genet Metab2010100213614220307994
- ZhangBKuntzMJGoodwinGWEdenbergHJCrabbDWHarrisRAcDNA cloning of the E1 alpha subunit of the branched-chain alpha-keto acid dehydrogenase and elucidation of a molecular basis for maple syrup urine diseaseAnn N Y Acad Sci19895731301362634344
- ImtiazFAl-MostafaAAllamRTwenty novel mutations in BCKDHA, BCKDHB and DBT genes in a cohort of 52 Saudi Arabian patients with maple syrup urine diseaseMol Genet Metab Rep201711172328417071
- AbiriMKaramzadehRMojbafanMIn silico analysis of novel mutations in maple syrup urine disease patients from IranMetab Brain Dis201732110511327507644
- LiXDingYLiuYEleven novel mutations of the BCKDHA, BCKDHB and DBT genes associated with maple syrup urine disease in the Chinese population: report on eight casesEur J Med Genet2015581161762326453840
- CouceMLRamosFBuenoMAEvolution of maple syrup urine disease in patients diagnosed by newborn screening versus late diagnosisEur J Paediatr Neurol201519665265926232051
- McCabeLLMcCabeERGenetic screening: carriers and affected individualsAnnu Rev Genomics Hum Genet20045576915485343
- PackmanWMehtaIRafieSYoung adults with MSUD and their transition to adulthood: psychosocial issuesJ Genet Couns2012215692703
- EnnsGMPackmanWThe adolescent with an inborn error of metabolism: medical issues and transition to adulthoodAdolesc Med2002132315329
- FrazierDMAllgeierCHomerCNutrition management guideline for maple syrup urine disease: an evidence- and consensus-based approachMol Genet Metab2014112321021724881969
- ServaisAArnouxJBLamyCTreatment of acute decompensation of maple syrup urine disease in adult patients with a new parenteral amino-acid mixtureJ Inherit Metab Dis201336693994423250513
- van CalcarSNutrition management of maple syrup urine diseaseBernsteinLERohrFHelmJRNutrition Management of Inherited Metabolic DiseasesSwitzerlandSpringer International Publishing2015173183
- AtwalPSMacmurdoCGrimmPCHaemodialysis is an effective treatment in acute metabolic decompensation of maple syrup urine diseaseMol Genet Metab Rep20154464826937409
- MarriageBJNutrition management of patients with inherited disorders of branched-chain amino acid metabolismAcostaPNutrition Management of Patients with Inherited Metabolic DisordersSudbury, MAJones and Bartlett Publishers2010175236
- WesselAEMogensenKMRohrFManagement of a woman with maple syrup urine disease during pregnancy, delivery, and lactationJPEN J Parenter Enteral Nutr201539787587924618664
- StraussKAMazariegosGVSindhiRElective liver transplantation for the treatment of classical maple syrup urine diseaseAm J Transplant20066355756416468966
- MuellyERMooreGJBunceSCBiochemical correlates of neuropsychiatric illness in maple syrup urine diseaseJ Clin Invest201312341809182023478409
- MazariegosGVMortonDHSindhiRLiver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United Network for Organ Sharing experienceJ Pediatr2012160111612121839471
- FeierFSchwartzIVBenkertARLiving related versus deceased donor liver transplantation for maple syrup urine diseaseMol Genet Metab2016117333634326786177
- StraussKAWardleyBRobinsonDClassical maple syrup urine disease and brain development: Principles of management and formula designMol Genet Metab201099433334520061171
- BurrageLCJainMGandolfoLLeeBHNagamaniSCSodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disordersMol Genet Metab20141131–213113525042691
