Abstract
In recent years, facilitated by rapid technological advances, we are becoming more adept at probing the molecular processes, which take place in the nucleus, that are crucial for the hierarchical regulation and organization of chromatin architecture. With an unprecedented level of resolution, a detailed atlas of chromosomal structures (histone displacement, variants, modifications, chromosome territories, and DNA looping) and mechanisms underlying their establishment, provides invaluable insight into physiological as well as pathological phenomena. In this review, we will focus on prostate cancer, a prevalent malignancy in men worldwide, and for which a curative treatment strategy is yet to be attained. We aim to catalog the most frequently observed oncogenic alterations associated with chromatin conformation, while emphasizing the TMPRSS2-ERG fusion, which is found in more than one-half of prostate cancer patients and its functions in compromising the chromatin landscape in prostate cancer.
Keywords:
Introduction to chromatin organization and three-dimensional topology
The nucleus is a fascinating organelle within a cellular entity, not only due to the fact that it contains the entire genetic blueprint required for a cell to survive and propagate, but – more importantly – how it is capable of organizing this vast sea of information in an efficient and effective manner. It has been known that the human genome consists of more than 3 billion base pairs, and in fact the total deoxyribonucleic acid (DNA) in a diploid human cell would sum up to approximately 2 m in length when completely stretched.Citation1 Moreover, the extent of compaction for metaphase chromosome is estimated to be between 10,000- and 20,000-fold.Citation2
To achieve this high level of proficiency and accuracy, the nucleus employs multiple levels of packaging methods to generate what is known as the higher-order structure of chromatin, which is composed of a combination of DNA and proteins that intertwine together to separate genes into regulatory hubs and to form a three-dimensional (3D) topology best suited for a cell’s functions.
About 40 years ago, the use of electron microscopy enabled identification of the classical beads-on-a-string type of structure of DNA, which has been generally accepted as the basic level of chromatin organization.Citation3–Citation6 Further demonstrated by biochemical and X-ray diffraction studies,Citation5,Citation7 the chromatin has been described to be formed by repeating units of nucleosomes, octameric structures consisting of four different histone proteins (two each of H2A, H2B, H3, and H4), which are wound by an estimated number of 147 base pairs of DNA, giving rise to 1.7 superhelical turns.Citation7,Citation8
The advent of fluorescence in situ hybridization (FISH) technology has provided evidence for the nonrandom spatial organization of the genome, allowing visualization of position and interaction of chromosomes, chromatin domains, as well as individual genes. It was revealed that gene density is one of the indicators of nuclear positional organization, which is present generally in a radial pattern where gene-dense chromosomal regions prefer to congregate in the nuclear interior, while gene-poor regions are located around the nuclear periphery.Citation9 It has been observed that chromosomes are segregated into subnuclear compartments, known as chromosome territories,Citation10 where the edge of the nucleus is host to mainly repressed genes packed into heterochromatin form,Citation11,Citation12 and the nuclear interior is concentrated in early replicating DNA and frequently transcribed genes.Citation13,Citation14 In addition, FISH experiments have also demonstrated that during differentiation, specific loci can reposition either toward or away from the nuclear periphery, which is concordant with repression or activation of those nearby genes.Citation15 More recently, a series of chromosome conformation capture (3C)-based approaches, which can achieve a high-resolution interrogation of the chromatin landscape, further confirmed that intrachromosomal associations in metazoan genome can serve to concentrate and segregate active gene-rich and gene-poor domains.Citation16
It is known that – indeed – there are topologically associated domains (TADs) that are pervasive throughout the genome and function to compartmentalize the genome into local and distinct regions, therefore modulating gene expression.Citation17
Role of chromatin organization in gene transcription
The structure of chromatin has been well-known to associate with the status of gene transcription. As early as the 1980s, scientists were able to demonstrate that the mere presence of nucleosomes can inhibit initiation by ribonucleic acid polymerase II (RNAPII) and thus stall transcription.Citation18 The mechanisms for regulation of the chromatin structure with respect to gene transcription are diverse, and may involve histone displacement, histone variant incorporation, posttranslational modifications, chromosome territories, and DNA looping ().Citation19 Each of these mechanisms has its unique influence on chromatin conformation, which in turn dictates gene transcription status.
Figure 1 Different chromatin remodeling regulates gene transcription.
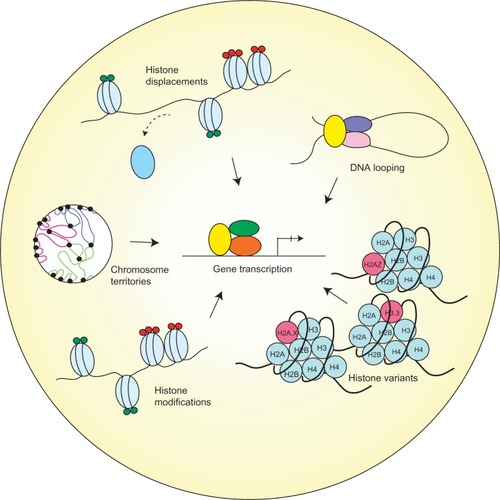
While packaging of the DNA into nucleosomes can inhibit transcription in vitro, this stereochemical constraint may be relieved by structural changes in nucleosomes.Citation18 Histones have been observed to exhibit high turnover properties from the core nucleosome. It is reported that histone dimers of H2A and H2B are relatively more susceptible to displacement when compared to H3 and H4.Citation20 Results from biochemical and genetic studies consistently reinforce the notion that histone eviction from the nucleosome typically occurs at promoters during gene activation, and such process may be mediated by events including but not limited to adenosine triphosphate (ATP)-dependent chromatin remodeling, as well as histone chaperones.Citation21
For instance, chromatin remodeling complexes, such as switch/sucrose nonfermentable (SWI/SNF)Citation22–Citation24 and chromatin structure remodeling (RSC) complex,Citation25,Citation26 and additionally active RNAP IICitation27 can all take part in evicting H2A and H2B to assist nucleosome unraveling. Thus, in a stepwise manner, these chromatin remodeling complexes can mediate repositioningCitation28 or ejectionCitation29 of nucleosomes at promoters to initiate transcription activation. Moreover, histone chaperone proteins (Asf1,Citation30,Citation31 Nap1,Citation32 and nucleophosminCitation33), which act by sequestering the evicted histones to prevent their reincorporation into the nucleosome, are also an indispensable component for proper histone displacement and ultimately gene transcription.
In addition to the physical exchange of histone proteins, the incorporation of variant histones can also lead to modifications in chromatin structure and transcriptional regulation. Unlike canonical core histones, generally these unconventional histone proteins are distinguished by the fact that they are expressed outside of the S phase and their deposition into the nucleosome is deemed DNA replication-independent.Citation34
As a result of changes in their amino acid sequence, variant forms of histones could acquire divergent biophysical properties predisposing them to localize in specific regions of the genome. One prominent histone variant is H2A.Z, which is an alternative form of H2A, and differs from its counterpart in that its N-terminal tail sequence and several key internal residues, which can effectively alter its ability to interact with H2B as well as the H3/H4 tetramer that eventually manifests in reduced nucleosome stability.Citation35,Citation36 The deposition of H2A.Z is reportedly carried out by ATP-dependent histone exchange reactions through SWR1,Citation37 or by the aforementioned chaperone protein Nap1.Citation38 Another well-studied histone variant is H3.3, and in spite of the fact that it only differs in four amino acids from its canonical form H3, H3.3 has its distinct deposition pattern where it is preferentially enriched in transcriptionally active chromatin and regulatory sites.Citation39,Citation40
On the other hand, certain variants, such as macroH2A (mH2A), have the ability to repress gene transcription by remodeling the chromatin to impede RNAPII binding. The name mH2A is derived from the structural feature of this histone variant, which contains a large nonhistone region (NHR), known as the macro domain, on its N-terminus.Citation41 As a consequence, the NHR of mH2A alters nucleosome structure and interferes with the transcription machinery.Citation42
Furthermore, a significant category of mechanisms contributing to chromatin organization is posttranslational modifications (PTMs) on histone proteins. There has been extensive research conducted to compile and characterize existing histone modifications, depicting a close relationship between histone PTMs and chromatin structure. Some of the most widely studied histone PTMs include acetylation, methylation, phosphorylation, ubiquitination, and sumoylation. They covalently modify the N-terminal and/or the C-terminal histone tails, while affecting the globular domains at a lesser extent.Citation43
These various forms of histone marks generate a code that can be interpreted by specialized proteins to regulate gene expression or to mediate DNA repair.Citation44 Modifications that reflect in active transcription have been elucidated and include acetylation of H3 and H4, and di- or trimethylation of H3 at lysine position 4 (H3K4me2 or me3). In contrast, modifications that instigate inactivation of transcription include methylation at H3K9 and H3K27.Citation19
In eukaryotes, individual chromosomes can occupy spatially defined territories in the interphase nucleus, and repositioning of these genomic regions has an impact on the regulation of gene expression. FISH analysis has shown that chromosome territories adjoin at their borders to create boundaries between chromatin domains. More recently, it is demonstrated that TADs are enclosed by sharp boundaries enriched for the insulator-binding protein CTCF, as well as the heterochromatin mark H3K9me3.Citation17 Since boundaries of these topological domains display properties of classical insulator and barrier features, it is therefore suggested that TADs may be linked to transcriptional control.
Concordantly, another study reported that the positions of TADs align with repressive epigenetic marks, as well as lamina-associated domains, and disrupting a TAD boundary can lead to the long-range deregulation in gene expression during X-chromosome inactivation.Citation45 Therefore, the evidence is convincing that TADs indeed play a role in shaping transcriptional landscapes by clearly defining which sequences belong to the same regulatory network.
Last, as DNA is packaged inside the nucleus, long-range chromatin interactions inevitably occur and – as a result – form loop structures, a majority of which take place between cis-regulatory elements and promoters. It is reported that the dynamic alterations of chromatin looping can either activate or suppress gene expression by facilitating the interactions between enhancers or silencers and their target genes.
One study revealed that only approximately 7% of looping is bridging its nearest gene, reflecting that this chromatin structure is not restrained by genomic proximity and is capable of engaging promoters with distal sites to form complex networks.Citation46 At the same time, these long-range interactions are not inhibited by CTCF and cohesin occupancy,Citation46 which argues against previous notions that CTCF’s binding to insulator sequences may prevent promoter-enhancer interactions.
Moreover, evidence suggests that the enhancer-promoter loop interactions are formed, in a cell type-specific manner, prior to the binding of transcription factors, indicating their critical role in laying the groundwork for transcriptional control during lineage specification.Citation47 Furthermore, in terms of thermodynamic properties of DNA looping, it is understood that this mechanism of bringing together multiple components into one functional unit serves to simultaneously increase specificity and affinity and reduce transcriptional noise.Citation48
Role of chromatin conformation in cancer
Due to the crucial role chromatin structure has on determining gene transcription, it is intuitive that chromatin conformation could be manipulated during oncogenic transformation of cancerous cells. It has been demonstrated that under the employment of tumor cells, these chromatin organization machineries become deregulated, disrupting the 3D architecture and undermining the genomic integrity. One of the most recurring phenomena that is associated with cancer development is chromosomal translocations.Citation49 In the past several decades, a copious number of translocation events have been identified to play pivotal roles in development of a wide range of hematological malignancies as well as solid tumors, which have in turn been utilized as valuable diagnostic and prognostic markers.
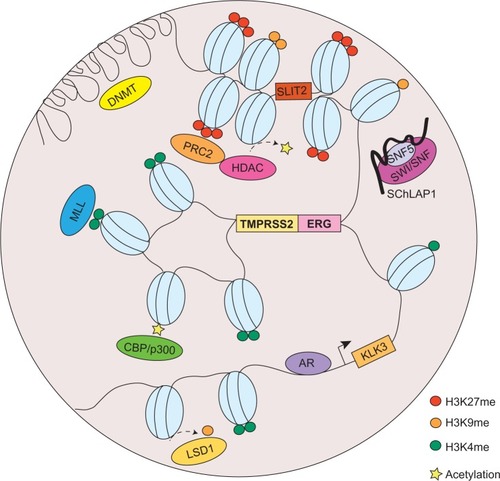
Aside from chromosomal translocations, a myriad of events have been implicated in cancer, most of which are deviations from the physiological occurrences of chromatin organization discussed previously. Here, we will catalog the most significant aberrations pertinent to chromatin topology that contribute to cancer development, with a particular emphasis on prostate cancer ().
Figure 2 Chromatin organization aberrations in prostate cancer.
Abbreviations: DNMT, DNA methyltransferase; PTMs, posttranslational modifications; RNA, ribonucleic acid; ncRNA, noncoding RNA; PRC2, polycomb repressive complex 2; HDAC, histone deacetylase; SWI/SNF, switch/sucrose nonfermentable; MLL, mixed-lineage leukemia; AR, androgen receptor; LSD1, lysine-specific demethylase 1.
The Philadelphia chromosome is recognized as one the most prominent cancer-associated cytogenetic abnormality that was first reported by Nowell and Hungerford in 1960.Citation50 It is a highly frequent oncogenic event found in more than 90% of chronic myelogenous leukemia. The translocation is characterized by a reciprocal interchange between chromosome 9 and chromosome 22, which inopportunely generates a BCR-ABL tyrosine kinase gene fusion product.Citation51 As a result of juxtaposing the breakpoint cluster region (BCR) promoter with the coding region of the ABL gene, the hyperactive BCR-ABL fusion protein confers myeloproliferative properties and leads to leukemogenesis.Citation52 Clinical successes obtained through pharmacological therapies directly inhibiting the activity of BCR-ABL (eg, imatinib mesylate) have provided a promising paradigm in which chromosomal organization could be a critical target for cancer development and, certainly, cancer treatment.
It was not until recently, however, that chromosomal translocations have been identified in solid tumors. In 2005, Tomlins et al made the breakthrough discovery of the fusion of the TMPRSS2 and ERG genes in prostate cancer.Citation53 According to their study, a striking proportion of 50% of prostate cancers were found to contain a merged product of the 5′ untranslated region of TMPRSS2 (21q22), an androgen-regulated gene, and the protein-coding sequences of ERG (21q22), an erythroblast transformation-specific (ETS) transcription factor (). The TMPRSS2-ERG rearrangement has been confirmed to be present in 36%–78% of prostate cancers.Citation54 In addition, other members of the ETS family, including ETV1 (7p21), ETV4 (17q21), and ETV5 (3q28), were also uncovered as fusion partners with TMPRSS2 in prostate cancer, but they were detected in lower frequency.Citation55 Unlike the BCR-ABL translocation, the fusion between TMPRSS2 and ETS genes does not generate a chimeric protein, but instead it promotes the overexpression of oncogenic factors directed by a corrupted promoter element. While solely TMPRSS2 has been identified as a fusion partner of ERG, other 5′ partners of ETS genes have also been observed. These include androgen-induced genes SLC45A3, KLK2, CANT1, and NDRG1, and an endogenous retroviral element HERV-K_22q11.23, which are functionally comparable to TMPRSS2, as well as androgen-repressed gene C15orf21.Citation56–Citation58
It was also reported that rearrangements in the rapidly accelerated fibrosarcoma (RAF) pathway also occur in advanced prostate cancer (SLC45A3-BRAF, ESRP1-RAF1), which can be targeted by RAF kinase inhibitors.Citation59 Moreover, a recent study was able to identify a median of 90 rearrangements in seven prostate cancer tumor samples.Citation60 Examples of disrupted genes due to rearrangement include CADM2, which is a cell adhesion molecule, and phosphatase and tensin homolog (PTEN), a well-established tumor suppressor, as well as a PTEN-interacting protein, MAGI2. These findings depict a convoluted network of genomic rearrangements and chromatin conformation, which synergistically confer deregulated gene expressions and contribute to tumorigenesis.
In addition to chromosomal translocations, modifications to histone could also place a huge impact on the 3 D structure of chromatin and has been widely implicated in cancer. In prostate cancer, H3K4 methylation and H3K27 methylation are among the most extensively investigated histone PTMs; while the former is generally associated with activation of proto-oncogenes, the latter is associated with silencing of tumor suppressors. The repressive epigenetic PTM, H3K27 trimethylation (H3K27me3), has been found to be significantly enriched in promoters of numerous tumor suppressor genes (eg, ADRB2,Citation61 SLIT2,Citation62 DAB2IP,Citation63,Citation64 etc), in metastatic prostate cancer. Meanwhile, H3K9me1 and me2, generally accompanied by heterochromatin assembly,Citation65 are also implicated in prostate cancer. Demethylation of H3K9 has been reported to refect in derepression of AR-regulated genes.Citation66
H3K4 mono- and dimethylation (H3K4me1, H3K4me2) have been thought of as markers for enhancer sites in directing the androgen receptor (AR) transcriptional program, by facilitating AR binding directly or indirectly through the recruitment of coactivators, such as FOXA1, GATA2, and MED1.Citation67 Moreover, an endeavor combining high-resolution nucleosome positioning with histone marks mapping showed strong evidence that H3K4me2-containing nucleosomes spaced 250–450 bp (base pair) apart can flank binding sites of AR prior to its ligand-mediated activation, while the binding site is occluded by a well-positioned nucleosome. Following AR activation, nucleosomes with altered H3K4me2 marks become destabilized at AR binding sites and are comparably more stable at the two flanking loci.Citation68
In addition, the study revealed that the labile H2A.Z variant was more likely to be present in the central nucleosome relative to the flanking nucleosomes, which further contributes to reduced stability of the nucleosome occupied at the AR binding site. Also, it has been shown that androgen treatment can increase the level of H2A.Z and that the incorporation of H2A.Z in enhancer and proximal promoter sites of the AR-induced gene prostate-specific antigen (PSA; or KLK3) can poise the gene for activation by AR.Citation69
Established and maintained by protein–protein interaction between transcription factors bound at enhancers and at promoters,Citation70 DNA looping and chromatin compartmentalization are essential processes governing gene transcription; hence, they are a frequent target for disruption during cancer development. In the case of prostate cancer, AR-mediated chromatin looping has been a longtime research interest in the field, and extensive efforts have been devoted to elucidate the process of how AR signaling may lead to changes in chromatin conformation during prostate tumorigenesis. Studies using chromatin immunoprecipitation (ChIP) techniques showed a striking feature of AR genome-wide binding pattern that, approximately 86%–95% of AR localization occurs in nonpromoter regions.Citation67,Citation71
This evidence strongly indicates that AR, as a transcription factor, is able to direct its specific transcriptional program from a distance – sometimes, even hundreds of kilobases –away from its target gene. Therefore, it is plausible to presume that a looping model is the mechanism by which AR can regulate its targets from afar. In fact, this model has been proven to be true through 3C-based assays, which demonstrated that distal AR enhancer regions form long-range physical contacts with transcription start sites of AR-regulated genes, such as PSA and TMPRSS2,Citation72,Citation73 as well as UBE2C, which is a critical enzyme involved in promoting growth of castration-resistant prostate cancer.Citation67,Citation74
Oncogene-mediated alterations in chromatin conformation
Oncogenes have long been implicated in cancer through chromatin alterations, and one route they take is histone modification. It was discovered in 2002 that EZH2, the enzymatic component of the polycomb repressive complex 2 (PRC2), was among the most upregulated genes in prostate cancer.Citation75 The tumorigenic role of EZH2 has been well-documented, and it involves epigenetic silencing of tumor suppressors and developmental regulators to maintain a dedifferentiated state for cancer cells.Citation76 EZH2 catalyzes trimethylation of H3K27, creating repressive chromatin structures over long genomic distances.Citation77,Citation78 It also recruits several other players, such as PRC1, DNA methyltransferase (DNMT), and histone deacetylase (HDAC) (), which are concordantly upregulated in prostate cancer.Citation79–Citation81 It has been revealed that 50% of hypermethylated genes in prostate cancer display preestablished EZH2-mediated H3K27me3 marks, which then leads to de novo DNA methylation.Citation82 Therefore, EZH2 acts in concert with additional epigenetic enzymes to implement chromatin compaction in a cooperative manner.
Furthermore, methylation at H3K9 has also shown to be deregulated in prostate cancer, through perturbed activities of lysine-specific demethylase 1 (LSD1) ().Citation66 However, the functions of LSD1 in prostate cancer appear to multifaceted, since it is capable to demethylate not only H3K9, but also H3K4. Since H3K4me1 and me2 are essential marks on AR enhancer sites, erasing these modifications consequently result in gene repression.Citation83 Moreover, an exome-sequencing study recently revealed that several members of the mixed-lineage leukemia (MLL) complex (MLL, MLL2, and ASH2L), which acts as H3K4-specific methyltransferase, can physically interact with AR and are significantly mutated in prostate cancerCitation84 (). From a translational standpoint, pharmacological targeting of these histone-modifying enzymes has been envisaged and shown clinical triumph.Citation85
While histone modifications are carried out by specific enzymes, the molecular process underlying the formation of chromatin looping may be effected through a network of coregulators (eg, MED12, SRC-1, p300/CBP, BRG1, etc) that are collectively responsible for sustaining the loop structure.Citation1 Additionally, the GATA, OCT, PAX, NKX, and LEF family proteins have been observed to have sequence motifs near nuclear hormone receptors, including AR and estrogen receptor (ER).Citation86 Disruption of chromosomal structures, therefore, can significantly impair proper gene transcription. A central protein in AR/ER signaling, the pioneer factor forkhead box A1 (FOXA1), has been regarded as a key mediator of AR/ER transcription regulation through chromatin remodeling and recruitment of AR/ER to target sites.Citation87
The fact that FOXA1 is overexpressed and mutated in hormone-dependent cancers, prostate cancer, and breast cancer, is in concordance with its predominant role in directing AR/ER signaling to drive cancer development.Citation88,Citation89 In addition, knowledge about the multiprotein Mediator complex, which is well-known for its role in bridging enhancer and promoter into close proximity,Citation90 has also contributed to our understanding about chromosome looping involving AR, wherein the silencing of a Mediator subunit MED1 can significantly impair AR transactivation.Citation91
Moreover, in the past decade, the AR signaling pathway has also been shown to play essential roles in altering chromatin conformation, primarily due to its involvement in a majority of chromosomal translocations identified in prostate cancer. Through FISH analyses, it was discovered that androgen stimulation can induce the spatial proximity between TMPRSS2 and ERG, thus highly augmenting the probability of forming a fusion product when under the stress of DNA double-strand breaks.Citation92 Further evidence demonstrated that AR binding at specific intronic loci near break sites in TMPRSS2, ERG, and ETV1 could result in rapid formation of intra- and interchromosomal interactions that in turn generate enough spatial proximity to predispose the genes for translocation.
In addition, the ensuing modifications of chromatin architecture sensitize these regions to genotoxic stress, making the translocation loci particularly susceptible to double-stranded breaks. The liganded AR, upon binding to DNA, can recruit enzymes – including activation-induced cytidine deaminase (AID) and LINE-1 repeat-encoded ORF2 endonuclease, as well as topoisomerase II beta (TOP2B) to create double-stranded breaks at break sites, which then become ligated through nonhomologous end joining.Citation93,Citation94
Aside from proteins playing an oncogenic function, there has been emerging evidence that long noncoding RNAs (lncRNAs; >200 nt)Citation95 may also adversely affect chromatin structures. For instance, it is recently reported that HOTAIR, a 2.2 kb lncRNA residing in the HOXC locus, serves as a crucial interface between DNA and the chromatin-modifying complex PRC2. As a result, in breast cancer, an overexpression of HOTAIR is causally linked to alterations in the chromatin state reimposed by PRC2 occupancy, consequently permitting a gene expression program that is conducive to cell motility and invasion by silencing key metastasis suppressor genes.Citation96
Another prominent member of the lncRNAs that is recently implicated in cancer is SChLAP1, which was identified as an overexpressed gene in prostate tumor samples.Citation97 Similar to HOTAIR, SChLAP1, in context of prostate cancer, can promote cancer invasion and metastasis. The molecular mechanisms underlying SChLAP1’s oncogenic function were also connected to a chromatin modifying complex, namely SWI/SNF (). Through antagonizing the genomic binding of SWI/SNF, SChLAP1 significantly impairs the transcriptional program directed by SWI/SNF that, as alluded to previously, is a complex that utilizes ATP to mobilize nucleosomes and remodel chromatin.Citation97
Numerous links have been established between SWI/SNF and carcinogenesis;Citation98 however, the latest discovery of SChLAP1, in addition to HOTAIR, sheds new light on the mechanistic basis of how deregulation of lncRNAs may result in defective chromatin organization, which ultimately contributes to oncogenesis. Furthermore, a recent study reports that two lncRNAs overexpressed in prostate cancer, PCGEM1 and PRNCR1 (PCAT8), can bind with AR and facilitate the enhancer-promoter loop formation required for AR transcriptional regulation. It was demonstrated that these lncRNAs can promote AR activation in a hormone-independent environment, providing novel mechanistic insight into the pathogenesis of castration-resistant prostate cancer.Citation99
Therefore, taken together, these lines of encouraging evidence keep propelling scientists forward to continuously uncover novel mechanisms associated with deregulation of chromosomal organization – to provide beneficial insight into strategies for diagnosing as well as treating cancer.
ERG overexpression and chromatin conformation
ERG is overexpressed in prostate cancer due to AR-mediated changes in chromosome rearrangement. As a consequence, ERG overexpression, in turn, can also lead to chromatin structure alterations, which further contribute to prostate cancer development in a feed-forward vicious cycle. The function of ERG involves physical interaction with a number of cofactors as well as transcription factors, including AR – ultimately leading to a transcriptional program favoring the dedifferentiation, invasion, and neoplastic transformation of prostate epithelial cells.Citation100
To characterize the molecular crosstalk between ERG and AR, studies showed that ERG can effectively attenuate AR signaling by the direct transcriptional repression of AR, and additionally ERG occupancy at AR target genes correlates with negative regulation, which is potentiated by ERG-induced EZH2 activity.Citation76,Citation101 It is becoming increasingly clear that ERG complexes with other molecules to coordinately organize chromatin structure. Several interacting partners of ERG have been identified in recent years, including EZH2Citation71,Citation102 and HDAC1,Citation103 which – along with ERG – form a repressor coregulatory network that is important for mediating androgen response in prostate cancer.Citation101 This notion is further supported by the fact that ERG overexpression also dictates changes in the genomewide DNA methylation landscape,Citation104 reflecting a complex regulatory program directed by ERG to impose structural alterations in the overall 3D chromatin topology.
Employing a combination of advanced technologies, including Hi-C, ChIP-seq, and RNA-seq, and integrative bioinformatic analyses, Rickman et alCitation100,Citation105 showed that an overexpression of ERG could induce dramatic changes in 3D chromatin topology, corresponding to the changes in the expression of a group of genes implicated in aggressive prostate cancer. Since ERG binding strongly associated with hotspots of differential chromatin interactions, an upregulation of ERG when fused to TMPRSS2 upon androgen stimulation consequently leads to altered regulation of transcription events.
Among these ERG-regulated genes are genes associated with invasion and migration (eg, FYN, PLAU, MMP3, MMP9, LEF1, and miR200c) and urogenital development (eg, HOXA, B, C gene cluster members, PYGO1, and NKX3.1).Citation100,Citation106–Citation108
Concluding remarks and therapeutic implications
Over the past several decades, we have witnessed a plethora of pioneering studies that established the essential role of chromatin conformation during normal biological processes and oncogenic cellular transformations.
Through investigations of molecular mechanisms governing the alterations in chromatin architecture, researchers have been able to strategically design therapeutic agents which, by abolishing the enzymatic activity of certain chromatin-modifying proteins, to achieve the correct 3D chromatin topology. Several drugs were recently approved by the FDA due to their improved efficacy in prolonging survival and reduced toxicity compared to conventional chemotherapy. Some prominent examples include DNA methylation inhibitor azacitidine (Vidaza®) and decitabine (Dacogen®) and HDAC inhibitors vorinostat (Zolinza®) and romidepsin (Istodax®), which were FDA-approved successively in the last 10 years, for the treatment of myelodysplastic syndrome and cutaneous T-cell lymphoma, respectively.Citation109
Currently, clinical trials are being conducted to examine the pharmacological efficacy of DNMT and HDAC inhibitors in prostate cancer, as adjuvant therapies to complement androgen deprivation.Citation110 In addition, a wide range of chemical inhibitors targeting enzymes, such as EZH2 (eg, DZNepCitation111 and GSK126Citation112) and LSD1 (eg, TCPCitation113 and ORY-1001Citation85), have demonstrated promising potential in various in vitro and in vivo studies for multiple cancer types.Citation85
These molecules hold hopeful prospective for treatment of prostate cancer, in which oncogenic contributors to chromosomal abnormalities are abundant. It is anticipated that future pharmaceutical therapies aimed to restore the physiological activity level of key chromatin modulators would provide desirable curative effects.
Acknowledgments
This work was supported in part by: the National Institutes of Health/National Cancer Institute training grant T32CA09560 (to YAY); National Institutes of Health R01CA172384 (to JY); the US Army Medical Research and Materiel Command grant W81XWH-13-1-0319 (to JY); and the Research Scholar Award RSG-12-085-01 (to JY) from the American Cancer Society.
Disclosure
The authors report no conflicts of interest in this work.
References
- WuDZhangCShenYNephewKPWangQAndrogen receptor-driven chromatin looping in prostate cancerTrends Endocrinol Metab2011221247448021889355
- WoodcockCLGhoshRPChromatin higher-order structure and dynamicsCold Spring Harb Perspect Biol201025a00059620452954
- OudetPGross-BellardMChambonPElectron microscopic and biochemical evidence that chromatin structure is a repeating unitCell1975442813001122558
- HewishDRBurgoyneLAChromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonucleaseBiochem Biophys Res Commun19735225045104711166
- KornbergRDChromatin structure: a repeating unit of histones and DNAScience197418441398688714825889
- OlinsALOlinsDESpheroid chromatin units (v bodies)Science197418341223303324128918
- FinchJTLutterLCRhodesDStructure of nucleosome core particles of chromatinNature197726956232936895884
- LugerKMäderAWRichmondRKSargentDFRichmondTJCrystal structure of the nucleosome core particle at 2.8 A resolutionNature199738966482512609305837
- BickmoreWAThe spatial organization of the human genomeAnnu Rev Genomics Hum Genet201314678423875797
- CremerTCremerCChromosome territories, nuclear architecture and gene regulation in mammalian cellsNat Rev Genet20012429230111283701
- CroftJABridgerJMBoyleSPerryPTeaguePBickmoreWADifferences in the localization and morphology of chromosomes in the human nucleusJ Cell Biol199914561119113110366586
- BridgerJMBickmoreWAPutting the genome on the mapTrends Genet199814104034099820029
- XingYJohnsonCVMoenPTJrMcNeilJALawrenceJNonrandom gene organization: structural arrangements of specific pre-mRNA transcription and splicing with SC-35 domainsJ Cell Biol19951316 Pt 2163516478557734
- CarterKCBowmanDCarringtonWA three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleusScience19932595099133013358446902
- KosakSTSkokJAMedinaKLSubnuclear compartmentalization of immunoglobulin loci during lymphocyte developmentScience2002296556515816211935030
- SimonisMKlousPSplinterENuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C)Nat Genet200638111348135417033623
- DixonJRSelvarajSYueFTopological domains in mammalian genomes identified by analysis of chromatin interactionsNature2012485739837638022495300
- KnezeticJALuseDSThe presence of nucleosomes on a DNA template prevents initiation by RNA polymerase II in vitroCell1986451951043955658
- LiBCareyMWorkmanJLThe role of chromatin during transcriptionCell2007128470771917320508
- KimuraHCookPRKinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2BJ Cell Biol200115371341135311425866
- WorkmanJLNucleosome displacement in transcriptionGenes Dev200620152009201716882978
- Owen-HughesTUtleyRTCôtéJPetersonCLWorkmanJLPersistent site-specific remodeling of a nucleosome array by transient action of the SWI/SNF complexScience199627352745135168662543
- BrunoMFlausAStockdaleCRencurelCFerreiraHOwen-HughesTHistone H2A/H2B dimer exchange by ATP-dependent chromatin remodeling activitiesMol Cell20031261599160614690611
- PhelanMLSchnitzlerGRKingstonREOctamer transfer and creation of stably remodeled nucleosomes by human SWI-SNF and its isolated ATPasesMol Cell Biol200020176380638910938115
- LorchYZhangMKornbergRDRSC unravels the nucleosomeMol Cell200171899511172714
- LorchYMaier-DavisBKornbergRDChromatin remodeling by nucleosome disassembly in vitroProc Natl Acad Sci U S A200610393090309316492771
- KireevaMLWalterWTchernajenkoVBondarenkoVKashlevMStuditskyVMNucleosome remodeling induced by RNA polymerase II: loss of the H2A/H2B dimer during transcriptionMol Cell20029354155211931762
- WhitehouseIFlausACairnsBRWhiteMFWorkmanJLOwen-HughesTNucleosome mobilization catalysed by the yeast SWI/SNF complexNature1999400674678478710466730
- LorchYZhangMKornbergRDHistone octamer transfer by a chromatin-remodeling complexCell199996338939210025404
- AdkinsMWHowarSRTylerJKChromatin disassembly mediated by the histone chaperone Asf1 is essential for transcriptional activation of the yeast PHO5 and PHO8 genesMol Cell200414565766615175160
- SchwabishMAStruhlKAsf1 mediates histone eviction and deposition during elongation by RNA polymerase IIMol Cell200622341542216678113
- WalterPPOwen-HughesTACôtéJWorkmanJLStimulation of transcription factor binding and histone displacement by nucleosome assembly protein 1 and nucleoplasmin requires disruption of the histone octamerMol Cell Biol19951511617861877565770
- SwaminathanVKishoreAHFebithaKKKunduTKHuman histone chaperone nucleophosmin enhances acetylation-dependent chromatin transcriptionMol Cell Biol200525177534754516107701
- KamakakaRTBigginsSHistone variants: deviants?Genes Dev200519329531015687254
- ZhangHRobertsDNCairnsBRGenome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone lossCell2005123221923116239141
- RaisnerRMHartleyPDMeneghiniMDHistone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatinCell2005123223324816239142
- MizuguchiGShenXLandryJWuWHSenSWuCATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complexScience2004303565634334814645854
- ParkYJChodaparambilJVBaoYMcBryantSJLugerKNucleosome assembly protein 1 exchanges histone H2A-H2B dimers and assists nucleosome slidingJ Biol Chem200528031817182515516689
- HenikoffSLabile H3.3+H2A.Z nucleosomes mark ‘nucleosome-free regions’Nat Genet200941886586619639024
- YuenBTKnoepflerPSHistone H3.3 mutations: a variant path to cancerCancer Cell201324556757424229707
- PehrsonJRFriedVAMacroH2A, a core histone containing a large nonhistone regionScience19922575075139814001529340
- DoyenCMAnWAngelovDMechanism of polymerase II transcription repression by the histone variant macroH2AMol Cell Biol20062631156116416428466
- BergerSLThe complex language of chromatin regulation during transcriptionNature2007447714340741217522673
- KouzaridesTChromatin modifications and their functionCell2007128469370517320507
- NoraEPLajoieBRSchulzEGSpatial partitioning of the regulatory landscape of the X-inactivation centreNature2012485739838138522495304
- SanyalALajoieBRJainGDekkerJThe long-range interaction landscape of gene promotersNature2012489741410911322955621
- JinFLiYDixonJRA high-resolution map of the three-dimensional chromatin interactome in human cellsNature2013503747529029424141950
- VilarJMSaizLDNA looping in gene regulation: from the assembly of macromolecular complexes to the control of transcriptional noiseCurr Opin Genet Dev200515213614415797196
- GöndörADynamic chromatin loops bridge health and disease in the nuclear landscapeSemin Cancer Biol2013232909823376421
- NowellPCHungerfordDAChromosome studies on normal and leukemic human leukocytesJ Natl Cancer Inst1960258510914427847
- RowleyJDLetter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa stainingNature197324354052902934126434
- LugoTGPendergastAMMullerAJWitteONTyrosine kinase activity and transformation potency of bcr-abl oncogene productsScience19902474946107910822408149
- TomlinsSARhodesDRPernerSRecurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancerScience2005310574864464816254181
- DemichelisFFallKPernerSTMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohortOncogene200726314596459917237811
- ClarkJPCooperCSETS gene fusions in prostate cancerNat Rev Urol20096842943919657377
- TomlinsSALaxmanBDhanasekaranSMDistinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancerNature2007448715359559917671502
- HermansKGBressersAAvan der KorputHADitsNFJensterGTrapmanJTwo unique novel prostate-specific and androgen-regulated fusion partners of ETV4 in prostate cancerCancer Res20086893094309818451133
- PfluegerDRickmanDSSbonerAN-myc downstream regulated gene 1 (NDRG1) is fused to ERG in prostate cancerNeoplasia200911880481119649210
- PalanisamyNAteeqBKalyana-SundaramSRearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanomaNat Med201016779379820526349
- BergerMFLawrenceMSDemichelisFThe genomic complexity of primary human prostate cancerNature2011470733321422021307934
- YuJCaoQMehraRIntegrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancerCancer Cell200712541943117996646
- YuJCaoQYuJThe neuronal repellent SLIT2 is a target for repression by EZH2 in prostate cancerOncogene201029395370538020622896
- ChenHTuSWHsiehJTDown-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancerJ Biol Chem200528023224372244415817459
- MinJZaslavskyAFedeleGAn oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaBNat Med201016328629420154697
- NakayamaJRiceJCStrahlBDAllisCDGrewalSIRole of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assemblyScience2001292551411011311283354
- MetzgerEWissmannMYinNLSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcriptionNature2005437705743643916079795
- WangQLiWZhangYAndrogen receptor regulates a distinct transcription program in androgen-independent prostate cancerCell2009138224525619632176
- HeHHMeyerCAShinHNucleosome dynamics define transcriptional enhancersNature Genet201042434334720208536
- DryhurstDMcMullenBFazliLRenniePSAusióJHistone H2A.Z prepares the prostate specific antigen (PSA) gene for androgen receptor-mediated transcription and is upregulated in a model of prostate cancer progressionCancer Lett20123151384722055461
- BulgerMGroudineMFunctional and mechanistic diversity of distal transcription enhancersCell2011144332733921295696
- YuJYuJManiRSAn integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progressionCancer Cell201017544345420478527
- WangQCarrollJSBrownMSpatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase trackingMol Cell200519563164216137620
- WangQLiWLiuXSA hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growthMol Cell200727338039217679089
- ChenZZhangCWuDPhospho-MED1-enhanced UBE2C locus looping drives castration-resistant prostate cancer growthEMBO J201130122405241921556051
- VaramballySDhanasekaranSMZhouMThe polycomb group protein EZH2 is involved in progression of prostate cancerNature2002419690762462912374981
- YuJYuJRhodesDRA polycomb repression signature in metastatic prostate cancer predicts cancer outcomeCancer Res20076722106571066318006806
- FrancisNJKingstonREMechanisms of transcriptional memoryNat Rev Mol Cell Biol20012640942111389465
- ZhaoJCYuJRunkleCCooperation between Polycomb and androgen receptor during oncogenic transformationGenome Res201222232233122179855
- CaoQManiRSAteeqBCoordinated regulation of polycomb group complexes through microRNAs in cancerCancer Cell201120218719921840484
- WeichertWRöskeAGekelerVHistone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomyBr J Cancer200898360461018212746
- NelsonWGYegnasubramanianSAgostonATAbnormal DNA methylation, epigenetics, and prostate cancerFront Biosci2007124254426617485372
- SchlesingerYStraussmanRKeshetIPolycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancerNat Genet200739223223617200670
- CaiCHeHHChenSAndrogen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1Cancer Cell201120445747122014572
- GrassoCSWuYMRobinsonDRThe mutational landscape of lethal castration-resistant prostate cancerNature2012487740623924322722839
- HelinKDhanakDChromatin proteins and modifications as drug targetsNature2013502747248048824153301
- LupienMBrownMCistromics of hormone-dependent cancerEndocr Relat Cancer200916238138919369485
- LupienMEeckhouteJMeyerCAFoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcriptionCell2008132695897018358809
- RobinsonJLHolmesKACarrollJSFOXA1 mutations in hormone-dependent cancersFront Oncol201332023420418
- JinHJZhaoJCOgdenIBerganRCYuJAndrogen receptor-independent function of FoxA1 in prostate cancer metastasisCancer Res201373123725373623539448
- KageyMHNewmanJJBilodeauSMediator and cohesin connect gene expression and chromatin architectureNature2010467731443043520720539
- WangQSharmaDRenYFondellJDA coregulatory role for the TRAP-mediator complex in androgen receptor-mediated gene expressionJ Biol Chem200227745428524285812218053
- ManiRSTomlinsSACallahanKInduced chromosomal proximity and gene fusions in prostate cancerScience20093265957123019933109
- LinCYangLTanasaBNuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancerCell200913961069108319962179
- HaffnerMCAryeeMJToubajiAAndrogen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangementsNat Genet201042866867520601956
- DjebaliSDavisCAMerkelALandscape of transcription in human cellsNature2012489741410110822955620
- GuptaRAShahNWangKCLong non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasisNature201046472911071107620393566
- PrensnerJRIyerMKSahuAThe long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complexNat Genet201345111392139824076601
- RobertsCWOrkinSHThe SWI/SNF complex – chromatin and cancerNat Rev Cancer20044213314214964309
- YangLLinCJinClncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programsNature2013500746459860223945587
- RickmanDSSoongTDMossBOncogene-mediated alterations in chromatin conformationProc Natl Acad Sci U S A2012109239083908822615383
- ChngKRChangCWTanSKA transcriptional repressor co-regulatory network governing androgen response in prostate cancersEMBO J201231122810282322531786
- KunderfrancoPMello-GrandMCangemiRETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancerPLoS One201055e1054720479932
- IljinKWolfMEdgrenHTMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogrammingCancer Res20066621102421024617079440
- KronKTrudelDPetheVAltered DNA methylation landscapes of polycomb-repressed loci are associated with prostate cancer progression and ERG oncogene expression in prostate cancerClin Cancer Res201319133450346123549870
- ElementoORubinMARickmanDSOncogenic transcription factors as master regulators of chromatin topology: a new role for ERG in prostate cancerCell Cycle201211183380338322918253
- TomlinsSALaxmanBVaramballySRole of the TMPRSS2-ERG gene fusion in prostate cancerNeoplasia200810217718818283340
- KimJWuLZhaoJCJinHJYuJTMPRSS2-ERG gene fusions induce prostate tumorigenesis by modulating microRNA miR-200cOncogene2013
- WuLZhaoJCKimJJinHJWangCYYuJERG is a critical regulator of Wnt/LEF1 signaling in prostate cancerCancer Res201373196068607923913826
- BoumberYIssaJPEpigenetics in cancer: what’s the future?Oncology (Williston Park)201125322022622821548464
- LinJWangCKellyWKTargeting epigenetics for the treatment of prostate cancer: recent progress and future directionsSemin Oncol201340339340123806502
- TanJYangXZhuangLPharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cellsGenes Dev20072191050106317437993
- McCabeMTOttHMGanjiGEZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutationsNature2012492742710811223051747
- SchenkTChenWCGöllnerSInhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemiaNat Med201218460561122406747
