Abstract
Background and Aim
Genetic factors are vital participants in the development and progression of myocardial infarction (MI). Adiponectin has been assumed to have a protective role in MI and adiponectin receptors variants could be a determinant for atherosclerosis. We aimed to evaluate the prevalence of ADIPOQ (rs2241766) and ADIPOR2 (rs10773989) polymorphisms and their association with mRNA levels and circulatory adiponectin levels in patients with MI.
Subjects and Methods
A total of 220 participants were classified into two groups: group 1 included 120 patients with MI, and group 2 involved 100 healthy participants as controls. Genotyping of ADIPOQ (rs2241766) and ADIPOR2 (rs10773989) polymorphisms were analyzed using an allele discrimination assay with real-time PCR and their relative expression or mRNA levels were determined by real-time PCR. Serum adiponectin level was determined using an ELISA technique.
Results
The ADIPOQ rs2241766 GG genotype and G allele and the CC genotype and C allele of ADIPOR2 rs10773989 were significantly prevalent in patients with MI and associated with increased risk of MI. We detected a marked reduction in serum adiponectin, ADIPOQ and ADIPOR2 mRNA levels in patients than control. The GG genotype of ADIPOQ rs2241766 and the CC genotype of ADIPOR2 rs10773989 had the lowest levels of their mRNA and adiponectin level in both patients and controls.
Conclusion
Adiponectin gene and receptor variants are potentially related to MI risk; furthermore, their expressions were markedly depressed in MI which suggests their use as potential biomarkers for MI.
Introduction
Coronary artery disease (CAD) has been considered a fundamental cause of death and morbidity worldwide. It might progress to myocardial infarction (MI) as a result of prolonged ischemia of the coronary artery.Citation1 MI is a multifactorial disease, evolving from the interaction of various genetic variations and environmental influences.Citation2 Different analyses have recognized various hazardous factors for MI such as gender, smoking and family history.Citation3,Citation4 Additionally diabetes mellitus (DM), hypertension and obesity are well-known risk factors for MI.Citation5 Moreover, genetic factors are reported as vital participants in the development and progression of MI.Citation6
The ADIPOQ gene on the long arm of chromosome 3q27 encodes adiponectin and comprises three exons and two introns.Citation7 Adiponectin (ADIPOQ), a 30 kDa protein that originates mainly from adipose tissues, plays a vital role in glucose metabolism and enhances sensitivity to insulin. It is also reported to have antidiabetic, anti-atherogenic and anti-inflammatory actions.Citation8 ADIPOQ has a significant role in the regulation of endothelial function and inhibition of angiogenesis.Citation9 Additionally, it represses the generation of tissue necrosis factor α (TNF-α) and inhibits propagation of vascular smooth muscle.Citation10 A decreased serum level of ADIPOQ was detected in patients with CAD and found to have a prognostic significance for MI.Citation11
The activity of ADIPOQ is moderated through two primary receptors ADIPOQ receptors: 1 and 2 (ADIPOR1, 2).Citation9 Human ADIPOQ receptors are expressed mainly in adipose tissue and found to be expressed in muscle cells as well.Citation12 Disturbance of these receptors reduces the attachment of adiponectin, thus inhibiting actions with subsequent elevation of tissue triglyceride and inflammation.Citation13 The level of ADIPOR2 protein in CAD patients’ subgroups was associated with adiponectin level.Citation14 Additionally, ADIPOR2 expression level in visceral tissues in contrast to ADIPOR1 was correlated with circulatory adiponectin concentrations.Citation13 Various analyses have investigated the correlation of ADIPOQ gene with MI.Citation15,Citation16 In this study we aimed to evaluate the prevalence of ADIPOQ (rs2241766) and ADIPOR2 (rs10773989) polymorphisms and their relation to gene expression or mRNA levels and circulatory adiponectin levels in patient with MI.
Subjects and Methods
This study was carried out by co-operation between the Medical Biochemistry and Molecular Biology and Cardiology Departments, Faculty of Medicine, Menoufia University. A total of 220 subjects were included in the study, they were categorized into two groups: Group 1 included 120 patients with confirmed diagnosis of MI. All patients were recruited from the Coronary Care Unit (CCU), Menoufia University Hospitals during the period from November 2019 to August 2020. Diagnosis of MI was based on full history taking (symptoms suggestive of myocardial ischemia: anginal pain), electrocardiogram (ECG) evidence of myocardial infarction (ST segment elevation in at least two contiguous leads) and cardiac biomarker (troponin) elevation.
Group 2 included 100 healthy subjects of matched age and sex that were carefully chosen as control subjects. They were neither hypertensive nor diabetic and they did not have any systemic illness by careful examination and investigations, without any personal or family history of CAD.
The exclusion criteria for our patients' group included patients with diabetes, heart failure, congenital heart diseases and patients with accompanied liver or renal diseases or other systemic illness.
The analysis was completed as per the Helsinki Declaration. All participants gave written informed consent, and the research protocol was assigned from the Ethics Committee of the Faculty of Medicine, Menoufia University.
Methods
Samples were collected after 12 hours of fasting and within the first 24 hours after MI, 10 milliliters (mL) of venous blood were taken from all contributors and processed as follow: 3 mL were put in a tube containing EDTA for DNA extraction for further assessment of adiponectin Q (ADIPOQ) rs2241766 and adiponectin receptor 2 (ADIPOR2) rs10773989 genetic polymorphisms. Another 2 mL were put in another EDTA containing tube for RNA extraction for processing adiponectin gene and its receptor expression. The remaining 5 mL were put in a plain tube, where they were centrifuged at 4000 rpm for 10 minutes for serum isolation. The serum was kept and stored at −80ºC til the time of genetic assessment. Laboratory investigations were done for all participants in this study including standard colorimetric procedures using kits supplied by the Spinreact (Spain) for measuring serum total cholesterol (TC) and triacylglycerol (TG) values and human kit (Germany) for measuring serum high density lipoprotein cholesterol (HDLc) levels. An approved equation (LDLc) = TC – (TG/5 +HDLc), as TG levels did not exceed 400 mg/dL, was used for calculation of low-density lipoprotein cholesterol (LDLc) values. Quantitative determination of serum human total adiponectin was done by enzyme linked immunosorbent assay (ELISA) manufactured by (R&D systems, Inc, USA).
Assessment of ADIPOQ rs2241766 and ADIPOR2 rs10773989 genetic variants were carried out by real time PCR. Adiponectin gene and adiponectin receptor expressions were done by quantitative real time PCR.
Genotyping Assay
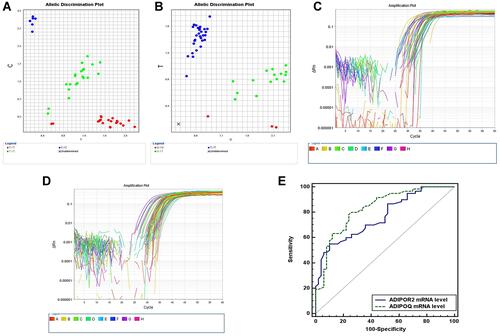
DNA was purified from whole blood with Qiagen DNA extraction kit (Hilden, Germany) following the manufacturer's protocol. Genotyping of ADIPOQ rs2241766 and ADIPOR2 rs10773989 were completed by allelic discrimination assay utilizing TaqMan probes (Applied Biosystems, USA). An overall mixture of 20 μL was conducted by applying 5 μL of sample DNA to a mixture of 10 μL of genotyping master mix, 1.25 μL of the primer/probe assay and 3.75 μL of DNAase-free water. The TaqMan probes were labelled with VIC and FAM fluorescent dyes. The probe sequence for ADIPOQ rs2241766 was TTCTACTGCTATTAGCTCTGCCCGG[T/G]CATGACCAGGAAACCACGACTCAAG. The probe sequence for ADIPOR2 rs10773989 was AATCTTACTGGGTTCCATT TTAAAA[T/C]AATGAGTCGTTTGGTTCTTGCTGCT. Cycling provisions were completed as: initial 50ºC for 60 seconds, then 95ºC for 10 minutes as a primary denaturation step followed by 45 cycles of 15 seconds at 95ºC and 60 seconds at 60ºC (cycling), and a final extension step for 60 seconds at 60ºC. The Sequence Detection System implements the fluorescence emitted during the plate read and the fluorescence (Rn) values were plotted dependent on the signals from each well. Each well of the 96-well reaction plate is represented as an individual point on the plot ( and ). Fluorescence detection and data analysis were carried out by 7500 Real-Time PCR instrument (Applied Biosystems) version 2.0.1.
Figure 1 (A) Allele discrimination plot of ADIPOR2 rs 10773989 T>C where homozygous T were colored in red and homozygous C were colored in blue and heterozygous TC were colored green. (B) Allele discrimination plot of ADIPOQ rs2241766 T>G where homozygous T were colored in blue and homozygous G were colored in red and heterozygous GT were colored green. (C) Amplification plot of ADIPOR2 mRNA. (D) Amplification plot of ADIPOQ mRNA. (E) ROC curve demonstrating sensitivity and specificity of both ADIPOQ and ADIPOR2 mRNA levels.
The Expression of Adiponectin Gene and Receptor
Total RNA was prepared from blood by QIAamp RNA Blood MiniKit (Qiagen, USA). Complementary DNA (cDNA) was synthesized by RT-PCR using (MyTaq T monestep RT PCR kit). Ten μL of RNA extract were applied to a mixture of 1 μL of reverse transcriptase enzyme, 4 μL of 5x TransAmp buffer and 5 μL of RNase-free water. The Applied Biosystems 2720 thermal cycler (Bioline, Singapore, USA) was used and advanced for single cycle of 10 minutes at 25ºC, then 15 minutes at 42ºC, and finally, 5 minutes at 85ºC for inhibition of reverse transcriptase enzyme. The formed cDNA was kept at −20ºC.
Quantitative Real-Time PCR (qRT-PCR)
The cDNA was utilized in SYBR green based qRT-PCR that was conducted with Quanti Tect SYBR Green PCR Kit with ready-made quanti Tect Primer Assay (Qiagen). For measurement of adiponectin mRNA levels, the consequent primers (Midland Texas) were utilized: ADIPOQ gene Sense 5ʹ-TGGTGAGAAGGGTGAGAA-3ʹ and Antisense: 5ʹ-AGATCTTGGTAAAGCGAATG-3ʹ; ADIPOR2 gene Sense 5ʹ-ATAGGGCAGATAGGCTGGTTGA-3ʹ and Antisense 5ʹ- GGATCCGGGCAGCATACA-3ʹ and primers for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Sense 5ʹ-TCCATGACAACTTTGGCATCGTGG-3ʹ and Antisense 5ʹ-GTTGCTGTTGAAGTCACAGGAGAC-3ʹ.
A mixture of 20 μL was conducted by applying 5 μL of the cDNA to a mixture of 10 μL of 2x SYBR® Low ROX Master Mix, 1 μL of each primer and 4 μL of RNase-free water. The reaction was proceeded as: 45 cycles; 30 seconds at 94ºC for denaturation, 30 seconds at 55ºC for annealing and 30 seconds at 72ºC for extension. Data was analyzed with Applied Biosystems 7500 software version 2.0.1. The mRNA levels were assessed by the relative quantification (RQ) utilizing the ΔΔCt method as the measurement of the definite gene, is standardized to an endogenous reference gene (GAPDH) and relative to a control ( and )
Statistical Analysis
Analysis of data was done by IBM SPSS software package version 20.0 (IBM Corp., Armonk, NY, USA). Categorical variables were compared between groups by Chi-square test (Fisher or Monte Carlo). For comparing two groups, Student’s t-test was conducted for normally distributed quantitative variables whereas Mann Whitney test was applied for abnormally distributed ones. For more than two groups we used Kruskal Wallis test for abnormally distributed quantitative variables. In terms of correlation between quantitative variables, Spearman coefficient was performed. Receiver operating characteristic curve (ROC) was applied to assess the diagnostic value of biomarkers. Odds ratio (OR) was calculated to determine the risk of MI. Univariate and multivariate regression analysis was performed to detect the most independent/affecting factor for MI. Significance of the results was considered at the 5% level.
Results
Criteria for selected patients and all measured laboratory parameters are presented in . Age and sex were matched between the studied groups (P>0.05). From a total of 120 patients, 64 (53.3%) were hypertensive, 39 (32.5%) had a positive family history and consequently they were markedly significant from selected control (neither hypertensive nor had a negative family history) (P<0.001) and proving the risk for occurrence of MI. Smoking was not a significant contributing risk factor for MI in this study as only 43 (35.8%) of patients had a smoking habit while 25 (25%) of the control group were smokers (P=0.083).
Table 1 Comparison Between the Two Studied Groups According to Different Parameters
Patients exhibited higher BMI levels than the control group (P<0.001). TC and LDLc were markedly increased in patients than in controls (P<0.001) while TG (P=0.174) and HDLc (P=0.105) were not significantly different between studied groups. MI patients showed lower levels of serum adiponectin compared with controls (7.91± 3.13 vs 10.87± 3.93, P<0.001), moreover, ADIPOQ and ADIPOR2 mRNA levels were also significantly lower in patients when compared with controls (0.45±0.33, 0.48±0.44 vs 1.08±0.72, 1.10±0.81, P<0.001, respectively) ().
shows the distribution of observed genotype frequencies of ADIPOQ rs2241766 and ADIPOR2 rs10773989. Genotype frequencies of both genes were consistent with Hardy-Weinberg equilibrium in both patients and controls (P>0.05) and so they were valid for statistical calculation. Patients showed dominant ADIPOQ rs2241766 mutant GG genotype and G allele which were markedly higher than in controls (11.7% vs 4% and 32.1% vs 15% respectively). Odds ratio (OR) for GG genotype was 4.544 [95% CI: 1.42–14.5] and for G allele OR 2.677 [95% CI: 1.67–4.30].
Table 2 Comparison Between the Two Studied Groups According to ADIPOQ and ADIPOR2 Polymorphisms
CC genotype and C allele of ADIPOR2 rs10773989 were significantly associated with MI patients where their frequencies were 20% and 43.3% while in controls they were 12% and 31% with P=0.033 and P=0.008, respectively. CC genotype showed an OR of 2.5 [95% CI: 1.11–5.61] and C allele an OR of 1.702 [95% CI: 1.15–2.52].
shows the receiver operating characteristics (ROC) curve for accurate determination of sensitivity and specificity of ADIPOQ and ADIPOR2 mRNA levels (). At area under the curve (AUC) 0.767 and cut-off point ≤0.64 for ADIPOR2 mRNA level, sensitivity was 74% while specificity was 64% for accurate identification of MI patients from controls. At area under the curve (AUC) 0.831 and cut-off point ≤0.72, ADIPOQ mRNA level had higher sensitivity 78.33% and higher specificity 76% thus explaining higher priority for ADIPOQ mRNA than ADIPOR2 for accurate prediction of MI.
Table 3 Sensitivity and Specificity of Both ADIPOR2 mRNA and ADIPOQ mRNA Levels to Predict MI Patients
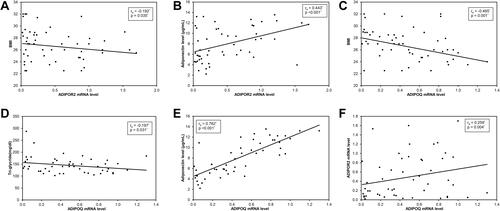
Spearman correlation of ADIPOR2 mRNA with differently measured outcomes showed negative correlation with BMI (r = −0.192, P= 0.035) () while positive correlation with adiponectin level (r = 0.443, P<0.001) (). ADIPOQ mRNA level also showed negative correlation with BMI (r = −0.465, P<0.001) () and with TG (r = −0.197, P=0.031) () and positive correlation with adiponectin level (r = 0.762, P<0.001) () and ADIPOR2 mRNA level (r = 0.259, P=0.004) ().
Figure 2 (A) Spearman negative correlation of ADIPOR2 mRNA level with BMI. (B) Positive correlation of ADIPOR2 mRNA level with adiponectin level. (C) Negative correlation of ADIPOQ mRNA level with BMI. (D) Negative correlation of ADIPOQ mRNA level with triglyceride. (E) Positive correlation of ADIPOQ mRNA with adiponectin level. (F) Positive correlation of ADIPOQ mRNA level with ADIPOR2 mRNA level.
Relation of studied adiponectin SNPs are completely described in and . CC genotype of ADIPOR2 rs10773989 had the lowest median levels of ADIPOR2 mRNA level and serum adiponectin vs TT and TC (P<0.001) and the lowest in patients. Using post hoc test for comparison among groups, it was also significant between groups (TT vs TC, TT vs CC and TC vs CC) except for comparing TC vs CC regarding relation with serum adiponectin. In the control group, also, CC genotype had the lowest level of ADIPOR2 mRNA level (P=0.001) vs TT and TC and utilizing post hoc test for comparison among groups, it was also significant between groups (TT vs TC, TT vs CC and TC vs CC) however, no detectable relation with adiponectin level (P=0.295) ().
Table 4 Relation Between ADIPOR2 rs10773989 with Its mRNA and Serum Adiponectin in Each Group
Table 5 Relation Between ADIPOQ rs2241766 with Its mRNA and Serum Adiponectin in Each Group
GG genotype of ADIPOQ rs2241766 had the lowest median levels of ADIPOQ mRNA level and adiponectin level vs TT and GT (P<0.001) in patients and post hoc test for comparison among groups showing also significance between groups (TT vs GT, TT vs GG and GT vs GG). Moreover, in the control group, also, GG genotype had the lowest level of both ADIPOQ mRNA level and adiponectin level. Comparison between groups also showed significance between groups (TT vs GT and TT vs GG) except (GT vs GG) was not significant either with mRNA levels or circulating adiponectin ().
Logistic regression univariate analysis for the significant risk factors affecting MI patients showed that BMI (P<0.001), TC (P<0.001), LDLc (P<0.001), ADIPOQ mRNA (P<0.001), ADIPOR2 mRNA (P<0.001), adiponectin level (P<0.001), ADIPOQ rs2241766 GG (P=0.011), GT (P=0.001), ADIPOR2 rs10773989 CC (P=0.026), TC (P=0.041) can significantly predict MI patients while in multivariate analysis, BMI (P=0.006), TC (P<0.001), LDLc (P=0.001), ADIPOQ mRNA (P=0.001), ADIPOQ rs2241766 GG (P=0.002), GT (P=0.001) can independently predict occurrence of MI from healthy individuals ().
Table 6 Univariate and Multivariate Analysis for the Risk Parameters Affecting Patients with MI
Discussion
Adiponectin has been assumed to have a protective role in CAD,Citation17 possibly by enhancing insulin sensitivity and diminishing hepatic triglyceride accumulation.Citation18 Adiponectin receptors in humans have been identified in monocytes and macrophagesCitation19 and ADIPOR2 variants may be a contributing agent for atherosclerosis regardless of insulin resistance.Citation20 Decreased concentrations of adiponectin have been related to increased risks of type 2 diabetes (T2DM), hypertension, and coronary atherosclerosis.Citation21
We have investigated ADIPOQ and ADIPOR2 variants and expressions or mRNA levels in 120 nondiabetic patients suffering from MI and 100 healthy subjects taken as a control group. We detected a marked reduction in serum adiponectin, ADIPOQ and ADIPOR2 mRNA levels in patients compared to controls. These findings support previous studies where Kollias et alCitation14 reported that a decrease in plasma adiponectin level and decrease in ADIPOR1 and ADIPOR2 protein levels in peripheral monocytes might aggravate the deteriorated antiatherogenic effect of adiponectin in overweight patients with CAD compared to similar weight control individuals. Liberale et alCitation22 demonstrated that adiponectin was negatively linked with chronic low-grade inflammation and decreased risk of adverse cardiovascular outcome in patients diagnosed to have severe carotid stenosis. Moreover, the same study reported that serum adiponectin levels ≤ 2.56 μg/mL in patients could develop more acute coronary syndromes within 12 months of follow-up. Gan et alCitation23 clarified that lowered adiponectin levels were related to the development of high-risk plaques in asymptomatic individuals.
Wang et alCitation24 reported that adiponectin has a protective role in countering coronary atherosclerosis inception in early onset CAD patients. High molecular weight adiponectin and high molecular weight total adiponectin ratio showed more intense inverse correlation with the severity of coronary atherosclerosis compared to the outcome of total adiponectin and could be a valuable potential biomarker in assessment of CAD risk.
Adiponectin is considered a protective factor for CAD, however its correlation with the atherosclerotic severity and its prognostic potential for the disease is still inconsistent in various populations.Citation25,Citation26
Our analysis showed also that ADIPOQ rs2241766 mutant GG genotype and G allele had an elevated risk of MI. Additionally, CC genotype and C allele of ADIPOR2 rs10773989 were shown to be associated with MI, which proves that the presence of adiponectin variants could increase the risk of MI. Moreover, these mutant alleles were associated with diminished levels of circulatory adiponectin and lower expression values of adiponectin mRNA and its receptor. These results support previous studies where a meta-analysis carried out by Zhou et alCitation27 displayed that the SNP rs2241766 T>G genotype was related with an elevated risk of CAD.
Significant associations of ADIPOQ rs2241766 T>G genotype with CVD were observed in Caucasians but not in Asians, hence proposing a potential influence of ethnicity in genetic background.Citation28
The latest meta-analysis which was carried out by Kanu et alCitation29 reported that ADIPOQ rs2241766 variant was related with the elevated risk of CAD in various genetic models (allelic, dominant and recessive).
A recent study carried out by Hussain et alCitation30 demonstrated that ADIPOQ rs2241766 is enrolled in the pathological process of CAD in the Iraqi population. Individuals with one or two G alleles were found to have a risk of CAD of 2 to 6.67 times compared to those of the wild gene respectively. Zhang et alCitation31 demonstrated that both ADIPOQ rs2241766 and ADIPOR2 rs10773989 polymorphisms, specifically in the genetic recessive model, are assumed to be an independent risk factor for MI in Han Chinese populations and also added that rs2241766, and rs10773989 were significant contributors, and exhibited a good predictive utility.
Heid et alCitation32 reported that ADIPOQ rs2241766 T>G gene polymorphism and haplotypes were correlated with lowered adiponectin levels and propose that the adiponectin gene participates in the inflammatory process via a modified transcriptional activity.
In contrary to our findings, Chang et alCitation33 stated that the G allele has a protective influence against CAD risk. However, no significant associations were reported in previous studies, eg, in Italians,Citation34 in a Chinese populationCitation35 and in Tunisians.Citation36 This discrepancy could be credited to ethnicity, genetic background, study design, and/or environmental factors.
The specific mechanism by which polymorphisms of the adiponectin gene add to CAD progression is not fully clarified. Yan et alCitation37 suggested that the site of this polymorphism is adjacent to exon and intron junctions, resulting in a silent mutation which might affect its expression via an mRNA splicing approach.
Adiponectin prompts fatty acid oxidation and reduces free fatty acids level, additionally; it may induce the sensitivity to insulin, hinders monocytes segregation to the endothelial cells and represses fat aggregations within the monocyte.Citation38
Lisowska et alCitation39 reported that adiponectin in addition to adipocytes is also synthesized by cardiomyocytes, skeletal muscle and osteoblasts, although ADIPOR2 are found to be expressed by cardiac and skeletal muscle. Also, Piñeiro et alCitation40 proposed that the native synthesis of this protein by cardiac muscle might affect cardiac metabolism and function.
Adiponectin can protect against atherosclerosis as its lacks exaggerated neointimal thickening, however adiponectin enhancement lessens this influence, mainly via the inhibitory impact on the propagation and immigration of vascular smooth muscle cells.Citation41
Siitonen et alCitation42 suggested a role for ADIPOR2 gene in predisposition to CAD and T2DM, probably by independent genetic effects. Diverse polymorphisms within the ADIPOR2 gene might function independently or interacting with other factors to prompt precise and perhaps tissue definite modifications in gene expression.
Additionally, in the multivariate analysis, we had indicated that BMI, TC, LDLc, ADIPOQ gene expression and ADIPOQ rs2241766 GG, GT can predict MI and Zhang et alCitation31 reported that age, TC, hypertension, rs2241766, and rs10773989 were significant contributors, and exhibited a good predictive utility for MI.
Monzo et alCitation43 concluded that adiponectin influences water homeostasis and congestion progress in heart failure, its use as a biomarker of severe congestion and its role in decongestion therapy should be further studied. Additionally, elevated adiponectin concentration was reported as an independent indicator of congestive heart failure severity.Citation44
We explored in this study a significant correlation of ADIPOQ and ADIPOR2 expressions and variants with BMI, triglycerides and circulatory adiponectin that may contribute to its predictive ability for MI. However as a limitation of this study, we did not measure adiponectin isoforms, specifically the high molecular weight (HMW) isoform considering that adiponectin isoforms seems to have different biological actions, particularly in the heart so we recommend measuring adiponectin isoforms in MI in further studies to approve adiponectin relation with MI.
Conclusion
This study explored a marked association of ADIPOQ and ADIPOR2 variants with their expressed mRNA values and further with serum adiponectin levels in MI so it might have a role in the pathogenesis of disease and can be regarded as a risk factor. Further studies are needed to evaluate adiponectin association with the severity of disease and the use of adiponectin supplements to prevent or lessen the disease.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- Xu S, Jiang J, Zhang Y, et al. Discovery of potential plasma protein biomarkers for acute myocardial infarction via proteomics. J Thorac Dis. 2019;11(9):3962–3972. doi:10.21037/jtd.2019.08.100
- Luan L, Hu H, Li SC. A review of studies of quality of life for Chinese-speaking patients with ischemic heart disease. Value Health Reg Issues. 2018;15:82–90. doi:10.1016/j.vhri.2017.08.013
- Barsova RM, Lvovs D, Titov BV, et al. Variants of the coagulation and inflammation genes are replicably associated with myocardial infarction and epistatically interact in Russians. Zhang H, ed. PLoS One. 2015;10(12):e0144190. doi:10.1371/journal.pone.0144190
- Hirokawa M, Morita H, Tajima T, et al. A genome-wide association study identifies PLCL2 and AP3D1-DOT1L-SF3A2 as new susceptibility loci for myocardial infarction in Japanese. Eur J Hum Genet. 2015;23(3):374–380. doi:10.1038/ejhg.2014.110
- Shah N, Kelly AM, Cox N, Wong C, Soon K. Myocardial infarction in the “young”: risk factors, presentation, management and prognosis. Heart Lung Circ. 2016;25(10):955–960. doi:10.1016/j.hlc.2016.04.015
- Rodríguez-Pérez JM, Posadas-Sánchez R, Blachman-Braun R, et al. HHIPL-1 (rs2895811) gene polymorphism is associated with cardiovascular risk factors and cardiometabolic parameters in Mexican patients with myocardial infarction. Gene. 2018;663:34–40. doi:10.1016/j.gene.2018.04.030
- Kyriakou T, Collins LJ, Spencer-Jones NJ, et al. Adiponectin gene ADIPOQ SNP associations with serum adiponectin in two female populations and effects of SNPs on promoter activity. J Hum Genet. 2008;53(8):718–727. doi:10.1007/s10038-008-0303-1
- Banerjee A, Khemka VK, Roy D, Poddar J, Roy TKS, Karnam SA. Role of serum adiponectin and vitamin D in prediabetes and diabetes mellitus. Can J Diabetes. 2017;41(3):259–265. doi:10.1016/j.jcjd.2016.10.006
- Méndez-Hernández A, Gallegos-Arreola MP, Moreno-Macías H, Espinosa Fematt J, Pérez-Morales R. LEP rs7799039, LEPR rs1137101, and ADIPOQ rs2241766 and 1501299 polymorphisms are associated with obesity and chemotherapy response in Mexican women with breast cancer. Clin Breast Cancer. 2017;17(6):453–462. doi:10.1016/j.clbc.2017.03.010
- Wang Y, Wang X, Lau WB, et al. Adiponectin inhibits tumor necrosis factor-α-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ Res. 2014;114(5):792–805. doi:10.1161/CIRCRESAHA.114.302439
- Li TD, Zeng ZH. Adiponectin as a potential therapeutic target for the treatment of restenosis. Biomed Pharmacother. 2018;101:798–804. doi:10.1016/j.biopha.2018.03.003
- Martinez-Huenchullan SF, Tam CS, Ban LA, Ehrenfeld-Slater P, Mclennan SV, Twigg SM. Skeletal muscle adiponectin induction in obesity and exercise. Metabolism. 2020;102:154008. doi:10.1016/j.metabol.2019.154008
- Blüher M, Williams CJ, Klöting N, et al. Gene expression of adiponectin receptors in human visceral and subcutaneous adipose tissue is related to insulin resistance and metabolic parameters and is altered in response to physical training. Diabetes Care. 2007;30(12):3110–3115. doi:10.2337/dc07-1257
- Kollias A, Tsiotra PC, Ikonomidis I, et al. Adiponectin levels and expression of adiponectin receptors in isolated monocytes from overweight patients with coronary artery disease. Cardiovasc Diabetol. 2011;10(1):14. doi:10.1186/1475-2840-10-14
- De Caterina R, Talmud PJ, Merlini PA, et al. Strong association of the APOA5-1131T>C gene variant and early-onset acute myocardial infarction. Atherosclerosis. 2011;214(2):397–403. doi:10.1016/j.atherosclerosis.2010.11.011
- Zhang M, Peng Y, Lyu S. Association between genetic variants in the adiponectin gene and premature myocardial infarction. Chin J Cardiol. 2016;44(7):577–582. doi:10.3760/cma.j.issn.0253-3758.2016.07.005
- Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165(3):574–590. doi:10.1111/j.1476-5381.2011.01395.x
- Turer AT, Scherer PE. Adiponectin: just along for the ride? Circ Res. 2016;119(3):407–408. doi:10.1161/CIRCRESAHA.116.309226
- Pang TTL, Narendran P. The distribution of adiponectin receptors on human peripheral blood mononuclear cells. In: Annals of the New York Academy of Sciences. Vol. 1150. Blackwell Publishing Inc.; 2008:143–145. doi:10.1196/annals.1447.021
- Halvatsiotis I, Tsiotra PC, Ikonomidis I, et al. Genetic variation in the adiponectin receptor 2 (ADIPOR2) gene is associated with coronary artery disease and increased ADIPOR2 expression in peripheral monocytes. Cardiovasc Diabetol. 2010;9:10. doi:10.1186/1475-2840-9-10
- Li S, Shin HJ, Ding EL, Van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–188. doi:10.1001/jama.2009.976
- Liberale L, Carbone F, Bertolotto M, et al. Serum adiponectin levels predict acute coronary syndrome (ACS) in patients with severe carotid stenosis. Vascul Pharmacol. 2018;102:37–43. doi:10.1016/j.vph.2017.12.066
- Gan L, Yang L, Yan G. Predict value of adiponectin for coronary atherosclerosis plaques according to computed tomography angiography in an asymptomatic population. Clin Imaging. 2018;51:174–179. doi:10.1016/j.clinimag.2018.05.019
- Wang Y, Zheng A, Yan Y, et al. Association between HMW adiponectin, HMW-total adiponectin ratio and early-onset coronary artery disease in Chinese population. Atherosclerosis. 2014;235(2):392–397. doi:10.1016/j.atherosclerosis.2014.05.910
- Khan UI, Wang D, Sowers MR, et al. Race-ethnic differences in adipokine levels: the Study of Women’s Health Across the Nation (SWAN). Metabolism. 2012;61(9):1261–1269. doi:10.1016/j.metabol.2012.02.005
- Mente A, Razak F, Blankenberg S, et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010;33(7):1629–1634. doi:10.2337/dc09-1392
- Zhou D, Jin Y, Yao F, Duan Z, Wang Q, Liu J. Association between the adiponectin +45T>G genotype and risk of cardiovascular disease: a meta-analysis. Heart Lung Circ. 2014;23(2):159–165. doi:10.1016/j.hlc.2013.07.010
- Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4(2):45–61. doi:10.1097/00125817-200203000-00002
- Kanu JS, Qiu S, Cheng Y, et al. Associations between three common single nucleotide polymorphisms (rs266729, rs2241766, and rs1501299) of ADIPOQ and cardiovascular disease: a meta-analysis. Lipids Health Dis. 2018;17(1). doi:10.1186/s12944-018-0767-8
- Hussain MK, Almayali AH, Baqir Aljabery HA, Kamil ZD. Adeponectin gene polymorphism, rs2241766, is associated with coronary artery disease in Iraqi population. Gene Rep. 2019;14:50–53. doi:10.1016/j.genrep.2018.11.007
- Zhang Z, Li Y, Yang X, Wang L, Xu L, Zhang Q. Susceptibility of multiple polymorphisms in ADIPOQ, ADIPOR1 and ADIPOR2 genes to myocardial infarction in Han Chinese. Gene. 2018;658:10–17. doi:10.1016/j.gene.2018.03.022
- Heid IM, Wagner SA, Gohlke H, et al. Genetic architecture of the APM1 gene and its influence on adiponectin plasma levels and parameters of the metabolic syndrome in 1727 healthy Caucasians. Diabetes. 2006;55(2):375–384. doi:10.2337/diabetes.55.02.06.db05-0747
- Chang Y-C, Jiang J-Y, Jiang Y-D, et al. Interaction of ADIPOQ genetic polymorphism with blood pressure and plasma cholesterol level on the risk of coronary artery disease. Circ J. 2009;73(10):1934–1938. doi:10.1253/circj.CJ-09-0228
- Filippi E, Sentinelli F, Romeo S, et al. The adiponectin gene SNP+276G>T associates with early-onset coronary artery disease and with lower levels of adiponectin in younger coronary artery disease patients (age ≤50 years). J Mol Med. 2005;83(9):711–719. doi:10.1007/s00109-005-0667-z
- Yang Y, Zhang F, Ding R, Wang Y, Lei H, Hu D. Association of ADIPOQ gene polymorphisms and coronary artery disease risk: a meta-analysis based on 12 465 subjects. Thromb Res. 2012;130(1):58–64. doi:10.1016/j.thromres.2012.01.018
- Ghazouani L, Elmufti A, Baaziz I, Chaabane I, Ben Mansour H. Contribution of adiponectin polymorphisms to the risk of coronary artery disease in a North-African Tunisian population. J Clin Lab Anal. 2018;32(7):e22446. doi:10.1002/jcla.22446
- Yan CJ, Li SM, Xiao Q, et al. Influence of serum adiponectin level and SNP +45 polymorphism of adiponectin gene on myocardial fibrosis. J Zhejiang Univ Sci B. 2013;14(8):721–728. doi:10.1631/jzus.BQICC707
- de Luis DA, Izaola O, Primo D, et al. Role of rs1501299 variant in the adiponectin gene on total adiponectin levels, insulin resistance and weight loss after a Mediterranean hypocaloric diet. Diabetes Res Clin Pract. 2019;148:262–267. doi:10.1016/j.diabres.2017.11.007
- Lisowska A, Tycinska A, Knapp M, et al. Adiponectin an independent marker of coronary artery disease occurrence rather than a degree of its advancement in comparison to the IMT values in peripheral arteries. Clin Chim Acta. 2012;413(7–8):749–752. doi:10.1016/j.cca.2012.01.006
- Piñeiro R, Iglesias MJ, Gallego R, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579(23):5163–5169. doi:10.1016/j.febslet.2005.07.098
- Kanu JS, Gu Y, Zhi S, et al. Single nucleotide polymorphism rs3774261 in the AdipoQ gene is associated with the risk of coronary heart disease (CHD) in Northeast Han Chinese population: a case-control study. Lipids Health Dis. 2016;15(1):6. doi:10.1186/s12944-015-0173-4
- Siitonen N, Pulkkinen L, Lindström J, et al. Association of ADIPOR2 gene variants with cardiovascular disease and type 2 diabetes risk in individuals with impaired glucose tolerance: the Finnish Diabetes Prevention Study. Cardiovasc Diabetol. 2011;10:83. doi:10.1186/1475-2840-10-83
- Monzo L, Kotrc M, Benes J, et al. Clinical and humoral determinants of congestion in heart failure: potential role of adiponectin. Kidney Blood Press Res. 2019;44(5):1271–1284. doi:10.1159/000502975
- Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112(12):1756–1762. doi:10.1161/CIRCULATIONAHA.104.530972
